Abstract
This article reviews the literature on management of chronic cyclical pelvic pain (CCPP). Electronic resources including Medline, PubMed, CINAHL, The Cochrane Library, Current Contents, and EMBASE were searched using MeSH terms including all subheadings and keywords: “cyclical pelvic pain”, “chronic pain”, “dysmenorrheal”, “nonmenstrual pelvic pain”, and “endometriosis”. There is a dearth of high-quality evidence for this common problem. Chronic pelvic pain affects 4%–25% of women of reproductive age. Dysmenorrhea of varying degree affects 60% of women. Endometriosis is the commonest pathologic cause of CCPP. Other gynecological causes are adenomyosis, uterine fibroids, and pelvic floor myalgia, although other systems disease such as irritable bowel syndrome or interstitial cystitis may be responsible. Management options range from simple to invasive, where simple medical treatment such as the combined oral contraceptive pill may be used as a first-line treatment prior to invasive management. This review outlines an approach to patients with CCPP through history, physical examination, and investigation to identify the cause(s) of the pain and its optimal management.
Keywords: cyclical pelvic pain, chronic pain, dysmenorrhea, nonmenstrual pelvic pain, endometriosis
Introduction
Chronic pelvic pain is defined as pelvic pain occurring for at least 6 months with sufficient intensity to interrupt normal activities of daily life and requiring medical or surgical treatment. Chronic cyclic pelvic pain (CCPP) is traditionally considered as a subset of chronic pelvic pain that occurs in relation to the menstrual cycle.1 However, CCPP also incorporates pelvic pain that occurs with a cyclic pattern, which may not be related to the menstrual cycle. For example, pelvic pain that occurs at the time of intercourse may give rise to a pattern of pain, which can be due to nonhormonal causes such as pelvic floor myalgia, provoked vestibulodynia, or pudendal neuralgia (PN). Other events can be responsible for CCPP such as ovulation giving rise to Mittelschmerz pain. Although pain occurring with interstitial cystitis (IC) or irritable bowel syndrome (IBS) occurs without a particular pattern, patients may present with CCPP as this pain can be exacerbated by menstruation. Gynecologists need to be aware of a range of differential diagnoses for the optimal management of patients with CCPP.
Methods
This review was produced by searching the electronic resources including Medline, PubMed, CINAHL, The Cochrane Library (including the Cochrane Database of Systematic Reviews), Current Contents, and EMBASE. The MeSH terms included all subheadings and keywords: “cyclical pelvic pain”, “chronic pain”, “dysmenorrhea”, “nonmenstrual pelvic pain”, and “endometriosis”.
Epidemiology
The exact epidemiology of CCPP is difficult to determine and there is paucity of data in this subset area of pelvic pain. Estimates from the reported prevalence of chronic pelvic pain which ranges from 4% to 25%2–4 and endometriosis, the most common gynecologic pathology causing chronic pelvic pain of 1%–7%5,6 would suggest that between 5%–10% of women may be affected. Primary dysmenorrhea of varying degree is reported to affect 60% of adult women7 and 72% of adolescents,8 with 12%–14% adults and 17% adolescents describing this as severe.9
Etiology
A large proportion of CCPP is due to hormonally driven pathology where the pain cycle is synchronous with the menstrual cycle. Although physiological causes including Mittelschmerz pain and primary dysmenorrhea are the most common cause of CCPP, these are typically mild. The most common gynecological pathology responsible for CCPP is endometriosis.1 Adenomyosis and fibroids can also give rise to CCPP, whereas cervical stenosis or an obstructive abnormality is a less common cause.
Other pelvic organs, including the gastrointestinal tract or urinary tract can be a source of cyclical pelvic pain, as well as the musculoskeletal or neurological system. There is an overlap between pelvic organs and muscles that share the same nerve supply, and a variety of contributing causes may coexist.10 Possible etiologies of chronic pelvic pain are listed in Table 1, and are discussed individually in the systems review.
Table 1.
Etiologies of chronic pelvic pain
| Gynecological | Physiologic |
| Primary dysmenorrheaa | |
| Mittelschmerza | |
| Pathologic | |
| Endometriosisa | |
| Adenomyosisa | |
| Fibroid (uterine leiomyoma)a | |
| Cervical stenosis or obstructive abnormalitya | |
| Pelvic venous congestion syndromea | |
| Ovarian remnant/residual ovary syndrome | |
| Pelvic adhesions | |
| Postpelvic inflammatory disease | |
| Endosalpingiosis | |
| Gastrointestinal tract | Irritable bowel syndromea |
| Inflammatory bowel disease | |
| Chronic constipation | |
| Urinary tract | Interstitial cystitis |
| Nervous system | Pudendal neuralgia |
| Provoked vestibulodynia | |
| Muscloskeletal | Pelvic floor myalgia |
| Myofascial pain | |
| Psychosocial | Depression |
| Physical/sexual abuse | |
| Drug seeking behavior |
Note: May cause cyclic pelvic pain in relation to menstrual cycle.
A pragmatic approach to women with chronic cyclical pelvic pain
History
The most important part of the assessment for CCPP is accurate and meticulous history taking. A detailed, chronological history of pain must be obtained. This should include timing, onset and nature of pain, as well as its location, radiation, precipitating and relieving factors. Associated symptoms denoting the gastrointestinal system, urinary tract, musculoskeletal system should be elicited. Specific questions about pain during urination or defecation will aid in the management of CCPP. History should include results of previous investigation and treatments, both successful and unsuccessful. Current impact of pain on the patient’s quality of life, and the amount of medication used can be used as indicators of response to treatment.
Menstrual and obstetric background is an essential part of the history, with particular attention to cycles of pain in relation to menstruation. Onset of associated symptoms, such as dysuria or change of bowel habit in relation to menstrual cycle should be noted. A history of previous trauma such as instrumental delivery or episiotomy should be elicited. Desire for future fertility is crucial, as it will determine feasible treatment options. Sexual history may reveal the presence of deep dyspareunia and determine risk factors for pelvic inflammatory disease (PID).
A history of depression and other psychological disorders should be obtained. Although there is no definite causal relationship between depression and pelvic pain, the incidence of depression is higher in chronic pain patients.11 Patients with chronic pain also seem to have a higher incidence of previous physical or sexual abuse.12,13 Previous stressful experiences may have altered the neuropsychological process of perceiving pain, leading to excessive pain. Establishing trust and good rapport with the patient will help in identifying these issues. It may be best to leave this until a subsequent visit, rather than addressing it at the patient’s first visit.
Physical examination
The aim of the examination is to look for pathology, as well as to reproduce the patient’s usual pain to help identify the cause. Given the intermittent nature of pain in CCPP, it is best to examine the patient when she is in pain. For gynecologists seeing patients with chronic pelvic pain, it is important to examine the patient as a whole, rather than just examining the uterus and cervix.
General inspection of the patient during history taking should include general well being, posture, gait, and scoliosis. Such musculoskeletal abnormalities may predispose patients to chronic pain and alter their pain perception.14 Ask the patient to empty her bladder, and perform an urinalysis. Examination of the abdomen should take place with the patient supine, to inspect for scars, masses, and distension. Palpate for organomegaly, fecal loading, or other masses. Eliciting allodynia (vigorous pain response to light localized pressure) may indicate psychologically driven pain or advanced chronic pain syndrome. The head raise test (Carnett’s test) is performed by asking the patient to lift their head whilst in the supine position. If pain is lessened by head raising then the pain is likely intraperitoneal as the rectus muscles protect the peritoneum from stretch. If, however, the pain is worsening by head raising then abdominal wall pathology is suspected.
The patient is then placed in lithotomy position and the vulva is inspected for irritation, inflammation, lesions, ulcers, or discharge. Particular attention should be given to vulvar vestibulitis, which is common in women with chronic pelvic pain. This condition can cause burning introital discomfort with intercourse. Perform a vaginal examination by inserting 1 finger into the introitus and have the patient contract and relax her pelvic floor. This assesses tone and muscle control. The muscles of the pelvic floor and their attachments should be palpated with gentle compression, including puborectalis, pubococcygeus, coccygeus, piriformis, and obturator internus for tenderness and rigidity. They should not be tender and the patient should be able to contract and relax these muscles voluntarily. The ischial spine and Alcock’s canal are gently palpated bilaterally for tenderness and/or fibrosis, which may aid in diagnosis of PN.15
Palpate the anterior vaginal wall to locate tenderness along the urethra and bladder base. Tenderness may indicate possible lower urinary tract involvement. The cervix is gently palpated for motion tenderness. The posterior vaginal wall is palpated, especially looking for rectovaginal nodularity or localized tenderness which may be indicative of endometriosis. A bimanual examination is performed, as gently as possible, to delineate the uterus and adnexa, looking for mobility, tenderness, and masses. A tender uterus may reflect the presence of adenomyosis. A speculum examination is performed if swabs or a Pap smear is required, but a well performed digital examination is preferred, as it is less likely to provoke pain and muscle spasms after examination. Per rectal examination is reserved for cases where compressing pathology is suspected or symptoms such as per rectal bleeding are present.
