Abstract
Neuroplasticity is characterized by growth and branching of dendrites, remodeling of synaptic contacts, and neurogenesis, thus allowing the brain to adapt to changes over time. It is maintained in adulthood but strongly repressed during aging. An age-related decline in neurogenesis is particularly pronounced in the two adult neurogenic areas, the subventricular zone and the dentate gyrus. This age-related decline seems to be attributable mainly to limited proliferation, associated with an age-dependent increase in quiescence and/or a lengthening of the cell cycle, and is closely dependent on environmental changes. Indeed, when triggered by appropriate signals, neurogenesis can be reactivated in senescent brains, thus confirming the idea that the age-related decrease in new neuron production is not an irreversible, cell-intrinsic process. The coevolution of neurogenesis and age-related memory deficits – especially regarding spatial memory – during senescence supports the idea that new neurons in the adult brain participate in memory processing, and that a reduction in the ability to generate new neurons contributes to the appearance of memory deficits with advanced age. Furthermore, the age-related changes in hippocampal plasticity and function are under environmental influences that can favor successful or pathological aging. A better understanding of the mechanisms that regulate neurogenesis is necessary to develop new therapeutic tools to cure or prevent the development of memory disorders that may appear during the course of aging in some individuals.
Keywords: aging, hippocampus, memory, neurogenesis, neuroplasticity, spatial learning
Introduction
The vast majority of cells in the adult central nervous system (CNS) are generated during the embryonic and early postnatal period. However, it is now well accepted that new neurons are continuously added in specific regions of the mammalian brain throughout adulthood. In rodents, monkeys, and humans, neurogenesis has been described within the hippocampal formation (HF) in the subgranular layer (SGL) of the dentate gyrus (DG), and in the subventricular zone (SVZ) (Gross, 2000; Abrous etal., 2005; Ming & Song, 2005; Christie & Cameron, 2006; Lledo etal., 2006; Scharfman & Hen, 2007) (Fig. 1).
Fig. 1.
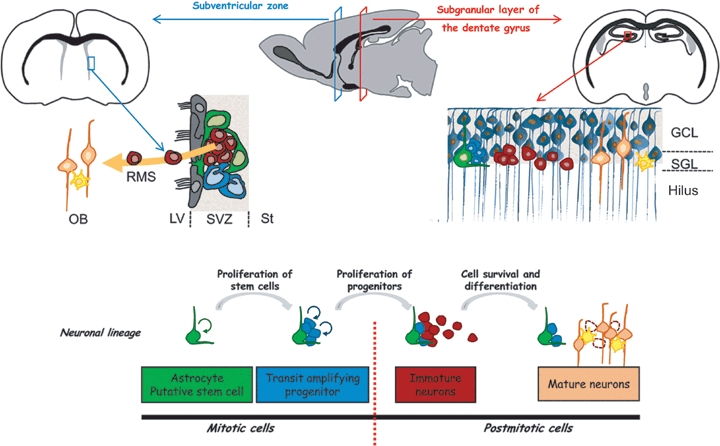
The main neurogenic areas of the adult brain. Adult neurogenesis has been described in the subventricular (SVZ)/olfactory bulb (OB) and in the hippocampal system. Representations of the different stages of adult neurogenesis that may be affected by aging; glial fibrillary acidic protein-positive astrocytes (green) have been identified as in vivo stem cells in the SVZ and stem-like cells in the dentate gyrus (DG). They divide slowly to give rise to transit amplifying progenitors (blue), which in turn generate immature cells (red) able to differentiate either into neurons (orange) or glial cells (yellow). A significant fraction of the newborn cells die during the maturation process (dotted cells). RMS, rostral migratory stream; LV, lateral ventricle; St, striatum; GCL, granule cell layer; SGL, subgranular layer.
The process of neurogenesis in the young adult brain can be divided into a series of distinct developmental steps, which can be examined separately and include the proliferation of precursor cells, the survival of newly born cells, the migration of these cells, and, finally, their differentiation into mature functional neurons. Precursor cells can be either stem cells, characterized by a slow-dividing cell cycle, long-term self-renewal potential, and multipotentiality, or progenitors, which exhibit a higher dividing rate of turnover and reduced self-renewal abilities. Progenitors are more differentiated than stem cells, and their multipotentiality is still a matter of debate.
In the DG, new neurons originate from cells located in the SGL at the border of the granule cell layer (GCL) facing hilus. These cells are slowly proliferating astrocytes [identified on the basis of their expression of glial fibrillary acidic protein (GFAP) and their ultrastructural properties], presumably radial glia (Garcia etal., 2004b; Seri etal., 2004). However, their stem cell nature is still a matter of debate as in vitro studies have shown that cells isolated specifically from the DG have only limited self-renewal abilities and are restricted to the neuronal lineage (Seaberg & van der Kooy, 2002). This lack of stem cell properties may be caused by the experimental in vitro conditions, yet we will refer to these cells as ‘stem-like’ cells rather than stem cells. The SGL-dividing astrocytes generate immature, GFAP-negative, intermediate-amplifying precursors that in turn divide. Their daughter cells migrate a short distance into the GCL. A significant fraction of the newly generated neuronal cells undergo programmed cell death (Gould etal., 1999; Sun etal., 2004; Dupret etal., 2007), while the surviving cells successfully differentiate, mostly into hippocampal granule cells, extending axons into the CA3 region (Stanfield & Trice, 1988; Hastings & Gould, 1999; Markakis & Gage, 1999; van Praag etal., 2002) and becoming functionally integrated in the hippocampal circuitry (Hastings & Gould, 1999; Carlen etal., 2002; van Praag etal., 2002; Jessberger & Kempermann, 2003; Kee etal., 2007; Toni etal., 2007).
In the SVZ, GFAP cells have been identified as stem cells (Doetsch etal., 1999a). They divide slowly to generate rapidly dividing transit amplifying cells, which in turn give rise to neuroblasts. These neuronal precursors born in the SVZ migrate tangentially along the rostral extension of the SVZ toward the olfactory bulb (OB), constituting the rostral migratory stream (RMS). As in the DG, a high proportion of the cells generated in the SVZ die after birth (Petreanu & Alvarez-Buylla, 2002; Winner etal., 2002). After reaching the core of the OB, surviving cells move radially into the granular and periglomerular layers where they differentiate into functional interneurons (Luskin, 1993; Lois & Alvarez-Buylla, 1994; Carlen etal., 2002; Huang & Bittman, 2002; Magavi etal., 2005).
Although the adult brain retains remarkable plastic capabilities, aging is classically associated with a decline in several forms of neuronal plasticity. Indeed, the ability of neurons to modify their connectivity by changing their number and spine shape in response to various environmental (e.g. brain damage) and physiological stimuli is less robust in the senescent brain (see, for review, Petit & Ivy, 1988; Burke & Barnes, 2006). In this context, it is logical that neurogenesis, which continues throughout life, decreases in both of the neurogenic areas with increasing age.
Even if the functional significance of this ongoing adult neurogenesis is not fully understood, evidence has been provided that newly produced neurons play an important role in functions associated with the neurogenic areas. Adult neurogenesis has been shown to be involved in several brain functions (e.g. memory and emotion) and pathologies (e.g. depression and addiction) (Abrous etal., 2005). Here, we will focus mainly on neurogenesis and memory. Indeed, the functional consequences of the age-related decline in neurogenesis have been examined only in relation to this function, known to be altered in the course of aging (Stevens & Cain, 1987; Grady & Craik, 2000; Hulshoff Pol etal., 2000; Kaneda etal., 2000). Briefly, in young subjects, an increasing number of studies have correlated changes in the rate of dentate neurogenesis with spatial memory ability, and changes in adult-born olfactory neurons to olfactory memory (Leuner etal., 2006; Lledo etal., 2006; Abrous & Wojtowicz, 2008). Based on these observations, it has been hypothesized that the age-related decline in neurogenesis, together with a decline in other type of structural and synaptic plasticity, may contribute to the normal physiological reduction in memory function associated with aging.
Consequently, in this review, we propose an overview of the current knowledge about the evolution of adult neurogenesis during aging and its functional consequences. We will first describe the changes in neurogenesis observed during aging and then focus on ‘intrinsic’ and extracellular mechanisms (i.e. the changes in the environmental niche for neurogenesis) that can explain the observed age-related modifications of neuronal production. Finally, we will focus on the relationship between the age-related decline in neurogenesis and age-dependent memory impairments, and discuss the possible role of decreased neurogenesis in the memory decline reported in some senescent subjects.
Neurogenesis in the aging brain
Multiple reports demonstrate that the production of new neurons in neurogenic brain regions declines dramatically with age. However, in most of these studies, neurogenesis was measured by labeling the newborn cells with a thymidine analogue such as bromodeoxyuridine (BrdU) or by expression of markers for proliferation and immature cells. Various BrdU dosages, injection frequencies, and survival times have been used, yielding different results concerning fate or numbers of newborn cells, and making it difficult to compare these studies in terms of absolute numbers. Moreover, the basal level of neurogenesis, the onset of its age-related down-regulation, and ‘how old is old’ vary significantly among species, strains (and median lifespan), sex, and experimental conditions. Consequently, all of these variables could explain the discrepancies between experiments that we will report.
In the following section, we will review the effect of aging on the different steps leading to the production of new neurons. Neurogenesis in the adult brain can be divided into three phases in accordance with the sequence of neurogenesis during development: (i) proliferation, when new cells are generated; (ii) survival of a portion of these new cells and their migration toward target areas; and (iii) terminal differentiation into a neuronal or glial phenotype. Substantial changes in some or all of the above events may underlie the reduction in hippocampal neurogenesis during aging (Fig. 2). The aging process could either deplete the number of precursors or alter their mitotic activity; both would ultimately lead to a reduction in the actual number of newly born cells. Additionally, the newborn cells could die before they differentiate into granule neurons or toward another cell phenotype.
Fig. 2.
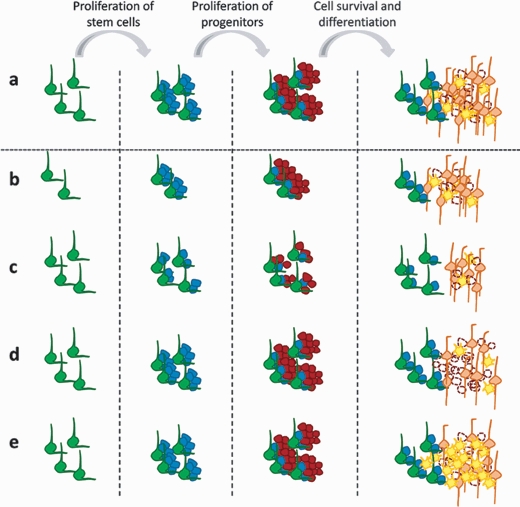
Different stages of adult neurogenesis and their potential modifications during aging. Neurogenesis in young adult (a) and aged (b–e) brain. The decline of neurogenesis observed during aging can be the consequence of: (b) a decrease of the number of precursors; (c) a reduction in the proliferative activity of the precursors as a consequence either of a lengthening of the cell cycle or an increase in their quiescence; (d) a decrease of the proportion of newly generated cells surviving after the first weeks of maturation; (e) a differentiation biased toward a glial phenotype. It is likely that a combination of all of these mechanisms is involved in the aging of neurogenic areas.
In the DG
The time course of cell proliferation has been extensively studied in rodents (see Table 1) using injected proliferation markers such as BrdU and tritiated thymidine associated with short survival periods (usually 24 h after the injection as this is sufficient for a newborn cell to complete at least one cell cycle) to label proliferating cells and their progeny. Alternatively, cell proliferation may be studied using intrinsic proliferation markers such as Ki67, proliferating cell nuclear antigen (CNA), the phosphorylated histone 3 (HH3), and the minichromosome maintenance deficient 2 mitotin (MCM-2) (Maslov etal., 2004) that are transiently expressed by cycling cells. All are considered to be reliable markers to assay the proliferative activity of precursors (Kee etal., 2002; Wojtowicz & Kee, 2006; Taupin, 2007). In the rat DG, cell proliferation reaches a peak during the second postnatal week (Schlessinger etal., 1975), and then declines dramatically with increasing age. In a pseudo-longitudinal study, cell proliferation was reported to decrease by 80% from adolescence (28 days) to adulthood (3 months), by 70% from adulthood to middle age (12 months) and by 60% from middle age to senescence (22 months) (Lemaire etal., 2000). Although many studies found an overall age-related decline in cell proliferation (Kuhn etal., 1996; Kempermann etal., 1998b; Cameron & McKay, 1999; Lemaire etal., 2006; Molofsky etal., 2006), the existence of a decline between middle age and senescence is still a matter of debate because some studies reported a significant effect (Bizon & Gallagher, 2003; Bondolfi etal., 2004; Rao etal., 2006) while others did not (Seki & Arai, 1995; Lichtenwalner etal., 2001; Nacher etal., 2003; Heine etal., 2004a; Rao etal., 2005; Driscoll etal., 2006; Kronenberg etal., 2006). Notably, BrdU-labeled precursors appeared to be lost equally at rostral and caudal levels, as well as in suprapyramidal and infrapyramidal blades of the GCL (Olariu etal., 2007). In contrast, cell proliferation in the hilus was not at all (Kuhn etal., 1996) or only slightly (Lichtenwalner etal., 2001) affected by aging, making the age-related decrease in proliferation specific to the GCL.
Table 1.