Investigations
After obtaining a thorough history and physical examination, focused investigations need to be arranged, tailored to each patient depending on the information obtained from the history and examination. It should commence with those that are simple, inexpensive, and widely available, such as urinalysis, midstream urine specimen for microscopy, culture and sensitivity, vaginal swabs, and serological investigation such as full blood count, C-reactive protein, or CA 125.
Diagnostic imaging should be performed only when indicated by history and physical findings. Transvaginal and transabdominal ultrasound are valuable modality to evaluate the female genital tract, particularly when assessing structural abnormalities such as fibroids, adenomyosis, and adnexal pathology such as ovarian cysts or hydrosalpinges. It is effective in distinguishing cystic from solid lesions and allows information on vascular characteristics of the mass. Ultrasound is readily available, relatively inexpensive, and does not expose the patient to radiation. The ultrasound transducer can also identify points of maximal tenderness to aid in diagnosis of CCPP. However, in endometriosis, it is important to remember that pelvic ultrasound will only aid in determining the presence of deeply infiltrating endometriosis or involvement of the ovaries with the presence of an endometrioma, which accounts for less than 20% of patients with endometriosis. Ultrasound is not helpful in diagnosing small volume peritoneal disease.16
Computed tomography has a limited role in imaging the female genital organs but can provide important information on involvement of the gastrointestinal tract,17 although transrectal ultrasonography offers higher sensitivity for pathology such as invasive rectovaginal endometriosis.18 Magnetic resonance imaging (MRI) is useful in characterizing pelvic masses as well as diagnosing adenomyosis, endometriomata, or deep endometriosis but its use is limited by high cost and varying availability.
Laparoscopy is indicated in patients with CCPP where a pelvic abnormality is suspected to find and treat contributing factors. Laparoscopy is the gold standard to diagnose pathology such as endometriosis or adhesions. As all surgical investigations carry morbidity, thorough clinical assessment may lead to avoidance of unnecessary surgical procedures.
Systems review
The majority of CCPP is due to gynecological causes, which are frequently hormonally driven. However, any abdominal or pelvic structures may cause CCPP. The following is an outline of common conditions that may cause CCPP in different systems. It is important to remember that there is significant overlap and interaction between different systems and more than 1 diagnosis for the pain may be present.
Gynecologic causes
Primary dysmenorrhea
Primary dysmenorrhea refers to menstrual pain without pelvic pathology. This is the most common cause of CCPP in women of reproductive age, affecting 60% of women7 and 72% of adolescents.8 It is often described as recurrent, crampy, colicky pain in the suprapubic region that occurs during menses associated with onset of menstrual flow with a typical duration of 2–3 days. It may be accompanied with nausea, fatigue, bloating and general malaise, and may radiate to the lower back and thighs.
Studies have shown that declining uterine progesterone levels in the late luteal phase during endometrial sloughing removes inhibition of arachidonic acid production, which is subsequently metabolized by the cyclooxygenase (COX)-2 pathway into eicosanoids19,20 (including leukotrienes and prostanoids). These vasoactive eicosanoids cause abnormal uterine contractions leading to decreased uterine blood flow and subsequent ischemia, causing pain. This is supported by Doppler ultrasound studies showing high uterine artery resistance on the first day of menses in patients with primary dysmenorrhea compared with a control group.21 Dawood demonstrated that the intensity of menstrual cramps and associated symptoms of dysmenorrhea are directly proportional to the amount of prostaglandins released in endometrial secretions,22,23 making excessive production of prostaglandins the problem rather than an increased sensitivity to the effect of prostaglandins. It has been suggested that vasopressin may also be involved in the etiology of primary dysmenorrhea, but its role remains controversial.24 How this would fit into therapeutic management is yet to be determined but may be in the form of additional analgesia for this particular problem.
The diagnosis of primary dysmenorrhea is made from a characteristic symptom history and the rapid improvement on appropriate therapy. As it is a diagnosis of exclusion, a detailed history and physical examination to look for symptoms and signs indicative of pelvic pathology (such as endometriosis, adenomyosis, fibroid, PID, and pelvic adhesions) are required. Physical examination in patients with primary dysmenorrhea will be normal and laboratory tests, imaging studies, and laparoscopy are not mandatory to exclude these disorders, but should be performed as indicated, if pelvic disease is suspected.
Nonsteroidal anti-inflammatory drugs (NSAID) and the combined oral contraceptive pill (COCP) are the mainstay of treatment for primary dysmenorrhea. There is class I and II evidence that NSAID are an effective treatment for primary dysmenorrhea25–27 although this is insufficient to determine which individual NSAID is most effective and safe. The COCP is an effective treatment for primary dysmenorrhea28,29 in particular for women wishing to use hormonal contraception, although limited evidence is available on the efficacy of different preparations. It acts via suppressing ovulation and thinning the endometrium to reduce prostaglandin production, uterine blood flow, and cramps.
Mittelschmerz pain
“Mittelschmerz” (German for “middle pain”) refers to pelvic pain in ovulatory women that occurs in the middle of a menstrual cycle. It is a sharp pelvic pain that may be felt low in the abdomen on either the right or left side. This pain is typically mild and unilateral, lasting for a few hours to few days, and is seldom severe enough to distress women. It is thought to be due to normal follicular enlargement just prior to ovulation, or to normal follicular rupture and the subsequent intraperitoneal spillage of follicular fluid and blood.30
Endometriosis
Endometriosis is the most common gynecological pathology causing CCPP accounting for 12%–32% of women of reproductive age undergoing laparoscopy for pelvic pain, and for 45%–70% in adolescents.31 Endometriosis is defined as the presence of endometrial glands and stroma outside of the endometrial cavity. Up to 75% of symptomatic endometriosis causes cyclic pelvic pain with menstruation,32 though it is often associated with several different pain symptoms including noncyclical, nonmenstrual pelvic pain.33–35 A range of other symptoms such as deep dyspareunia, dyschezia, subfertility, and abnormal menstrual bleeding may be present and may reflect the site and depth of endometriotic infiltration. In particular, deep dyspareunia and dyschezia are suggestive of posterior deep infiltrating endometriosis at the rectovaginal septum.36 It is important to note that the severity of symptoms does not correlate with the severity of endometriosis.37
Physical examination in endometriosis is important, as examination findings such as uterosacral nodularity or palpable induration or tenderness in the posterior cul de sac or uterosacral ligaments may alter management. In advanced disease, lateral displacement of the cervix may be seen on speculum examination due to unilateral shortening of a diseased uterosacral ligament, or an ovarian endometrioma may be palpable during bimanual examination.
Laparoscopy and biopsy with histological findings of endometrial glands and stroma in the peritoneal tissues or other tissue biopsies is the gold standard in making the diagnosis of endometriosis.38 Although ultrasound is helpful in assessing the extent of disease by detecting endometriomas and deeply invasive uterosacral and rectal disease, it is not helpful in the diagnosis of small volume peritoneal disease.16 Recent updates on nerve fibers in the endometrium of women with endometriosis may offer a new diagnostic investigation of endometriosis.39
In determining the treatment of endometriosis, the patient’s desire of future fertility, side effect profile, and personal preference needs to be accounted for. The best medical treatments for endometriosis include the COCP and progestins in their various forms since they may be used in the long term, have acceptable side effect profiles and are inexpensive. Other treatments including, androgenic agents such as danazol, and centrally acting drugs like gonadotrophin-releasing hormone (GnRH) analogs are limited by cost, duration of activity and more significant side effect profiles, without offering significantly greater benefits than the COCP.40 COCPs are effective41 but will of course prevent conception and may predispose to side effects such as migraine and thromboembolic disorders in some individuals. The use of the combined oral contraceptive on a continuous basis is effective in arresting menstruation, thus preventing dysmenorrhea. Progestogens have a variety of mechanisms of action including suppression of ovarian activity and have been demonstrated to be effective.42,43 The side effects may include irregular menstrual bleeding, weight gain, mood swings, and decreased libido. Androgenic agents that disrupt the menstrual cycle and often induce amenorrhea may be effective in endometriosis44 but the side effects such as acne, greasy skin, and deepening of the voice can be unacceptable to patients and occasionally irreversible. GnRH analogs can be used to suppress ovarian function and will result in symptomatic relief of the pain symptoms of endometriosis45 but the side effects such as the development of menopausal symptoms and the loss of bone mineral density limit its long-term use. Further development of new medical interventions such as Selective Estrogen Receptor Modulators, antiprogestogens, and antiangiogenic agents may provide further medical treatment options with fewer side effects in the future. Because of the chronic nature of endometriosis, medical treatment needs to be long term with low morbidity or side effects, making the COCP or progestogens the favored choice.