Influence of aging on cell proliferation within the subventricular zone (SVZ) and the dentate gyrus (DG)
| Proliferation | ||||||
|---|---|---|---|---|---|---|
| Reference | Species | Strains | Sex | Ages | Area | Results |
| (Bizon & Gallagher, 2003) | Rat | Long-Evans | Male | 7, 13, 25 months | DG | #BrdU cells: 7 m > 13 m > 25 m |
| (Bondolfi etal., 2004) | Mouse | C57BL/6 | Male | 2, 12, 18 months | DG | #BrdU cells: 2 m > 12 m > 18 m |
| (Brunson etal., 2005) | Rat | SD | Male | 3 and 12 months | DG | #BrdU cells: 3 m > 12 m |
| (Cameron & McKay, 1999) | Rat | SD | ? | 5 and 26 months | DG | #BrdU cells: 5 m > 25 m |
| (Cuppini etal., 2006) | Rat | SD | Male | 2, 5, 12 months | DG | #BrdU cells: 2 m > 5 m > 12 m |
| (Driscoll etal., 2006) | Rat | FBNF1 | Female | 3, 12, 24 months | DG | #Ki67 cells: 3 m > 12 m = 24 m |
| (Enwere etal., 2004) | Mouse | C57BL/6 | Male | 2 and 24 months | SVZ | #BrdU, Ki67, Mash cells: 2 m > 24 m |
| RMS | #BrdU cells: 2 m > 24 m | |||||
| (Gould etal., 1998) | Macaque | M. mulatta and fascicularis | Male and female | 5, 7–16, 23 years | DG | #BrdU cells: 5y > 7–16y > 23y |
| (Hattiangady & Shetty, 2008) | Rat | F344 | Male | 4, 12, 24 months | DG | %Sox 2/BrdU cells: 15 > 2.5 > 1%Sox 2/Ki67 cells: 25 > 8 > 4 |
| (Heine etal., 2004a) | Rat | Wistar | Male | 2 weeks, 6 weeks, 12, 24 months | DG | #BrdU cells: 2w > 6w > 12 m = 24 m |
| (Kempermann etal., 1998b) | Mouse | C57BL/6 | Female | 6 and 18 months | DG | #BrdU cells: 6 m > 18 m |
| (Kim etal., 2004) | Rat | SD | Male | 1, 2, 14 months | DG | #BrdU cells: 1 m > 2 m > 14 m |
| (Kronenberg etal., 2006) | Mouse | C57BL/6 | Male | 1.5, 9, 12, 24 months | DG | #BrdU cells: 1.5 m > 9 m > 12 m = 24 m |
| (Kuhn etal., 1996) | Rat | F344 | Female | 6 and 21 months | DG | #BrdU cells: 6 m > 21 m |
| SVZ | #BrdU cells: 6 m = 21 m | |||||
| (Lemaire etal., 2000) | Rat | SD | Male | 1, 3, 10, 22 months | DG | #BrdU cells: 1 m > 3 m > 10 m > 22 m |
| (Lemaire etal., 2006) | Rat | Wistar | Male | 4 and 26 months | DG | #BrdU cells: 4 m > 26 m#Ki67 cells: 4 m > 26 m |
| (Lichtenwalner etal., 2001) | Rat | BNxF344 | Male | 5, 18, 28 months | DG | #BrdU cells: 5 m > 18 m = 28 m |
| (Luo etal., 2006) | Mouse | ? | ? | 2, 10, 22 months | SVZ | #BrdU cells: 2 > 22 |
| (Maslov etal., 2004) | Mouse | C57BL/6 | Male | 4 and 26 months | SVZ | #BrdU cells: 4 m > 26 m |
| (McDonald & Wojtowicz, 2005) | Rat | SD | Male | 1 and 12 months | DG | #BrdU cells: 1 m > 12 m |
| (Molofsky etal., 2006) | Mouse | C57BL/6 | Male | 2 and 24 months | SVZ | #BrdU cells: 2 m > 24 m |
| (Nacher etal., 2003) | Rat | F344 | Female | 3, 10, 20 months | DG | #BrdU cells: 3 m > 10 m = 20 m |
| (Olariu etal., 2007) | Rat | SD | Male | 2.5 and 10 months | DG | #BrdU cells: 2.5 > 10 m#PCNA cells: 2.5 > 10 m |
| (Rao etal., 2005) | Rat | F344 | Male | 4, 12, 24 months | DG | #BrdU cells: 4 m > 12 m = 24 m |
| (Rao etal., 2006) | Rat | F344 | Male | 4, 12, 24 months | DG | #BrdU cells: 4 m > 12 m = 24 m#Ki67 cells: 4 m > 12 m > 24 m |
| (Seki & Arai, 1995) | Rat | Wistar | Male | 1, 2, 4, 6, 12, 18 months | DG | #BrdU cells: 1 m > 2 m > 2 m > 4 m > 6 m > 12 m = 18 m |
| (Simon etal., 2005) | Tree shrew | 3–10, 11–20, 21–30 months | DG | #BrdU cells: 3–10 m > 11–20 m > 21–30 m | ||
| (Tanaka etal., 2007) | Mouse | ICR | Males | 2 and 9 months | DG | #Ki67 cells: 2 m > 9 m |
| SVZ | #Ki67 cells: 2 m > 9 m | |||||
| (Tropepe etal., 1997) | Mouse | SW/COBS | Males | 2–4 and 23–25 months | SVZ | #BrdU cells: 2–4 m > 23–25 m |
Despite a drastic drop in proliferation, the short-term survival pattern of the newborn cells, that is, the proportion of cells that do not die shortly after their birth, seems to be unaffected by aging (see Table 2). A time course study comparing juvenile (38 days) and middle-aged rats (12 months old) has shown a significant peak in the number of BrdU-labeled cells observed 7 days after the injection, independent of the animal's age (McDonald & Wojtowicz, 2005). Following this peak, the number of BrdU-labeled cells rapidly starts to decrease with time. By comparing the ratio between the number of BrdU-positive cells just after BrdU injection and the number of BrdU-labeled cells surviving a few weeks after the injection, several studies have revealed that there is no effect of age on the rate of survival of newborn progeny (Kempermann etal., 1998b; Lichtenwalner etal., 2001; Bondolfi etal., 2004; McDonald & Wojtowicz, 2005; Rao etal., 2006). Even 5 months after their birth, the survival of adult-born neurons is independent of the age of the animals (Rao etal., 2005).
Table 2.
Influence of aging on the newborn cell survival and differentiation within the subventricular zone (SVZ) and the dentate gyrus (DG)
| Reference | Species | Strains | Sex | Ages | Delay | Area | Results |
|---|---|---|---|---|---|---|---|
| Survival of newly generated cells | |||||||
| (Bizon etal., 2004) | Rat | Long-Evans | Male | 7 and 25 months | 3 weeks | DG | #BrdU cells:7 m > 25 m |
| (Bondolfi etal., 2004) | Mouse | C57BL/6 | Male | 2, 12, 18, 24 months | 4 weeks | DG | #BrdU cells: 2 m > 12 m > 18 m = 24 mSurvival rate: 2 m = 12 m = 18 m = 24 m |
| (Cuppini etal., 2006) | Rat | SD | Male | 2, 5, 12 months | 15 days | DG | #BrdU cells: 2 m > 5 m > 12 m |
| (Jin etal., 2003a) | Mouse | CD1 | Male | 3 and 20 months | 1 week | DG | #BrdU cells: 3 m > 20 m#BrdU cells: 3 m > 20 m |
| (Heine etal., 2004a) | Rat | Wistar | Male | 6 weeks, 12, 24 months | 4 weeks | DG | #BrdU cells: 6w > 12 m = 24 m |
| (Kempermann etal., 1998b) | Mouse | C57BL/6 | Female | 6 and 18 months | 4 weeks | DG | #BrdU cells: 6 m > 18 mSurvival rate: 6 m = 18 m |
| (Kuhn etal., 1996) | Rat | F344 | Female | 6, 12, 27 months | 4 weeks | DG | #BrdU cells: 6 m > 12 m = 27 m |
| (Lichtenwalner etal., 2001) | Rat | BNxF344 | Male | 5, 18, 28 months | 4 weeks | DG | #BrdU cells: 5 m > 18 m = 28 m |
| (McDonald & Wojtowicz, 2005) | Rat | SD | Male | 1 and 12 months | 60 days | DG | #BrdU cells: 1 m > 12 mSurvival rate: 1 m = 12 m |
| (Merrill etal., 2003) | Rat | F344 | Female | 2 and 21 months | 10 days | DG | #BrdU cells: 2 m > 21 m |
| (Rao etal., 2005) | Rat | F344 | Male | 4, 12, 24 months | 5 months | DG | Survival rate: 4 m = 10 m = 24 m |
| (Rao etal., 2006) | Rat | F344 | Male | 4, 12, 24 months | 10 days | #BrdU cells: 4 m > 12 m = 24 m | |
| (Segovia etal., 2006) | Rat | Wistar | Male | 2 and 25 months | 6 weeks | DG | #BrdU cells: 2 > 25 m |
| (Tropepe etal., 1997) | Mouse | SW/COBS | Male | 2–4 & 23–25 months | 31 days | OB | #BrdU cells: 2–4 m > 23–25 m |
| (van Praag etal., 2005) | Mouse | C57BL/6 | Male | 3 and 19 months | 35 days | DG | #BrdU cells: 3 m > 19 m |
| (Wati etal., 2006) | Rat | SD | Male | 3–4 and 28 months | 1 week | DG | #BrdU cells: 3–4 m > 28 m |
| Phenotype of newly generated cells | |||||||
| (Bizon etal., 2004) | Rat | Long-Evans | Male | 7 and 25 months | 3 weeks | DG | %BrdU/NeuN: 83 > 67%BrdU/GFAP: 0/5 |
| (Bondolfi etal., 2004) | Mouse | C57BL/6 | Male | 2, 12, 18, 24 months | 4 weeks | DG | %BrdU/NeuN: 68 > 39 = 33 = 30%BrdU/S100: 9/15/21/20 |
| (Driscoll etal., 2006) | Rat | FBNF1 | Female | 3, 12, 24 months | 5 weeks | DG | %BrdU/NeuN cells: 3 m > 12 m > 24 m |
| (Enwere etal., 2004) | Mouse | C57BL/6 | Male | 2 and 24 months | 4 weeks | OB | %BrdU/TH: 2 m > 24 m%BrdU/calretin: 2 m > 24 m |
| (Heine etal., 2004a) | Rat | Wistar | Male | 12 and 24 months | 4 weeks | DG | %BrdU/NeuN: 12 m > 24 m |
| (Kempermann etal., 1998b) | Mouse | C57BL/6 | Female | 6 and 18 months | 4 weeks | DG | %BrdU/NeuN: 6 m > 18 m%BrdU/S100 : 6 m < 18 m |
| (Lichtenwalner etal., 2001) | Rat | BNxF344 | Male | 5, 18, 28 months | 4 weeks | DG | %BrdU/NeuN: 5 m > 18 m = 28 m |
| (McDonald & Wojtowicz, 2005) | Rat | SD | Male | 1 and 12 months | 3, 7, 8, 14, 21, 60 days | DG | %BrdU/DCX: 1 m > 12 m%BrdU/CaBP: 1 m > 12 m |
| (Molofsky etal., 2006) | Mouse | C57BL/6 | Male | 2 and 24 months | 4 weeks | OB | %of NeuN/BrdU: 2 m > 24 m |
| (Nacher etal., 2003) | Rat | F344 | Female | 3, 10, 20 months | 3 weeks | DG | %BrdU/rCRMP4: 3 m > 10 m > 20 m |
| (Rao etal., 2005) | Rats | F344 | Male | 4, 12, 24 months | 10 days5 months | DG | %BrdU/DCX: 4 m = 12 m = 24 m%BrdU/DCX: 4 m = 12 m = 24 m%BrdU/NeuN: 4 m = 12 m = 24 m |
| (van Praag etal., 2005) | Mouse | C57BL/6 | Male | 3 and 19 months | 35 days | DG | %BrdU/NeuN: 3 > 19 m%BrdU/S100b: 3 m < 19 m |
The column ‘Delay’ refers to the interval between the labeling of newly generated cells by BrdU injections and the sacrifice of the animals.
In addition to changes in proliferation, the capacity of the newly born cells to migrate radially from their birthplace in the SGL toward the GCL also slows down with increasing age (Heine etal., 2004a; Rao etal., 2005). In fact, in aged animals, only a small fraction of the newly born cells is able to migrate to the GCL within 4 weeks (Heine etal., 2004a). However, 5 months after their birth, the position of newly born cells within the GCL of aged subjects is similar to that of their young counterparts (Rao etal., 2005). This delayed migration may be because of an age-related decline in the expression of a highly polysialylated neural cell adhesion molecule, which is associated with the migration and maturation of immature precursor cells (Seki & Arai, 1995; Ni Dhuill etal., 1999).
The phenotype of 2-week-old to 6-week-old newborn cells is determined by double labeling of BrdU-labeled cells with immature neuronal markers (e.g. doublecortin), mature neuronal markers (e.g. calbindin or neuron-specific nuclear protein, NeuN), or glial markers (e.g. GFAP, see Table 2). Most studies have shown a strong reduction in the differentiation into neuronal phenotypes in aging subjects (Kempermann etal., 1998b; Lichtenwalner etal., 2001; Nacher etal., 2003; Bizon etal., 2004; Bondolfi etal., 2004; Heine etal., 2004a; McDonald & Wojtowicz, 2005; Driscoll etal., 2006; Molofsky etal., 2006). In the most drastic case reported, almost 70% of newly born cells differentiated into neurons (Bizon etal., 2004). However, some groups were not able to reproduce these results and have reported only small (McDonald & Wojtowicz, 2005) or undetectable changes in the proportion of new cells differentiating into neurons (Rao etal., 2006). These discrepancies are likely because of the fact that either the animals were significantly younger or the survival period between BrdU injection and animal perfusion was different. In some studies, an increase in astrocytic differentiation was also observed in aged animals (Bizon etal., 2004; Bondolfi etal., 2004).
Additionally, analysis of dendritic growth in 12-day-old BrdU–DCX double-labeled neurons revealed that dendritic maturation was considerably reduced during aging. At that time, newly born neurons exhibited diminished dendritic branching and total dendritic length compared with their age-matched counterparts in young DG (Rao etal., 2006). However, another study labeling adult-born cells with a GFP retrovirus found that dendritic length, branching, and spine density of 4-week-old cells were similar in young and aged brain (van Praag etal., 2005). The discrepancy between these two studies could be related to the age of the cells studied (2 weeks vs. 4 weeks). Another possibility is that in one study (van Praag etal., 2005), dendritic analysis was performed after running, a condition that might accelerate the maturation of adult-born neurons. Clearly, some additional experiments are needed to better understand the effect of aging on dendritic maturation.
In the SVZ
Within the SVZ, the existence of age-related changes in cell proliferation is not so clear and may show important variations among species (see Tables 1 and 2). In mice, a two- to threefold reduction of cell proliferation in the SVZ has been reported using BrdU labeling in young adults (2–5 months) compared to aged (20–27 months) mice (Tropepe etal., 1997; Jin etal., 2003a; Enwere etal., 2004; Maslov etal., 2004; Molofsky etal., 2006). In rats, however, no difference in the density of BrdU-labeled cells was observed between 6-month-old and 21-month-old rats (Kuhn etal., 1996). Aside from possible species-specific differences, this discrepancy can likely be explained by the fact that in the SVZ, newborn cells quickly leave the proliferation area to migrate toward the OB. Consequently, slight differences in the BrdU injection protocol may strongly affect the labeling index of SVZ cells and make the differences between young and aged individuals undetectable. Indeed, the use of intrinsic markers of cell proliferation expressed by proliferating and/or relatively quiescent cells such as MCM-2, Ki67, and HH3 showed a substantial reduction of neurogenesis in the SVZ of aged rats compared with young adult rats (Zhang etal., 2006; Tanaka etal., 2007). Electron microscopy studies have revealed that the proliferation defect is specific to neuroblasts and transitory amplifying progenitor cells, which are restricted to the anterior dorsolateral horn of the SVZ, while the number of SVZ stem cells remains relatively constant (Luo etal., 2006). Moreover, in vitro experiments suggest that an age-related decrease occurs in the number of restricted progenitors, but not in the number of stem cells (Tropepe etal., 1997), thus indicating that SVZ progenitors and stem cell numbers are differentially regulated with age. In agreement with this, the number of BrdU–GABA double-labeled neurons in the granule and glomerular layers of the OB was halved in aged mice (Enwere etal., 2004). This was accompanied by a reduction of BrdU-labeled periglomerular neurons expressing tyrosine hydroxylase (TH, 71%) or calretinin (59%).