Surgical treatment of endometriosis aims to remove visible areas of endometriosis and restore anatomy by division of adhesions. This is the preferred option for those seeking fertility or presenting with advanced disease with the presence of endometrioma or abnormal physical examination findings suggestive of deeply invasive endometriosis. Endometriomas are not amenable to medical treatment, and need to be removed surgically even if symptoms improve with medical treatment.46 Patients with deeply invasive disease in the rectovaginal space and uterosacral ligaments benefit most with surgical excision, with reduction of dysmenorrhea, as well as dyspareunia and nonmenstrual pelvic pain.47–49 Regardless of the stage of endometriosis, randomized controlled trials comparing the effect of surgery to conservative management have shown that surgery and excision of endometriosis results in symptomatic improvement.32,50,51
Since Doyle52 described a technique to destroy the nerve pathways thought to be responsible for carrying pain fibers from the pelvis in 1955, surgical interruption of pelvic nerve pathways has been increasingly used for symptomatic treatment of endometriosis. Laparoscopic uterine nerve ablation (LUNA) involves the transection of the uterosacral ligaments at their insertion into the cervix, where presacral neurectomy (PSN) involves the total removal of the presacral nerves lying within the boundaries of the interiliac triangle. Both procedures interrupt the majority of the cervical sensory nerve fibers thus diminishing uterine pain. Although observational studies have supported the use of both procedures53–55 results from randomized controlled trials demonstrate that the use of LUNA has no place in the management of chronic pelvic pain. Although PSN may be of benefit, it comes with the considerable risk of side effects including long-term bowel and bladder dysfunction and its use is recommended in patients who have been well counseled and it should be performed by experienced surgeons.50,56
Adenomyosis
Adenomyosis refers to a disorder in which endometrial glands and stroma are present within the uterine myometrium. It is estimated to affect 20% of women of reproductive age, though the exact prevalence is unknown as only histological examination at hysterectomy makes the diagnosis definite.57 Although menorrhagia is the most common presenting symptom of adenomyosis, 25% of women present with cyclic pelvic pain with menstruation,57 where pain may be due to bleeding and swelling of endometrial islands confined by myometrium.
The ectopic endometrial tissue appears to induce hypertrophy and hyperplasia of the surrounding myometrium, which results in a diffusely enlarged uterus. Ultrasound is helpful in the diagnosis with a sensitivity of 65% and specificity of 65%–98%.58,59 Although MRI has a higher sensitivity of 70% and specificity of 86%–98% for adenomyosis, cost and availability limits its use. Although medical treatment with progestogens and the COCP may relieve dysmenorrhea and menorrhagia, symptoms usually reoccur with cessation of treatment, making hysterectomy the only definitive treatment. Levonorgestrel-releasing intrauterine system have been shown to provide symptomatic relief up to 3 years60 and may offer an alternative to surgery. Conservative surgery such as endometrial ablation or resection has been helpful in some patients, though follow-up has been limited to 3 years.61,62
Fibroid (uterine leiomyoma)
CCPP may be due to fibroids, frequently accompanied by menorrhagia. Uterine fibroids are the most common pelvic tumor in females, affecting 20%–40% of women of reproductive age.63 Although abnormal uterine bleeding is a common symptom from fibroids, cyclical pelvic pain may arise from uterine contraction and heavy menstrual flow. Fibroids may be responsible for deep dyspareunia, particularly anterior and fundal fibroids.64
Clinical diagnosis is often possible with ultrasound being the investigation of choice to confirm the clinical findings.65 Treatment needs to be tailored to the individual patient depending on the symptoms, size and location of fibroids, and desire for future fertility. Medical treatments include COCPs, progestogens, levonorgestrel-releasing intrauterine system, or GnRH analogs, though long-term failure rates are high.66 Conservative surgical treatments for women who want to retain their fertility offer a viable alternative to hysterectomy and the route is dependent on the location and size of the fibroid with hysteroscopic resection of submucous fibroids being the treatment of choice for smaller lesions and laparoscopy and laparotomy being alternatives for lesions located elsewhere in the uterus, dependent on surgical skills. Hysterectomy is a definitive surgery, whereas uterine artery embolization and surgical uterine artery ligation are alternative treatment options.
Cervical stenosis, intrauterine adhesions, and congenital obstructive Mullerian abnormality
Cervical stenosis from previous surgery (including dilatation and curettage, large loop excision of the transformation zone, or cone biopsy) or tumor (endometrial or cervical cancer) can be a cause of CCPP. New onset dysmenorrhea after cervical procedures strongly suggests that cervical stenosis has developed and warrants thorough physical examination and dilatation of the cervix to allow access to, and drainage from the uterine cavity. Pending patient comfort can be performed as an outpatient procedure with dilators, or hysteroscopy or an ultrasound-guided procedure under general anesthesia. Alternative treatment options such as hysteroscopic resection of endocervical tissue67 or laser vaporization68 have been described.
Pelvic pain from intrauterine adhesions (Asherman syndrome) is commonly cyclic and associated with menstrual dysfunction. Pain is usually associated with decreased or absent menstrual flow, the mechanism may be due to outflow obstruction with backflow into the fallopian tubes, creating a hematosalpinx and retrograde menstruation, or as a result of pockets of residual endometrium responding to hormonal stimuli, with no route for egress.69 Hysteroscopy is the gold standard for diagnosis of intrauterine adhesions.70,71
Townsend et al72 first described postablation tubal sterilization syndrome (PATSS), which may be responsible for a new onset of CCPP. It occurs after total endometrial ablation from intrauterine contracture that forms from the collapsing of intrauterine walls after removal of distension medium, where persisting endometrium in cornua can cause symptomatic cornual hematometra (CH) or retrograde menstruation, which in patients who have had tubal ligation can cause painful distension of the proximal tube. Retrospective studies have demonstrated the incidence of CH or PATSS may occur in up to 10% of patients who undergo endometrial ablation, requiring salpingectomy or hysterectomy as a treatment;73 however, this is likely to be an overestimation due to selection bias when large-scale prospective studies of endometrial ablation are considered.
Congenital anomalies of the female genital organs are rare causes of CCPP with the mechanism for pain often due to complete or total outlet obstruction. These problems present in adolescents in conjunction with primary amenorrhea or abnormal menstruation. Imperforate hymen, Mullerian anomalies such as vaginal agenesis in Mayer-Rokitansky- Küster-Hauser syndrome, lateral fusion defects such as a rudimentary horn or vertical fusion defects such as cervical or vaginal agenesis can present with CCPP although pain can be noncyclical where there is persistent mechanical stretch on the tissues. A thorough history and physical examination looking for hematometra or hematocolpos, with pelvic ultrasound correlation is warranted. As renal anomalies are found in 20%–30% of women with Mullerian defects,74 radiological renal investigation such as renal ultrasound is indicated in all women with Mullerian defects.
Ovarian remnant and residual ovary syndrome
CCPP is a common presenting symptom from ovarian remnant or residual ovary syndrome. Ovarian remnant syndrome occurs in patients who have undergone bilateral oophorectomy with symptoms from the ovarian remnant, which was inadvertently left behind. Residual ovary syndrome occurs in patients whose ovaries were intentionally preserved at the time of surgery, who present subsequently with pathology. Patients often present with cyclic pelvic pain with mechanoreceptors often engaged due to the confined space of the entrapped tissue which may result in a mass that can be palpated or demonstrated on an ultrasound or other imaging technique. Of the patients who undergo hysterectomy with conservation of ovaries for benign indications, 2%–3% present with residual ovary syndrome requiring further operation, where most pain can be attributed to functional cysts and benign tumors. Malignant tumors of the ovary may be responsible in 12% of cases.75
Pelvic venous congestion syndrome
Pelvic venous congestion, a controversial entity, has also been proposed as a cause of pelvic pain with menstrual exacerbation.76 It refers to a condition in which characteristic symptoms of shifting location of pain, deep dyspareunia, and exacerbation of pain after prolonged standing occur. These symptoms are associated with radiological findings of pelvic varicosities that display reduced blood flow, though dilated ovarian veins are frequently seen on imaging in asymptomatic parous women.77
Other gynecological causes
Pelvic adhesions, chronic PID, and endosalpingiosis will usually cause more persistent chronic than cyclic pain, and these conditions are not responsive to hormonal treatments. Despite the common attribution of unilateral pelvic pain to an ovarian cyst, ovarian cysts do not usually cause pain,78 unless complicated by hemorrhage, rupture, or torsion.
Pelvic adhesions can be the result of previous surgery, infection, inflammation (endometriosis), or assisted reproductive technology procedures. An ongoing debate continues as to the cause of the pain in the presence of adhesions. In spite of several studies, correlation between pelvic pain and adhesions still remains uncertain. For patients in this category, a multidisciplinary team approach is frequently required. PID can be complicated by chronic pelvic pain in 18%–33% of women regardless of the mode of antibiotic therapy.79,80 The exact mechanism of post-PID chronic pain is unknown, although it is thought to be from pelvic adhesions or neurological priming. Endosalpingiosis is the finding of ciliated tubal epithelium outside of the fallopian tubes. Once the diagnosis is histologically confirmed, management is symptomatic, and medical or surgical treatments are often ineffective.81
Gastrointestinal tract
Irritable bowel syndrome
IBS, which affects 10%–20% of adults in developed countries,82–84 is a functional gastrointestinal disorder characterized by abdominal pain and bowel symptoms such as bloating, urgency, diarrhea, and constipation. Cross-sectional studies have shown that approximately one-third of women with chronic pelvic pain have IBS.85,86 IBS is most prevalent during menstruating years and is exacerbated during menstruation,87,88 hence presenting with a cyclical pattern of pain, accompanied with dyspareunia and dysmenorrhea.89,90 Colonoscopy should be considered in high-risk patients to exclude other pathology such as malignancy or inflammatory bowel disease.91,92 Treatment involves dietary manipulation and antispasmodic agents with fiber supplementation to relieve symptoms.93,94
Patients with inflammatory bowel disease present with intermittent crampy abdominal pain along with fatigue, diarrhea, weight loss, and/or rectal bleeding, and appropriate endoscopy with biopsy is required to make a diagnosis. Although chronic constipation is common in women, chronic cyclic pain from constipation is not a common symptom.95
The urinary tract
Interstitial cystitis
IC is a noninfectious chronic inflammatory condition of the bladder, where the etiology and pathophysiology are poorly understood. IC can present as CCPP with associated urinary symptoms (frequency, dysuria, urgency, or nocturia) that can fluctuate during the menstrual cycle with premenstrual flare,96–98 or worsen during or after intercourse.96 There is a large overlap between IC and chronic pelvic pain, as demonstrated by a prospective multicenter trial showing 84% of women with chronic pelvic pain having urological symptoms consistent with IC.99 This condition has been reported to commonly coexist with endometriosis and may make the diagnosis of either more difficult.100
Urinalysis and a midstream urine sample should be obtained to exclude infection. Cystoscopy with hydrodistention of the bladder may aid in diagnosis, demonstrating glomerulation and submucosal hemorrhage, as well as being therapeutic, giving symptomatic relief in 20%–30% for several months.101 A potassium sensitivity test where the bladder is filled with potassium chloride solution, leading to more pain when compared with saline, is not recommended for routine use, due to low specificity.102 Although there is no curative therapy, management should include simple analgesics, bladder retraining together with physiotherapy, and dietary modification to exclude acidic foods. Other medical treatments include antihistamines, azathioprine, corticosteroids, and heparin. A small prospective study looking at a subgroup of women with CCPP and IC with negative laparoscopy for endometriosis, demonstrated symptomatic improvement in 83% women when treated with the oral contraceptive pill.98
Musculoskeletal system
Pelvic pain can arise from muscles of the pelvic floor as well as muscles of the abdominal wall, back, hips, and upper thighs. There is increasing recognition that spasms of these muscles can be primary or secondary causes of pelvic pain. Up to 85% of patients with chronic pelvic pain have musculoskeletal dysfunction such as muscular spasm of the pelvic floor103,104 or postural changes such as scoliosis and pelvic rotation.105 Abnormal postures from such musculoskeletal dysfunction increase muscular tensions and spasm with consequent muscle shortening which then exacerbates or prolongs pain. Although it causes more constant pelvic pain than cyclic pain, it is important to consider musculoskeletal pain as a possible etiology for chronic pelvic pain and examine for posture, scoliosis, pelvic asymmetry, as well as performing vaginal examination of the pelvic floor muscles.
Pelvic floor myalgia is usually associated with involuntary spasm of the pelvic floor muscles. This pain can be either continuous or episodic and may manifest as a sense of aching, heaviness, or burning.106 It is not associated with the menstrual cycle, although the pain may be exacerbated by menses.107 Sometimes sexual intercourse or certain activities can be the trigger for a flare. Since dyspareunia is the most common symptom of this problem, a pattern of pain with intercourse should be sought, and hence it can be considered as a cyclic cause of pain.
Manual therapy by experienced physiotherapists is the best treatment for women affected and involves a personalized program of stretching, direct digital compression, and relaxation of affected muscles. In refractory cases, injection of botulinum toxin type A into pelvic floor muscles may be useful in reducing pelvic floor spasm and pelvic pain.108,109
Neurological
Patients with PN present with severe sharp pain along with the anatomical territory of the pudendal nerve (vagina, vulva, labia, perineum, and anorectal region). As pain is aggravated by intercourse, the patient may present with a distinct pattern of pain, with dyspareunia and vulvar pain.110 It is also precipitated by prolonged sitting, and relieved by standing. The pathophysiology of PN is not clear, but is believed that a neuronal insult from stretching and compression is a significant contributor. A multidisciplinary working party has described clinical diagnostic criteria for PN in 2006 (Nantes criteria111), where the 5 essential diagnostic criteria are: (1) pain along the anatomical distribution of the pudendal nerve; (2) the pain is aggravated by sitting; (3) the patient is not awakened at night by the pain; (4) there is no objective sensory loss on clinical examination; and (5) the pain is improved by an anesthetic pudendal nerve block. Treatment options range from conservative physical therapy to nerve blockade, surgical decompression and neuromodulation, though there are very few randomized controlled trials on efficacies for treatment techniques for PN.
Provoked vestibulodynia is a pain in the vaginal vestibule evoked by any kind of stimulation such as intercourse and light touch. It is reported to affect up to 12%–15% of women of reproductive age at some time of their life.112,113 It is one of the diagnoses under the umbrella of “vulvodynia” – a group of problems, rather than a specific diagnostic category. As the most common cause of dyspareunia,114,115 provoked vestibulodynia can present with a nonmenstrual pattern of pelvic pain. It may result from intraepithelial neural hyperplasia that leads to nociception in this area, though the pathophysiology remains poorly understood.116,117 Treatment options include physiotherapy with biofeedback, medical treatment with tricyclic antidepressants, topical anesthetics, local steroid injections, and vestibulectomy. Although randomized controlled trials and prospective trials showing vestibulectomy is more effective than behavioral treatment,118,119 due to its invasiveness and risk of worsening or reoccurrence of pain, surgery needs to be carefully considered.
Psychosocial
Although physical or sexual abuse is a rare cause of pelvic pain, it often makes it more difficult for the women to deal with pelvic pain, which may be reflected as CCPP. Untreated or unresolved abuse may interfere with the patient’s ability to cope with pain, and needs to be addressed appropriately. Depression is the most common emotional consequences of chronic pain and may become an obstacle to treat the actual pathology if left undiagnosed. Counseling and cognitive behavioral therapy by an appropriate health care professional, together with medical management of CCPP, may be the most effective treatment in affected women.
Management
Management of CCPP need to be tailored to the specific cause identified through history, examination and investigation, as well as the severity of pain and the patient’s desire for fertility. It is possible to undertake management without a specific diagnosis, such as with NSAID and COCP in patients not wishing an invasive management, and this approach may also be cost effective. The evidence of management of chronic pain in general is poor with studies usually including small numbers with limited follow-up of 6 months to a maximum of 5 years.
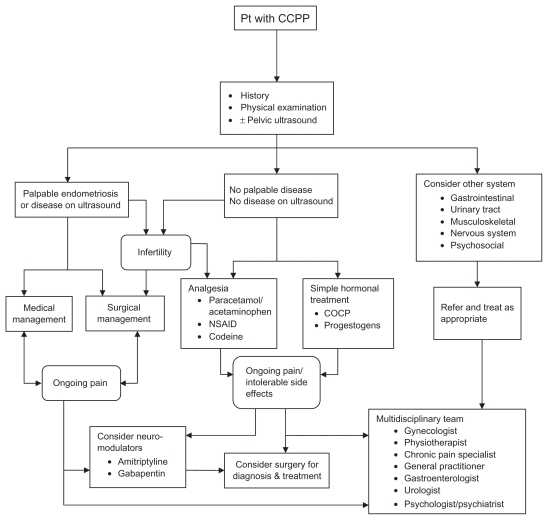
Given that the nature of CPPP may last for decades, guidelines rather than solid evidence must prevail for management. Table 2 lists RCT’s of management strategies for chronic pain and it is apparent that there are very few Class 1 studies in this area, given how commonplace the condition is. Given this information, Figure 1 proposes a pragmatic management approach for the optimal management of CCPP in premenopausal women. This flow chart should be considered as a guide only, as other variables such as the patient’s age and treatment preference or previous surgery may influence the choice of managements. There is a paucity of randomized controlled trials comparing therapeutic options for optimal management of CCPP, whereas stronger evidences are available for endometriosis-associated CCPP. Figure 1 is derived based on these randomized controlled trials, which are shown in Table 2.
Table 2.