Conclusions
Cell proliferation is the stage of neurogenesis in the DG and the SVZ that is most affected by aging. In contrast, migration, survival, and neuronal fate choice seem to be less dramatically affected, as they seem only to be delayed in the aged DG. Given the ‘paucity’ of data in the SVZ, it is unknown whether aging affects migration (tangentially along the RMS or radially within the OB), cell survival, or cell differentiation. Remarkably, after a pronounced increase in the first few postnatal weeks, the volume of the GCL and the total number of granule cells in the DG appear to be essentially stable throughout life (West, 1993; West etal., 1994; Rapp & Gallagher, 1996; Rasmussen etal., 1996; Kempermann etal., 1998b; Merrill etal., 2001; Rapp etal., 2002; Jin etal., 2003a; Heine etal., 2004a), suggesting that aging affects the turnover of granule cells rather than their absolute number. This hypothesis is strengthened by the fact that apoptosis slows down profoundly in the DG (Heine etal., 2004a). However, it is obvious that further studies are needed to: (i) distinguish the effects of aging on the activity of slowly cycling stem or stem-like cells and the more rapidly cycling transit progenitors; (ii) better characterize the effects of aging on the development of new cells in an old environment; and (iii) study the electrophysiological properties of these neurons.
What are the mechanisms involved in the decrease of neurogenesis?
Age-related changes in neurogenesis could be the consequence of the inability of old precursors to respond appropriately to external stimuli because of changes in their intrinsic properties. Alternatively, age-related changes that occur in local and systemic environments might be responsible for the decline in neurogenesis.
Old cells?
Overall, the data available from the bibliography suggest that the main difference between juvenile/young and aged rats is a decrease in the rate of cell proliferation. However, the mechanisms responsible for this effect are still unclear. Lengthening of the cell cycle occurring throughout the lifelong proliferative period could be responsible for the reduction in the rate of neuronal production that occurs with age. Alternatively, decreased neurogenesis could result from a progressive loss of precursors. Finally, those precursors might become quiescent with age, even though they could still retain the potential for reactivation. Ultimately, the age-related decline in neurogenesis may be the consequence of not just one of these phenomena but, more likely, a combination of them.
In the DG
As there are no specific markers for stem/progenitor cells in the adult brain, it is difficult to determine their precise number throughout life. Cumulative BrdU labeling, as well as endogenous markers of cell proliferation, suggest that the size of the dividing precursor pool is three to four times smaller in middle-aged animals compared to young adult animals (Olariu etal., 2007). GFAP-, nestin-, and vimentin-expressing radial-like cells are believed to be the precursor cells that divide asymmetrically to generate a daughter radial cell and a DCX-expressing direct progenitor (Seri etal., 2004; Encinas etal., 2006). Similar to what is observed with cumulative labeling studies, the number of these cells in the GCL is also dramatically decreased with age, becoming very low in both middle-aged and aged animals (Alonso, 2001; Nacher etal., 2003). However, these approaches do not take into consideration the existence of quiescent precursors. Using Sox-2 as a putative marker of stem cells, it has been shown that the overall number of Sox-2 cells remains constant in young, middle-aged, and aged rats, yet the percentage of these cells expressing proliferation markers (BrdU and Ki67) is drastically reduced with age (Hattiangady & Shetty, 2008). These results suggest that aging is not associated with a decrease in the total number of precursors in the SGL, but, rather, with a decrease in the proportion of active precursors, as a result of increased quiescence of these cells, likely because of age-related changes in the surrounding milieu. Moreover, a comparison of Sox-2/BrdU data with Sox-2/Ki67 results suggests a lengthening of the cell cycle of NSCs between young adult and middle-aged F344 rats as well as between middle-aged and aged F344 rats (Hattiangady & Shetty, 2008). However, this result was not confirmed by a study carried out on young adult and 10-month-old Sprague–Dawley rats, using a protocol of multiple BrdU and tritiated thymidine injections coupled with endogenous proliferation markers, specifically designed to measure the size of the proliferating population and its cell cycle duration (Olariu etal., 2007). Additional studies on older animals are now required to definitively corroborate or rule out the possibility of changes in the cell cycle with advancing age.
In the SVZ
Only a few studies have examined the mechanisms involved in the decrease of neurogenesis in the aged SVZ, leading to contradictory results. On one hand, sequential labeling with BrdU and tritiated thymidine has revealed a lengthening of the cell cycle in the SVZ of older mice (Tropepe etal., 1997; Jin etal., 2003a). On the other hand, the use of a combination of proliferation markers specific for the different stages of the cell cycle to identify the fraction of the proliferative progenitors that are in S-phase at a given time has demonstrated that aging does not change the rate of division of SVZ cells (Maslov etal., 2004). However, consistent with an increase in the length of the cell cycle, the time course for repopulation after a complete depletion of constitutively proliferating cells in the SVZ by an AraC treatment (see Doetsch etal., 1999b for protocol) is markedly different in aged animals compared to their younger counterparts. Complete recovery was not seen until 14 days in aged animals compared to only 4–8 days in young adult animals (Enwere etal., 2004). These experiments suggest that the reduction in proliferation detected in the SVZ might result from a lengthening of the cell cycle rather than from a reduction in the number of precursors. However, it is unknown if this lengthening is an intrinsic property of the aging SVZ stem/progenitor cells or if it is the consequence of changes in the environment, for example, the influence of inhibitory factors and/or absence of a stimulatory factor.
Conclusion
When taken together, these results suggest that the drastic decline of neurogenesis observed during aging may be attributable to several mechanisms (Fig. 2), for example, a decreased number of proliferating cells or limited proliferation potential resulting from increased quiescence.
Old environment?
In this context, either newly born cells may have lost their intrinsic capacity to respond to the mitotic stimuli provided by the environment (e.g. growth factor receptors) or, alternatively, the local environment may have changed so that the mitotic stimuli are no longer provided (see Table 3). The rapid reduction in the rate of proliferation and migration during aging is intriguing, as it suggests that the fate of newborn cells is strongly influenced by local environmental factors rather than by intrinsic or genetic cues. This hypothesis is strongly supported by the fact that: (i) during aging, positive regulators of neurogenesis are known to decrease, whereas signals identified as neurogenesis inhibitors are increased; and (ii) neurogenesis in the aging brain can be boosted by increasing the level of pro-neurogenic factors or by decreasing the levels of anti-neurogenic factors (see below). It has been suggested that this effect does not result from accelerating the cell cycle of the precursors, but from an increase in the number of precursors in aged DG (Olariu etal., 2007). The additional dividing cells could potentially come from precursors that have become quiescent over time, that is, cells that are not dividing but have retained their capacity to divide. Consequently, in the senescent brain, the adult neural precursor cells can apparently stop dividing for long periods of time, but still proliferate when prompted by the right signal.
Steroids
Corticosteroids.
Corticosteroids have been identified as one of the factors having the strongest negative regulatory effects on adult hippocampal neurogenesis. Corticosteroids are released into the blood circulation following the activation of the hypothalamo–pituitary–adrenal (HPA) axis, primarily by stress. Corticosterone, the main corticosteroid in rodents, regulates its own secretion through negative feedback by interacting with two receptors present in the DG, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) (van Eekelen etal., 1991; Sapolsky etal., 2000). Mineralocorticoid receptors have a higher affinity for corticosterone than GRs, and are primarily found in limbic structures, including the HF (van Eekelen etal., 1988), whereas GRs are expressed ubiquitously. Acute (Cameron & Gould, 1994) or chronic (Ambrogini etal., 2002) treatment with corticosterone has been associated with a strong down-regulation of cell proliferation in the adult DG. Different paradigms of stress, which dramatically increase corticosterone levels, decrease either cell proliferation (Gould etal., 1997, 1998; Czeh etal., 2001; Pham etal., 2003; Heine etal., 2004b; Simon etal., 2005) or cell survival and neuronal differentiation in the DG (Pham etal., 2003; Thomas etal., 2007). This effect is reversible, as 3 weeks after the termination of the stress stimulus, a total recovery is observed (Heine etal., 2004b). On the other hand, short-term suppression of corticosterone secretion by adrenalectomy in adulthood increases granule cell neurogenesis (Gould etal., 1992; Cameron & Gould, 1994). This change can be prevented by corticosterone replacement or by stimulation of MR or GR (Gould etal., 1992; Rodriguez etal., 1998; Montaron etal., 2003). In the young DG, GRs are expressed by 50% of the stem-like astrocytes, early progenitors, and immature new neurons, whereas MRs seem to be expressed only by more mature, calbindin-positive granule cells (Garcia etal., 2004a). However, the presence of MRs in mature neurons is inconsistent with the fact that the stimulation of these receptors is sufficient to reverse the adrenalectomy-induced increase in cell proliferation (Montaron etal., 2003; Wong & Herbert, 2005).
The age-related decline in neurogenesis has been associated with increased exposure to corticosterone, resulting from increased basal levels, mainly during the dark phase of the circadian cycle, and prolonged stress-induced secretion (Meaney etal., 1992; Sapolsky, 1992; Lupien etal., 1994). This intriguing correlation has led to the hypothesis that chronically elevated corticosterone levels are responsible for reduced neurogenesis in the aging DG. Consequently, the effects of adrenalectomy in senescent rats were studied. Adrenalectomy in senescent rats dramatically reduces corticosterone levels (below 0.3 µg dL−1) (Montaron etal., 1999), and 1 week later, increases cell proliferation and, consequently, neurogenesis (Cameron & McKay, 1999; Montaron etal., 1999). This effect depends on corticosterone secretion because it can be prevented by treatment with the hormone (Montaron etal., 1999). This set of experiments was the first to indicate that the age-related decrease in neurogenesis may not be solely caused by a limitation of the stem cells themselves, but rather, to inadequate environmental signals. Adrenalectomy at midlife blocked the age-related increase in both basal- and stress-induced corticosterone secretion and, subsequently, increased cell proliferation and neurogenesis in senescent animals (Montaron etal., 2006). When adrenalectomy is performed during early postnatal life and treatment with corticosterone is given orally, the rate of neurogenesis in middle-aged rats does not differ from that of age-matched, sham-operated controls (Brunson etal., 2005). However, differences in corticosterone levels [10 µg dL−1 in middle-aged, adrenalectomized rats (Brunson etal., 2005) compared to less than ≤ 2 µg dL−1 in 3-month-old rats and to aged rats adrenalectomized at either middle age or senescence (Montaron etal., 1999, 2006)] might explain this discrepancy. Using an approach that takes into account the individual differences in the activity of the HPA axis, we have shown that in aged animals, the weight of the adrenal glands, considered as a reliable index of the chronic activity of the HPA axis, is inversely correlated with the proliferation and survival of BrdU-labeled cells in the GCL (Montaron etal., 2006). In other words, hyperactivity of the HPA axis is associated with a low level of cell proliferation and survival. When compared to young animals, aged rats have higher expression of GR in early precursors, and calretinin-positive immature neurons express both GR and MR (Garcia etal., 2004a). This shift of the GR and MR expression profile toward a more immature stage of neuronal development suggests increased steroid sensitivity of the aged DG to corticoid impregnation.
In conclusion, long-term exposure to high levels of corticosterone throughout the animal's life can damage the precursor cell population, permanently decreasing the size of the dividing population in aged animals. Alternatively, corticosterone may be directly affecting proliferation without damaging the precursors, resulting in a reversible decline in neurogenesis.
Neurosteroids.
Neurosteroids are a subclass of steroids synthesized de novo in the brain, in particular, in the HF (Baulieu & Robel, 1997). In young adult rats, intracerebroventricular (icv) infusion of allopregnanolone, a neurosteroid which acts as a positive allosteric modulator of the GABAA receptors that are present on neuroblasts, decreases hippocampal cell genesis. In contrast, other neurosteroids such as pregnenolone sulfate (Preg-S) and dehydroxyepiandrosterone acting as negative allosteric modulators of GABAA, increase hippocampal neurogenesis (Karishma & Herbert, 2002; Mayo etal., 2003). The effects of Preg-S on neurogenesis are probably mediated by GABAA receptors because the Preg-S-induced increase in neurogenesis can be blocked by prior icv administration of muscimol, a GABAA agonist. Pregnenolone sulfate may act directly on these receptors because it is able to stimulate the proliferation of neural spheres in vitro (Mayo etal., 2003). In aged animals, acute icv infusion of Preg-S considerably increases the rate of cell proliferation and neurogenesis (Mayo etal., 2003). Interestingly, it has been proposed that age-related alterations are caused by an abnormally strong inhibitory GABAergic input (Marczynski, 1998; Segovia etal., 2006). Together with the observation that Preg-S increases neurogenesis, this suggests that the age-related decline in neurogenesis could be related to an enhancement of GABA transmission.
Glutamate
Granule cells are glutamatergic in nature and receive glutamatergic afferents mainly from the entorhinal cortex through the perforant pathway (Vizi & Kiss, 1998). In the young adult DG, the blockade of NMDA receptors by competitive or noncompetitive receptor antagonists enhances the number of newly generated granule neurons. This suggests an inhibitory action of glutamate on neurogenesis (Cameron etal., 1995; Nacher etal., 2001). Moreover, NMDA receptors and corticosterone are believed to work synergistically in inhibiting cell proliferation (Cameron etal., 1998). In aged rats, neurogenesis can be reactivated by intraperitoneally injecting NMDA receptor antagonists, which elicit a significant increase in the number of stem-like cells, proliferating cells, and new neurons in the DG (Nacher etal., 2003).
Growth factors
Epidermal growth factor (EGF) signaling.
Among the various members of the EGF family of ligands that are able to interact with EGF receptor (EGFR), only three have been shown to be expressed in the brain: (i) transforming growth factor alpha (TGFα), which binds only the EGF-R and is supposed to be the main endogenous ligand in the brain (Wilcox & Derynck, 1988; Seroogy et al., 1991, 1993; Lazar & Blum, 1992); (ii) heparin-binding EGF-like growth factor (HB-EGF), also able to bind a related receptor, ErbB4; and (iii) amphiregulin, to a lesser extent (Opanashuk et al., 1999). In the adult SVZ, icv administration of EGF expands the precursor population. This is accompanied by a differentiation bias toward the astrocyte phenotype, ultimately leading to a reduction in the total number of newborn neurons that reach the OB (Kuhn et al., 1997). Heparin-binding EGF-like growth factor seems to have a different effect as it is able to increase both cell proliferation in the SVZ and the number of newly born neurons reaching the OB (Jin et al., 2002a, 2003b). In the DG, HB-EGF (Jin et al., 2002a, 2003a) but not EGF (Kuhn et al., 1997) increases cell proliferation and neuronal differentiation (Jin et al., 2002a), while the role of TGFα is still unknown.
During aging (between 2 months and 24 months in mice), both EGFR and TGFα expression (at least in the SVZ) decline by 50% and 70%, respectively (Enwere etal., 2004). This suggests that the expansion potential of NSC progeny may be reduced because of a reduction in EGFR signaling. The consequences of HB-EGF infusion in the aged brain are similar, yet more significant, than the effects observed in young adults. Indeed, HB-EGF increases cell proliferation by ~1.6- and 5.5-fold in young and aged DG, respectively, and ~2.4- and ~2.7-fold in the young and aged SVZ, respectively. In the end, the number of BrdU-labeled cells is comparable in untreated 3-month-old and treated 20-month-old mice (Jin etal., 2003a). On the other hand, EGF is less efficient at increasing cell proliferation in the aged SVZ when compared to HB-EGF (~1.8-fold increase in young adult and ~1.4-fold in aged animals) (Enwere etal., 2004). The differences observed between these EGFR ligands are likely because of the different receptors and intracellular pathways involved.