Randomized controlled trials comparing therapeutic options for CCPP
| RCT | Intervention | Result and efficacy | Side effects | |
|---|---|---|---|---|
| Chronic pelvic pain with endometriosis | ||||
| COCP | Vercellini et al125 | Goserelin depot vs low-dose COCP for 6 months |
Both groups had significantly reduced deep dyspareunia (goserelin superior with mean difference of 1.8; 95% CI, 0.1–3.5) and nonmenstrual pain (no difference) COCP group more effective at reducing dysmenorrhea (mean difference 4.3; 95% CI, 3.7–4.8) |
Migraine Bloating Fluid retention Venous thromboembolism |
| Harada et al126 | Monophasic COCP vs placebo for 4 months | COCP group had significantly reduced verbal rating score for dysmenorrhea and volume of endometrioma on ultrasonography | ||
| GnRHa | Rock et al45 | Zoladex (goserelin acetate implant) vs danazol for 6 months | Zoladex is as effective as danazol in the treatment of endometriosis, 53% reduction in AFS endometriosis score with Zoladex vs 46% with danazol | Hypoestrogenism (Vasomotor symptoms, reduction in bone mineral density and osteoporosis) Cost |
| Gregoriou et al127 | GnRHa alone vs GnRHa plus HRT for 6 months | Both groups equally effective in reducing pelvic pain with no difference of reduction of CPP in both group (P < 0.001) GnRH plus HRT group had significantly less hypoestrogenism side effects (P < 0.001) |
||
| LNG-IS | Petta et al121 | LNG-IS vs GnRHa for 6 months | LNG-IS is as effective as GnRHa in treatment of endometriosis-associated CPP with no difference of reduction of CPP in both group (P > −0.999), without hypoestrogenic side effects of GnRHa | Spotting Breakthrough bleeding Amenorrhea |
| Progestagens | Vercellini et al120 | Depot medroxy progesterone acetate (MDA) vs OCOP + Danazol for 12 months | MDA group had reduced dysmenorrhea (mean difference VAS score 2.1; 95% CI, 0.8–3.4). Nonmenstrual pelvic pain and dyspareunia significantly decreased in both groups 72.5% patients in MDA group was satisfied at 1 year vs 57.5% in COCP + Danazol group (X2 = 1.37, P = 0.24) |
Amenorrhea Spotting, breakthrough bleeding Bloating Breast tenderness Depression Decreased libido |
| Surgery | Sutton et al50 | Laser ablation of endometriosis plus LUNA vs expectant management |
62.5% of laser ablation and LUNA had improved pain VAS scores and 22.6% in expectant group at 6 months (z = 2.92, P < 0.01) | No operative or laser complications |
| Abbott et al33 | Full excision of endometriosis vs diagnostic laparoscopy | Pain improved in 80% with immediate full excision vs 32% in diagnostic laparoscopy (X2 = 9.3, P = 0.002) | No difference in surgical morbidity in both groups | |
| Wright et al128 | Excision vs ablation of endometriosis in mild endometriosis (rAFS classification 1-2) | Both excision and ablation reduced pain scores and pelvic tenderness No significant difference in the mean difference of pain scores presurgery and postsurgery (8.7 in ablation group and 11.2 in excision group, P = 0.57) |
No difference in surgical morbidity in both groups | |
| Jarell et al51 | Excision of endometriosis vs expectant management | Pain was significantly reduced at 1 year (P < 0.05), with no significant difference between both groups (45% in excision group and 33% in expectant management) | No difference in surgical morbidity in both groups | |
| Chronic pelvic pain alone | ||||
| COCP | No RCT | |||
| LNG-IS | No RCT | |||
| GnRHa | Ling129 | Depot Leuprolide vs placebo for 3 months | Depot Leuprolide group had reduced dysmenorrhea (pain score 1.0 vs 2.7, P < 0.001) and pelvic pain (pain score 1.9 vs 2.9, P < 0.001) | Hypoestrogenism (Vasomotor symptoms, reduction in bone mineral density) Cost |
| Pudendal nerve block | No RCT | |||
| Botulinum toxin type A | Abbott et al108 | Bolulinum toxin type A vs placebo in patients with CPP and pelvic floor muscle spasm | Botulinum toxin A group had reduced dyspareunia (VAS score 66 vs 12, P < 0.001), nonmenstrual pelvic pain (VAS score 51 vs 22, P = 0.043) and pelvic floor pressure (49 vs 32, P < 0.001) | Cost Vaginal bleeding from injection site |
| Adhesiolysis | Keltz et al130 | Right paracolic adhesiolysis vs no lysis | Patients undergone right paracolic adhesiolysis had greater pain reduction than those who did not (P = 0.014) | No difference in surgical morbidity in both groups |
Abbreviations: COCP, combined oral contraceptive pill; CI, confidence interval; GnRHa, gonadotrophin-releasing hormone analog; LNG-IS, levonorgestrel-releasing intrauterine system; CPP, cyclical pelvic pain; LUNA, laparoscopic uterine nerve ablation. RCT, randomized controlled trials; HRT, hormone replacement therapy; VAS, visual analog scale; AFS, American Fertility Society.
Figure 1.
Flowchart describing a proposed management of chronic cyclic pelvic pain.
If abnormal findings are detected at physical examination, such as rectovaginal nodularity or uterosacral thickening or ultrasonic demonstration of endometrioma raise a suspicion of advanced endometriosis, surgical management may be considered as a first-line treatment. If fertility is also an issue, then medical options with hormonal treatments are contraindicated and only surgery or assisted reproduction can be implemented. If the patient prefers noninvasive management, medical treatment can be offered with surgery considered if pain persists.
When there is no palpable disease and no demonstrable sonographic abnormalities, medical treatment should be considered first line, if the patient is willing. Since the majority of causes are hormonally driven gynecological causes, empiric treatment with the combined oral contraceptive is appropriate in women with a normal physical examination who do not want to conceive, if there are no contraindications to the COCP. A trial of a monophasic preparation with a monthly cycle for 3 months is preferred. If there is good symptom relief from this, patients are advised to go on to a continuous regime for 2 months where the active pill is continuously taken followed by a withdrawal bleed. If this is well tolerated without a withdrawal bleed, the length of cycle can be extended to an indefinite time tailored to symptoms such as break through bleeding. Simple analgesics such as NSAID or paracetamol/acetaminophen should be commenced as first-line analgesia in conjunction with the oral contraceptive pill. Continuous progestogens such as medroxyprogesterone acetate, norethindrone acetate, or the levonorgestrel-releasing intrauterine system can be used as second-line medical treatment, with their efficacy demonstrated in randomized controlled trials for a variety of pelvic pain symptoms.120,121 GnRH analogs have a very limited role in the management of CCPP, due to their side effects of loss of bone mineral density and development of menopausal symptoms limiting long-term use.
Long-term opioid therapy should be reserved for patients with severe cases, not responding to simple analgesia and hormonal medication, due to their side effects such as opioid bowel syndrome tolerance and dependence. Of note, improvement in symptoms with medical treatment such as the COCP is not an absolute confirmation of a diagnosis since treatment effects are often not specific. As an example, hormonal treatment of endometriosis may also improve pelvic congestion syndrome, IBS, or IC/painful bladder syndrome.97,122 If there is ongoing pain despite medical treatment or the patient develops intolerable side effects from treatment, surgery should be considered to provide diagnosis and treatment. Neuromodulators such as amitriptyline and gabapentin may also be considered in conjunction with a pain specialist with frequent patient review. Side effects of these medications are important to recognize.
In refractory CCPP, a combination of treatments is usually required over time. The multifactorial nature of chronic pelvic pain needs to be discussed with the patient and a good rapport as well as a partnership needs to be developed to plan a management program with regular follow-up. Promotion of a multidisciplinary approach in managing CCPP may expand treatment options,123 and create more realistic expectations of treatment outcomes. This will include the gynecologist, physiotherapist, general practitioner, chronic pain team with pain specialist, and pain psychologist. Repetitive surgery is not optimal management and exposes the patient to significant risk of morbidity, without necessarily increasing the benefit of surgery.124 If the history and examination suggests that there is a nongynecological component to the pain, referral to the relevant health care professional such as a gastroenterologist or urologist needs to be considered.