Insulin-like growth factor-I (IGF-I).
Among the growth factors that may regulate neurogenesis, IGF-I is of particular interest given its expression pattern throughout life. Insulin-like growth factor-I is strongly expressed during development (Bondy, 1991; Baker et al., 1993), but its expression is subsequently gradually reduced in the adult brain. However, it persists in adult neurogenic areas (Rotwein et al., 1988; Anlar et al., 1999), most likely because of its local production by glial cells (Fernandez-Galaz et al., 1997; Du & Dreyfus, 2002). Insulin-like growth factor-I has considerable influence in adult hippocampal neurogenesis, as it stimulates both cell proliferation and neuronal differentiation (Aberg et al., 2000; Trejo et al., 2001; Anderson et al., 2002). During aging, IGF-I and IGF-I receptor levels undergo a secondary decline (Sonntag et al., 1999; Lai et al., 2000; Shetty et al., 2005), especially in the HF. Consequently, the reduced IGF-I concentration observed from middle age may contribute to initiating the age-related decline in neurogenesis. Indeed, the decrease of IGF-I levels with age correlates with the evolution of neurogenesis and can be reversed by icv infusion of IGF-I (Lichtenwalner et al., 2001). This treatment triples the number of newborn neurons in aged rats through a remarkable increase in cell proliferation.
Fibroblast growth factor 2 (FGF-2).
Fibroblast growth factors (FGFs), in particular FGF-2 (also called basic FGF), have been shown to play an important role during CNS development by controlling neurogenesis, neuron survival, and differentiation (Walicke etal., 1986; Morrison etal., 1988; Vicario-Abejon etal., 1995; Nakagami etal., 1997; Vaccarino etal., 1999). The expression of FGFs and FGF receptors (FGFRs) persists in the adult brain. Strikingly, the astrocytes of the DG, the SVZ, the RMS, and the OB display the most robust FGF receptor 2 (FGFR-2) expression in the adult brain (Chadashvili & Peterson, 2006). This result points out the possible role of FGF signaling in neurogenesis. In adult SVZ, a proliferative effect of FGF-2 as well as an enhancement of migration to the OB has been clearly demonstrated (Kuhn etal., 1997; Wagner etal., 1999; Jin etal., 2003a). In the adult DG, similar experiments increase proliferation in the hilus but are ineffective in the SGL (Kuhn etal., 1997; Jin etal. 2003a). On the other hand, over-expression of FGF-2 by gene transfer in lifelong FGF-2-deficient mice up-regulates DG cell proliferation (Yoshimura etal., 2001).
The hippocampal concentration of FGF-2 decreases between young adult age and middle age, but shows no change between middle age and old age. This decrease is more particularly associated with the decline of a subpopulation of astrocytes that express FGF-2 and could be the consequence of an age-related impairment in FGF-2 synthesis by astrocytes (Shetty etal., 2005). In this manner, an age-related decrease in FGFR-2 levels is observed in the SVZ, RMS, OB, and HF, but not in non-neurogenic regions of the brain (Chadashvili & Peterson, 2006). Unlike what is observed in young adults, FGF-2 icv infusion for 2 weeks or 3 days increases neurogenesis in both the DG and the SVZ of middle-aged (Rai etal., 2007) and aged mice (Jin etal., 2003a). The enhanced production of new neurons was associated with an enhanced dendritic growth (Rai etal., 2007). Thus, age-related declines in hippocampal neurogenesis are likely linked to reduced FGF-2 concentrations.
Vasculature and vascular endothelial growth factor (VEGF).
The vasculature is an important component of adult neurogenic niches. Blood vessels are conduits for the delivery of long-distance paracrine factors (e.g. hormones, growth factors.) from distant sources, and by this means, they could play an essential indirect role in the regulation of neurogenesis. Furthermore, in the DG (Palmer etal., 2000; Heine etal., 2005) as well as in the SVZ (Bovetti etal., 2007), new cells are clustered in close proximity to blood vessels where VEGF expression is high and angiogenesis is ongoing (Palmer etal., 2000). It is, thus, believed that neurogenesis and angiogenesis are mechanistically linked, and that VEGF, which is normally expressed in cerebral microvessels, is the linking factor between these two events (Palmer etal., 2000; Jin etal., 2002b). Consistent with this hypothesis, several studies performed in young adult rodents have highlighted the importance of VEGF in adult bulbar and hippocampal neurogenesis (Jin etal., 2002b; Fabel etal., 2003; Sun etal., 2003; Cao etal., 2004; Greenberg & Jin, 2004).
Brain aging is associated with a reduction in the cerebral microvasculature (Sonntag etal., 1997), a loss of microvascular plasticity (Riddle etal., 2003), and reduced VEGF synthesis (Shetty etal., 2005). This is specifically true in the DG where the total volume of the SGL occupied by RECA-1+ capillaries undergoes a more than 25% decrease in aged compared to young adult animals (Hattiangady & Shetty, 2008). Thus, limited angiogenesis, decreased cerebral blood flow, and decreased concentration of the associated growth factor, VEGF, in the aged brain may contribute to the decline in cell genesis. Using Sox2 as a marker of neural stem/progenitor cells, it has been shown that the distance between endothelial cells and the putative stem cells is increased with aging. This may in turn reduce the accessibility of these cells to endothelial-cell-derived and blood-transported factors (Hattiangady & Shetty, 2008).
Cell cycle regulators
The polycomb transcriptional repressor Bmi-1 is required for the self-renewal and postnatal maintenance of hematopoietic (Lessard & Sauvageau, 2003; Park etal., 2003) and neural stem cells (Molofsky etal., 2003, 2005). The absence of Bmi-1 in Bmi-1-deficient mice induces a premature senescence of stem cells and, consequently, a severe reduction in the rate of proliferation in the SVZ, both in vitro and in vivo. Conversely, Bmi-1 over-expression can prevent senescence and extend the replicative lifespan of primary cells (Molofsky etal., 2005). Bmi-1 acts through the repression of two inhibitors of cell proliferation whose induction has also been associated with cellular senescence: p16Ink4a, a cyclin-dependent kinase inhibitor, and p19Arf, which promotes p53 activation (Jacobs etal., 1999; Sherr, 2001). Indeed, deletion of Ink4a or Arf from Bmi-1−/– mice partially rescued stem cell self-renewal and stem cell proliferation defects in the SVZ (Bruggeman etal., 2005; Molofsky etal., 2005).
p16INK4a Gene expression increases with age in a variety of tissues (Zindy etal., 1997; Krishnamurthy etal., 2004), including the SVZ where p16INK4a expression is not detectable in the SVZ of 60-day-old mice, but becomes detectable by 1 year of age and is further increased at 2 years of age (Molofsky etal., 2006). On the other hand, p19Arf expression in the SVZ is not affected by aging. The effects of p16INK4a on the generation of new neurons in the SVZ increase with age and have been studied employing p16INK4a-deficient mice. While no effect was observed in young adults, p16INK4a deficiency significantly increases the frequency of newly generated OB neurons in aged animals. Notably, p16INK4a deficiency does not affect the ratio of non-neuronal cells in the OB or neurogenesis in the DG (Molofsky etal., 2006). Thus, in certain regions such as the SVZ but not the DG, stem cell function is regulated by a balance between Bmi-1, which promotes stem cell maintenance and regenerative capacity, and tumor suppressors like p16INK4a, which reduce regenerative capacity and promote aging. During aging, this balance is probably affected by as yet unidentified factors resulting in a reduction of precursor function and neurogenesis in at least certain regions of the nervous system.