Conclusion
CCPP is a common problem in women. Although most are due to gynecological causes, other systems such as gastrointestinal, urinary, muscular, and neurological systems may be involved. Thorough history and examination is the key to accurate diagnosis and successful management. Empiric treatment with the oral contraceptive pill with simple analgesia is used as first line if there is no demonstrable disease on examination or pelvic ultrasound. Surgery is a preferred management for diagnosis and treatment if advanced endometriosis is suspected or fertility is to be retained. In refractory cases, the demands of wide ranging differential diagnoses and therapies may be met by a multidisciplinary approach with other health professionals.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Muse KN. Cyclic pelvic pain. Obstet Gynecol Clin North Am. 1990;17(2):427–440. [PubMed] [Google Scholar]
- 2.Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Prevalence and incidence of chronic pelvic pain in primary care: evidence from a national general practice database. Br J Obstet Gynecol. 1999;106(11):1149–1155. doi: 10.1111/j.1471-0528.1999.tb08140.x. [DOI] [PubMed] [Google Scholar]
- 3.Grace VM, Zondervan KT. Chronic pelvic pain in New Zealand: prevalence, pain severity, diagnoses and use of the health services. Aust N Z J Public Health. 2004;28(4):369–375. doi: 10.1111/j.1467-842x.2004.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 4.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–327. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 5.Barbieri R. Etiology and epidemiology of endometriosis. Am J Obstet Gynecol. 1990;162:565–567. doi: 10.1016/0002-9378(90)90430-f. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood T, Templeton A, Thompson L. Menstrual symptoms in women with pelvic endometriosis. Br J Obstet Gynaecol. 1991;98:321–327. doi: 10.1111/j.1471-0528.1991.tb10370.x. [DOI] [PubMed] [Google Scholar]
- 7.Burnett MA, Antao V, Black A, et al. Prevalence of primary dysmenorrhea in Canada. J Obstet Gynaecol Can. 2005;27(8):765–770. doi: 10.1016/s1701-2163(16)30728-9. [DOI] [PubMed] [Google Scholar]
- 8.Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–660. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 9.Kessel N, Coppen A. The prevalence of common menstrual symptoms. Lancet. 1963;2:61–64. doi: 10.1016/s0140-6736(63)90063-1. [DOI] [PubMed] [Google Scholar]
- 10.De Groat WCBA, Yoshimura N. Neurophysioloy of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System. Nervous Control of the Urogenital System. Vol. 3. London: Harwood Academic Publishers; 1993. pp. 227–289. [Google Scholar]
- 11.Bodden-Heidrich R, Kuppers V, Beckmann MW, Rechenberger I, Bender HG. Chronic pelvic pain syndrome (CPPS) and chronic vulvar pain syndrome (CVPS): evaluation of psychosomatic aspects. J Psychosom Obstet Gynecol. 1999;20(3):145–151. doi: 10.3109/01674829909075588. [DOI] [PubMed] [Google Scholar]
- 12.Rapkin AJ, Kames LD, Darke LL, et al. History of physical and sexual abuse in women with chronic pelvic pain. 1990;76(1):92–96. [PubMed] [Google Scholar]
- 13.Reinhard MJ. The Long Term Neuropsychiatric Effects of Early Trauma. Malibu, CA: Pepperdine University; 2004. [Google Scholar]
- 14.King PM, Myers CA, Ling FW, Rosenthal RH. Musculoskeletal factors in chronic pelvic pain. J Psychosom Obstet Gynaecol. 1991;12:87–98. [Google Scholar]
- 15.Stav K, Dwyer PL, Roberts L. Pudendal neuralgia. Fact or fiction? Obstet Gynecol Surv. 2009;64(3):190–199. doi: 10.1097/ogx.0b013e318193324e. [DOI] [PubMed] [Google Scholar]
- 16.Abbott JA, Vancaillie TG. The role of ultrasound in the management of endometriosis and pelvic pain. Aust Soc Ultrasound Med Bull. 2002;5(1):14–19. [Google Scholar]
- 17.Gorell H, Cyr D, Wang K, Grer B. Rectosigmoid endometriosis: diagnosis using endovaginal sonography. J Ultrasound Med. 1989;8:459–461. doi: 10.7863/jum.1989.8.8.459. [DOI] [PubMed] [Google Scholar]
- 18.Fedele L, Bianchi S, Portuese A, Borruto F, Dorta M.Transrectal ultrasonography in the assessment of rectovaginal endometriosis; endoscopic ultrasound features and clinical implications Endoscopy 2000327525–530.10917184 [Google Scholar]
- 19.Bley KR, Hunter JC, Eglen RM, et al. The role of IP prostanoid receptors in inflammatory pain. Trends Pharmacol Sci. 1998;19:141–147. doi: 10.1016/s0165-6147(98)01185-7. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour HN, Kelly RW, Fraser HM, et al. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 21.Altunyurt S, Gol M, Altunyurt S, Sezer O, Demir N. Primary dysmenorrhea and uterine blood flow: a color Doppler study. J Reprod Med. 2005;50(4):251–255. [PubMed] [Google Scholar]
- 22.Dawood MY. Hormones, prostaglandin and dysmenorrhea. In: Dawood MY, editor. Dysmenorrhea. Baltimore: Williams and Wilkins; 1981. pp. 20–52. [Google Scholar]
- 23.Chan WY, Dawood MY, Fuchs F. Prostaglandins in primary dysmenorrhea. Comparison of prophylactic and nonprophy-lactic treatment with ibuprofen and use of oral contraceptives. Am J Med. 1981;70:535–541. doi: 10.1016/0002-9343(81)90576-3. [DOI] [PubMed] [Google Scholar]
- 24.Dawood MY. Primary dysmenor rhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108:428–441. doi: 10.1097/01.AOG.0000230214.26638.0c. [DOI] [PubMed] [Google Scholar]
- 25.Chantler I, Mitchell D, Fuller A. Diclofenac potassium attenuates dysmenorrhea and restores exercise performance in women with primary dysmenorrhea. J Pain. 2009;10(2):191–200. doi: 10.1016/j.jpain.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Daniels SE, Talwalker S, Torri S, Snabes MC, Recker DP, Verburg KM. Valdecoxib, a cyclo-oxygenase-2-specific inhibitor, is effective in treating primary dysmenorrhea. Obstet Gynecol. 2002;100:350–358. doi: 10.1016/s0029-7844(02)02085-9. [DOI] [PubMed] [Google Scholar]
- 27.Daniels S, Gitton X, Zhou W, Stricker K, Barton S. Efficacy andtolerability of Lumiracoxib 200 mg once daily for treatment of primary dysmenorrhea: results from two randomized controlled trials. J Womens Health. 2008;17(3):423–437. doi: 10.1089/jwh.2007.0416. [DOI] [PubMed] [Google Scholar]
- 28.Hendrix SL, Alexander NJ. Primary dysmenorrhoea treatment with a desogestrel-containing low dose oral contraceptive. Contraception. 2002;66(6):393–399. doi: 10.1016/s0010-7824(02)00414-6. [DOI] [PubMed] [Google Scholar]
- 29.Davis AR, Westhoff CL, O’Connell K, Callagher N. Oral contraceptives for dysmenorrhoea in adolescents girls. A randomised trial. Obstetrics Gynecol. 2005;106(1):97–104. doi: 10.1097/01.AOG.0000165826.03915.65. [DOI] [PubMed] [Google Scholar]
- 30.O’Herlihy C, Robinson H, De Crespigny L. Mittelschmerz is a preovulatory symptom. Br Med J. 1980;280:986. doi: 10.1136/bmj.280.6219.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laufer MR, Goitein L, Bush M, et al. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J Pediatr Adolesc Gynecol. 1997;10:199–202. doi: 10.1016/s1083-3188(97)70085-8. [DOI] [PubMed] [Google Scholar]
- 32.Howard F. Endometriosis and endosalpingiosis. In: Howard FM, Perry CP, Carter JE, El-Minawi AM, editors. Pelvic Pain: Diagnosis and Management. New York: Lippincott; 2000. pp. 125–150. [Google Scholar]
- 33.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82:878–884. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 34.Fedele L, Bianchi S, Bocciolone L, Di Nola G, Parazzini F. Pain symptoms associated with endometriosis. Obstet Gynecol. 1992;79:767–769. [PubMed] [Google Scholar]
- 35.Porpora MG, Koninckx PR, Piazze J, Natili M, Colagrande S, Cosmi EV. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6:429–434. doi: 10.1016/s1074-3804(99)80006-1. [DOI] [PubMed] [Google Scholar]
- 36.Chapron C, Barakat H, Fritel X, Dubuisson JB, Breart G, Fauconnier A. Presurgical diagnosis of posterior deep infiltrating endometriosis based on a standardized questionnaire. Hum Reprod. 2005;20(2):507–513. doi: 10.1093/humrep/deh627. [DOI] [PubMed] [Google Scholar]
- 37.Garry R, Clayton R, Hawe J. The effect of endometriosis and its radical laparoscopic excision on quality of life indicators. Br J Obstet Gynecol. 2000;107(1):44–54. doi: 10.1111/j.1471-0528.2000.tb11578.x. [DOI] [PubMed] [Google Scholar]
- 38.Muse K. Clinical manifestations and classification of endometriosis. Clin Obstet Gynecol. 1998;31:813–822. doi: 10.1097/00003081-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibres in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril. 2007;88(4):795–803. doi: 10.1016/j.fertnstert.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 40.Bedaiwy M, Casper R. Treatment with leuprolide acetate and hormonal add-back for up to 10 years in stage IV endometriosis patients with chronic pelvic pain. Fertil Steril. 2006;86:220–222. doi: 10.1016/j.fertnstert.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Vercellini P, Domigliana E, Vigano P, Abbiati A, Daguiati R, Crosignani PG. Endometriosis; current and future medical therapies. Best Pract Res Clin Obstet Gynaecol. 2008;22:275–306. doi: 10.1016/j.bpobgyn.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Telimaa S, Puolakka J, Ronnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1:13–23. doi: 10.3109/09513598709082692. [DOI] [PubMed] [Google Scholar]
- 43.Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of evidence. Fertil Steril. 1997;68:393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- 44.Razzi S, Luisi S, Calonaci F, Altomare A, Bocchi C, Petraglia F. Efficacy of vaginal danazol treatment in women with recurrent deeply infiltrating endometriosis. Fertil Steril. 2007;88:789–794. doi: 10.1016/j.fertnstert.2006.12.077. [DOI] [PubMed] [Google Scholar]
- 45.Rock J, Truglia J, Caplan R for the Zoladex endometriosis study group. Zoladex in the treatment of endometriosis: a randomised comparison with danazol. Obstet Gynecol. 1993;82:198–205. [PubMed] [Google Scholar]
- 46.Adamson G, Subak C, Pasta D, Hurd S, von Franque O, Rodriguez B. Comparison of CO2 laser laparoscopy with laparotomy for treatment of endometriomata. Fertil Steril. 1992;67:965–973. [PubMed] [Google Scholar]
- 47.Anaf V, Simon P, Nakadi I, et al. Relationship between endometriotic foci and nerves in rectovaginal endometriotic nodules. Hum Reprod. 2000;15(8):1744–1750. doi: 10.1093/humrep/15.8.1744. [DOI] [PubMed] [Google Scholar]
- 48.Wright J. The diagnosis and management of infiltrating nodular rectovaginal endometriosis. Curr Opin Obstet Gynecol. 2000;12:283–287. doi: 10.1097/00001703-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Abbott JA, Hawe JA, Clayton R, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis; a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18(9):1922–1927. doi: 10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- 50.Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized double blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 51.Jarrell J, Mohindra R, Ross S, Taenzer P, Brant R. Laparoscopy and reported pain among patients with endometriosis. J Obstet Gynaecol Can. 2005;27:474–485. doi: 10.1016/s1701-2163(16)30531-x. [DOI] [PubMed] [Google Scholar]
- 52.Doyle JB. Paracervical uterine denervation by transection of the cervical plexus for the relief of dysmenorrhoea. Am J Obstet Gynecol. 1955;70:1. doi: 10.1016/0002-9378(55)90282-9. [DOI] [PubMed] [Google Scholar]
- 53.Ewen SP, Sutton CJG. A combined approach to painful heavy periods: laparoscopic laser uterine nerve ablation and endometrial resection. Gynaecol Endosc. 1994;3(3):167–168. [Google Scholar]
- 54.Feste JR. Laser laparoscopy: a new modality. J Reprod Med. 1985;30:413–417. [PubMed] [Google Scholar]
- 55.Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstet Gynecol. 1997;90(6):974–975. doi: 10.1016/s0029-7844(97)00484-5. [DOI] [PubMed] [Google Scholar]
- 56.Sutton C, Pooley AS, Jones KD, Dover RW, Haines P. A prospective, randomized, double-blind controlled trial of laparoscopic uterine nerve ablation in the treatment of pelvic pain associated with endometriosis. Gynaecol Endosc. 2001;10(4):217–222. [Google Scholar]
- 57.McElin TW, Bird CC. Adenomyosis of the uterus. Obstet Gynecol Annu. 1974;3:425. [PubMed] [Google Scholar]
- 58.Dueholm M, Lundorf E, Hansen ES, Sorensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001;76:588–594. doi: 10.1016/s0015-0282(01)01962-8. [DOI] [PubMed] [Google Scholar]
- 59.Bazot M, Cortez A, Darai E, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod. 2001;16:2427–2433. doi: 10.1093/humrep/16.11.2427. [DOI] [PubMed] [Google Scholar]
- 60.Sheng J, Zhang WY, Zhang JP, Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79(3):189–193. doi: 10.1016/j.contraception.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4(4):323–336. doi: 10.1093/humupd/4.4.323. [DOI] [PubMed] [Google Scholar]
- 62.McCausland V, McCausland A. The response of adenomyosis to endometrial ablation/resection. Hum Reprod Update. 1998;4(4):350–359. doi: 10.1093/humupd/4.4.350. [DOI] [PubMed] [Google Scholar]
- 63.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 64.Ferrero S, Abbamonte LH, Giordano M, Parisi M, Ragni N, Remorgida V. Uterine myomas, dyspareunia, and sexual function. Fertil Steril. 2006;86(5):1504–1510. doi: 10.1016/j.fertnstert.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002;186(3):409–415. doi: 10.1067/mob.2002.121725. [DOI] [PubMed] [Google Scholar]
- 66.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83(4):566–572. doi: 10.1097/00006250-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Wortman M, Daggett A. Hysteroscopic endocervical resection. J Am Assoc Gynecol Laparosc. 1996;4:63–68. doi: 10.1016/s1074-3804(96)80111-3. [DOI] [PubMed] [Google Scholar]
- 68.Luesley DM, Williams DR, Gee H, Chan KK, Jordan JA. Management of post-conization cervical stenosis by laser vaporization. Obstet Gynecol. 1986;67:126–128. [PubMed] [Google Scholar]
- 69.Kodaman PH, Arici A. Intra-uterine adhesions and fertility outcome: how to optimize success? Curr Opin Obstet Gynecol. 2007;19:207–214. doi: 10.1097/GCO.0b013e32814a6473. [DOI] [PubMed] [Google Scholar]
- 70.Thomson AJ, Abbott JA, Deans R, Kingston A, Vancaillie TG. The management of intrauterine synechiae. Curr Opin Obstet Gynecol. 2009;21(4):335–341. doi: 10.1097/GCO.0b013e32832e07fc. [DOI] [PubMed] [Google Scholar]
- 71.Deans R, Abbott J. A review of intrauterine adhesions. J Min Invasive Gynecol. 2010 doi: 10.1016/j.jmig.2010.04.016. In press. [DOI] [PubMed] [Google Scholar]
- 72.Townsend DE, McCausland VM, McCausland AM. Post-ablation tubal sterilization syndrome. Obstet Gynecol. 1993;82:422–424. [PubMed] [Google Scholar]
- 73.McCausland AM, McCausland VM. Frequency of symptomatic cornual hematometra and postablation tubal sterilization syndrome after total rollerball endometrial ablation: a 10 year follow-up. Am J Obstet Gynecol. 2002;186(6):1274–1280. doi: 10.1067/mob.2002.123730. [DOI] [PubMed] [Google Scholar]
- 74.Li S, Qayyum A, Coakley FV, Hricak H. Association of renal agenesis and mullerian duct anomalies. J Comp Ass Tomogr. 2000;24(6):829–834. doi: 10.1097/00004728-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68(1):159–164. doi: 10.1016/0301-2115(96)00250-3. [DOI] [PubMed] [Google Scholar]
- 76.Beard RW, Reginald PW, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95:153–161. doi: 10.1111/j.1471-0528.1988.tb06845.x. [DOI] [PubMed] [Google Scholar]
- 77.Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES., Jr Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176(1):119–122. doi: 10.2214/ajr.176.1.1760119. [DOI] [PubMed] [Google Scholar]
- 78.Christensen J, Boldsen J, Westergaard J. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153–157. doi: 10.1016/s0010-7824(02)00353-0. [DOI] [PubMed] [Google Scholar]
- 79.Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol. 1980;138:880–892. doi: 10.1016/0002-9378(80)91077-7. [DOI] [PubMed] [Google Scholar]
- 80.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory diseases: results from the PID evaluation and clinical health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929–937. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 81.Laufer MR, Heerema AE, Parsons KE, Barbieri RL. Endosalpingiosis: clinical presentation and follow-up. Gynecol Obstet Invest. 1998;46(3):195–198. doi: 10.1159/000010032. [DOI] [PubMed] [Google Scholar]
- 82.Jones R, Lydeard S. Irritable bowel syndrome in the general population. Br Med J. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Talley N, Zinsmeister A, van Dyke C, Melton L. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 84.Sandler R. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990;99:409–415. doi: 10.1016/0016-5085(90)91023-y. [DOI] [PubMed] [Google Scholar]
- 85.Zondervan K, Yudkin P, Vessey M, et al. Chronic pelvic pain in the community symptoms, investigations, and diagnoses. Am J Obstet Gynecol. 2001;184:1149–1155. doi: 10.1067/mob.2001.112904. [DOI] [PubMed] [Google Scholar]
- 86.Williams RE, Hartmann KE, Sandler RS, Miller WC, Steege JF. Prevalence and characteristics of irritable bowel syndrome among women with chronic pelvic pain. Obstet Gynecol. 2004;104(3):452–458. doi: 10.1097/01.AOG.0000135275.63494.3d. [DOI] [PubMed] [Google Scholar]
- 87.We W, Cheskin L, Heller B, et al. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98:1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]
- 88.Moore J, Barlow DH, Jewell D, Kennedy SH. Do gastrointestinal symptoms vary with the menstrual cycle? Br J Obstet Gynaecol. 1998;105:1322–1325. doi: 10.1111/j.1471-0528.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 89.Crowell M, Dubin N, Robinson J, et al. Functional bowel disorders in women with dysmenorrhea. Am J Gastroenterol. 1994;89:1973–1977. [PubMed] [Google Scholar]
- 90.Longstreth G, Preskill D, Youkeles L. Irritable bowel syndrome in women having diagnostic laparoscopy or hysterectomy. Dig Dis Sci. 1990;35:1285–1290. doi: 10.1007/BF01536421. [DOI] [PubMed] [Google Scholar]
- 91.Cash BD, Chey WD. Irritable bowel syndrome – an evidence- based approach to diagnosis. Aliment Pharmacol Ther. 2004;19:1235–1245. doi: 10.1111/j.1365-2036.2004.02001.x. [DOI] [PubMed] [Google Scholar]
- 92.Longstreth GF. Definition and classification of irritable bowel syndrome: current consensus and controversies. Gastroenterol Clin North Am. 2005;34:173–187. doi: 10.1016/j.gtc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099–1104. doi: 10.1136/gut.30.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaiwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136–147. doi: 10.7326/0003-4819-133-2-200007180-00013. [DOI] [PubMed] [Google Scholar]
- 95.Gale JD. The use of novel promotility and prosecretory agents for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation. Adv Ther. 2009;26(5):519–530. doi: 10.1007/s12325-009-0027-4. [DOI] [PubMed] [Google Scholar]
- 96.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465–469. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- 97.Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241–250. [Google Scholar]
- 98.Lentz GM, Bavendam T, Stenchever MA, Miller JL, Smalldridge J. Hormonal manipulation in women with chronic, cyclic irritable bladder symptoms and pelvic pain. Am J Obstet Gynecol. 2002;186:1268–1271. doi: 10.1067/mob.2002.123729. [DOI] [PubMed] [Google Scholar]
- 99.Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynaecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obst Gynecol. 2002;187(5):1395–1400. doi: 10.1067/mob.2002.127375. [DOI] [PubMed] [Google Scholar]
- 100.Haggerty CL, Schulz R, Ness RB PID evaluation and clinical health study investigators. Lower quality of life among women with chronic pelvic pain after pelvic inflammatory disease. Obstet Gynecol. 2003;102:934–939. doi: 10.1016/s0029-7844(03)00695-1. [DOI] [PubMed] [Google Scholar]
- 101.Sant GR, Hanno PM. Intersitial cystitis: current issues and controversies in diagnosis. Urology. 2001;57(Suppl 6A):82–88. doi: 10.1016/s0090-4295(01)01131-1. [DOI] [PubMed] [Google Scholar]
- 102.Hanno P. Is the potassium sensitivity test a valid and useful test for the diagnosis of interstitial cystitis? Int Urogynecol Pelvic Floor Dysfunct. 2005;16:428. doi: 10.1007/s00192-005-1306-5. [DOI] [PubMed] [Google Scholar]
- 103.Baker PK. Musculoskeletal origins of chronic pelvic pain. Diagnosis and treatment. Obstet Gynecol Clin North Am. 1993;20:719–742. [PubMed] [Google Scholar]
- 104.Prendergast SA, Weiss JM. Screening for musculoskeletal causes of pelvic pain. Clin Obstet Gynecol. 2003;46:773–782. doi: 10.1097/00003081-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 105.Haugstad GK, Haugstad TS, Kirste UM, et al. Posture, movement patterns, and body awareness in women with chronic pelvic pain. J Psychosom Res. 2006;61:637–644. doi: 10.1016/j.jpsychores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Alvarez DJ, Rockwell PG. Trigger points: diagnosis and management. Am Fam Physician. 2002;65(4):653–660. [PubMed] [Google Scholar]
- 107.McDonald JS, Elliott ML. Gynecologic pain syndromes. In: Loeser JD, Butler SH, Champman CR, et al., editors. Bonica’s Management of Pain. 3rd ed. Philadephia: Lippincott Williams and Wilkins; 2001. pp. 1414–1447. [Google Scholar]
- 108.Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaillie TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923. doi: 10.1097/01.AOG.0000237100.29870.cc. [DOI] [PubMed] [Google Scholar]
- 109.Abbott J. Botulinum toxin in the female pelvis – a new answer to old problems? J Min Invasive Gynaecol. 2009;16(2):130–135. doi: 10.1016/j.jmig.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Shafik A. Pudendal canal syndrome as a cause of vulvodynia and its treatment by pudendal nerve decompression. Eur J Obstet Gynecol Reprod Biol. 1998;80:215–220. doi: 10.1016/s0301-2115(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 111.Labat JJ, Riant T, Robert R, et al. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria) Neurourol Urodyn. 2008;27:306–310. doi: 10.1002/nau.20505. [DOI] [PubMed] [Google Scholar]
- 112.Harlow BL, Wise LA, Stewart EG. Prevalence and predictors of chronic lower genital tract discomfort. Am J Obstet Gynecol. 2001;185:545–550. doi: 10.1067/mob.2001.116748. [DOI] [PubMed] [Google Scholar]
- 113.Bergeron S, Bouchard C, Fortier M, et al. The surgical treatment of vulvar vestibulitis syndrome: a follow-up study. J Sex Marital Ther. 1997;23:317–325. doi: 10.1080/00926239708403935. [DOI] [PubMed] [Google Scholar]
- 114.Friedrich EG., Jr Vulvar vestibulitis syndrome. J Reprod Med. 1987;32:110–114. [PubMed] [Google Scholar]
- 115.Goetsch MF. Vulvar vestibulitis: prevalence and historic features in a general gynecologic practice population. Am J Obstet Gynecol. 1991;164:1609–1614. doi: 10.1016/0002-9378(91)91444-2. [DOI] [PubMed] [Google Scholar]
- 116.Westrom L, Willen R. Vestibular nerve proliferation in vulvar vestibulitis syndrome. Obstet Gynecol. 1998;91:572–576. [PubMed] [Google Scholar]
- 117.Edwards L. New concepts in vulvodynia. Am J Obstet Gynecol. 2003;189:S24–S40. doi: 10.1067/s0002-9378(03)00790-7. [DOI] [PubMed] [Google Scholar]
- 118.Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297–306. doi: 10.1016/S0304-3959(00)00449-8. [DOI] [PubMed] [Google Scholar]
- 119.Granot M, Zimmer EZ, Friedman M, et al. Association between quantitative sensory testing, treatment choice, and subsequent pain reduction in vulvar vestibulitis syndrome. J Pain. 2004;5:226–232. doi: 10.1016/j.jpain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Vercellini P, De Giorgi O, Oldani S, Cortesi I, Panazza S, Crosignani PG. Depot medroxyprogesterone acetate versus an oral contraceptive combined with very-low-dose danazol for long-term treatment of pelvic pain associated with endometriosis. Am J Obstet Gynecol. 1996;175(2):396–401. doi: 10.1016/s0002-9378(96)70152-7. [DOI] [PubMed] [Google Scholar]
- 121.Petta CA, Ferriani RA, Abrao, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod. 2005;20(7):1993–1998. doi: 10.1093/humrep/deh869. [DOI] [PubMed] [Google Scholar]
- 122.Mathias JR, Clench MH, Reeves-Darby VG. Effect of leuprolide acetate in patients with moderate to severe functional bowel disease. Double-blind, placebo-controlled study. Dig Dis Sci. 1994;39(6):1155–1162. doi: 10.1007/BF02093778. [DOI] [PubMed] [Google Scholar]
- 123.Kames LD, Rapkin AJ, Naliboff BD, Afifi S, Ferrer-Brechner T. Effectiveness of an interdisciplinary pain management program for the treatment of chronic pelvic pain. Pain. 1990;41:41–46. doi: 10.1016/0304-3959(90)91107-T. [DOI] [PubMed] [Google Scholar]
- 124.Butrick CW. Chronic pelvic pain: How many surgeries are enough? Clin Obstet Gynecol. 2007;50(2):412–424. doi: 10.1097/GRF.0b013e31804b195f. [DOI] [PubMed] [Google Scholar]
- 125.Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani P. A gonadotropin-releasing hormone agonist versus a lowdose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993;60(1):75–79. [PubMed] [Google Scholar]
- 126.Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrheal associated with endometriosis: a placebo-controlled double-blind, randomized trial. Fertil Steril. 2008;90(5):1583–1588. doi: 10.1016/j.fertnstert.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 127.Gregoriou O, Konidaris S, Vitoratos N, Papadias C, Papoulias I, Chryssicopoulos A. Gonadotropin-releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Int J Fertil Womens Med. 1997;42(6):406–411. [PubMed] [Google Scholar]
- 128.Wright J, Lotfallah H, Jones K, Lovell D. A randomized trial of excision versus ablation for mild endometriosis. Fertil Steril. 2005;83(6):1830–1836. doi: 10.1016/j.fertnstert.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 129.Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol. 1999;93(1):51–58. doi: 10.1016/s0029-7844(98)00341-x. [DOI] [PubMed] [Google Scholar]
- 130.Keltz MD, Gera PS, Olive DL. Prospective randomized trial of right- sided paracolic adhesiolysis for chronic pelvic pain. J Soc Lap Surg. 2006;10(4):443–446. [PMC free article] [PubMed] [Google Scholar]