Conclusion
The studies conducted so far have clearly showed that corticosteroids exert a deleterious influence on hippocampal neurogenesis during aging (see also the third section). Although the list of factors influencing the course of neurogenesis is growing, little is known about their influence in the aging brain. This is because not only of the inherent difficulty of in vivo aging studies, but also to the controversy over the effects of some factors cited earlier in young rats, for example, glutamate and GABA have also been shown to promote neurogenesis (Deisseroth etal., 2004; Ge etal., 2007). Furthermore, in vivo‘pharmacological’ studies involve changes in local networks (other neurons and other types of cells in the DG), in the structure (HF) and in connected structures. Thus, the observed effects are certainly not only the result of a direct action on the precursors or their lineage.
The changes observed during senescence cannot be explained by only one of the listed factors, and are a consequence of intricate regulation of different factors working in concert and often dependent on each other. They can play either permissive or instructive roles, and it is likely that there is a strictly orchestrated regulation of all of them, where some could be involved at early stages of aging and others could take effect only at later times, leading to the aging of neurogenic areas. This environmental-dependent regulation of neurogenesis supports the idea that the age-related loss of new neurons is not an irreversible cell-intrinsic process and shows that, when triggered by appropriate signals, neurogenesis can be reactivated in senescent brain.
A reduction of neuronal plasticity is hypothesized to be the cornerstone of the appearance of age-related deficits. The possibility of preventing it by manipulating the basal level of neurogenesis raises new hope of improving brain function during aging. Consequently, in the last part of this review, we will examine the known relationships between the age-related decline in neurogenesis and age-dependent memory impairment, and see to what extent the low rate of neurogenesis can contribute to the appearance of deficits.
Functional consequences of the age-related decrease in neurogenesis
Several lines of evidence based on the structure–function relationships support the involvement of neurogenesis in memory processing in the young adult brain. In particular, adult-born olfactory neurons have been shown to be involved in olfactory memory, while adult-born hippocampal neurons have been related to complex forms of spatial or associative memories (Aimone etal., 2006; Leuner etal., 2006; Lledo etal., 2006; Abrous & Wojtowicz, 2008). The fact that adult neurogenesis strongly decreases with age raises the important question of whether this decline in plasticity participates in the appearance of age-related dysfunction and whether a reduced number of new cells in an old brain can make a relevant functional contribution. Apart from an elegant study by Enwere etal. (2004) showing that the age-related decline in adult-born periglomerular neurons is associated with deficits in fine odor discrimination, most studies on aging have focused on the role of adult hippocampal neurogenesis in hippocampal functioning and, especially, in spatial memory. The spatial learning deficits observed in senescent animals are similar to those caused by hippocampal alterations (Rosenzweig & Bennett, 1996; Morris, 2006); this has raised the critical issue as to whether an alteration of hippocampal neurogenesis could be responsible for the age-related loss of spatial memory abilities. From an operational standpoint, the water maze has been the only test used, with the exception of one study. This paradigm requires that animals learn multiple extra-maze visual cues, allowing them to build a dynamic spatial representation of their surroundings for navigating to a platform hidden underneath the surface of the water. The requirement of having to learn complex relationships of extra-maze visual cues is the aspect of the test that renders it sensitive to hippocampal dysfunction.
In the following sections, we will review the evidence linking neurogenesis and memory during ‘normal’ aging. By this term, we refer to the natural process of memory decline that does not involve neurodegenerative processes such as those observed in Alzheimer's disease. A distinction will be made between successful and pathological aging, the latter – and not the former – being characterized by the appearance of memory deficits.
Spatial memory abilities and neurogenesis in old rats
It has long been recognized that spatial learning is particularly vulnerable to the effects of aging. However, memory alteration is extremely variable within a population, and not all experimental animals exhibit memory disorders (Gage etal., 1988; Markowska etal., 1989; Rapp & Amaral, 1992; Gallagher etal., 1993). In particular, some old animals show a clear impairment of spatial reference memory using the water maze, while others exhibit memory capacities similar to those of younger individuals (Gage etal., 1988; Markowska etal., 1989; Rapp & Amaral, 1992; Gallagher etal., 1993). The impairments observed in some aged animals are similar to those observed after hippocampal lesions (Redish & Touretzky, 1998; Stoelzel etal., 2002) and have been associated with defects in hippocampal circuitry and plasticity (Petit & Ivy, 1988; Markowska etal., 1989; Gallagher etal., 1990; Rapp & Amaral, 1992; Rowe etal., 2007). The hypotheses being pursued relate the ability to perform hippocampus-related functions to hippocampal neurogenesis in basal (off the learning phase) and dynamic (in the course of learning) conditions.
The rate of basal neurogenesis determines learning performance and memory
The existence of a correlation between spatial memory ability, cell proliferation, cell survival, and neurogenesis was examined under basal conditions. In these circumstances, spatial memory performance of aged rats was found to predict the level of hippocampal neurogenesis. Indeed, memory abilities were positively correlated to the number of proliferating cells, surviving cells, and new neurons evaluated 3 weeks after training. In other words, animals with preserved spatial memory [aged unimpaired (AU)] exhibited a higher level of proliferating cells, 1-month-old surviving cells, and new neurons compared to animals displaying spatial memory impairments [aged impaired (AI)]. This quantitative relationship between a reservoir of new neurons and memory capability – revealed by linking, for a given individual, the levels of memory performances, and the levels of neurogenesis – reinforces the contention that neurogenesis participates in learning and memory. A similar correlation was observed using two different hippocampal-dependent tasks: a modified version of the water maze and a transverse patterning discrimination (visual discriminations) task (Driscoll etal., 2006). In these two tasks, performance was shown to be correlated with both hippocampal volume (measured by in vivo MRI) and neurogenesis assessed by DCX. However, two other studies failed to demonstrate a correlation between cell proliferation and spatial memory (Bizon & Gallagher, 2003; Merrill etal., 2003), and another one reported that greater numbers of 3-week-old BrdU-positive cells were associated with worse memory performance (Bizon etal., 2004). Various experimental differences in BrdU injection time (i.e. immediately or 1 week after the completion of the behavioral study as longer intervals are required to make sure that the effects observed are specific to the basal rate of neurogenesis and not a consequence of the recent training; see also below), number of subjects, rat strain, and gender of the animals could explain this apparent controversy.
Influence of learning on neurogenesis
Hypothetically, age-related memory deficits could result not only from an alteration of the new neuronal pool, but also from changing the dynamics of hippocampal neurogenesis. To this end, the influence of spatial learning on the birth and/or survival of adult-born cells has been examined. Indeed, in young rats, spatial learning has been reported to influence the production and fate of the newly born cells, depending on the time at which the cells were generated relative to learning. Learning increases the survival of cells born before the beginning of learning (Gould & Tanapat, 1999; Dupret etal., 2007), while the survival of cells generated during the early phase of learning (when performance improves rapidly) is decreased by the late phase of learning (when performance is stabilized) (Dobrossy etal., 2003). Furthermore, this late phase also shows an increase in cell proliferation (Dobrossy etal., 2003). The learning-induced decrease in BrdU cell number was found to reflect the elimination of young neurons by apoptosis (Dupret etal., 2007). These three events – survival of relatively mature neurons, apoptosis of more immature cells, and proliferation of precursors – are in fact interrelated events that may mediate learning. Indeed, blocking learning-induced apoptosis inhibits cell survival and cell proliferation, and impairs memory performance (Dupret etal., 2007).
In aged rats, spatial learning also influences the fate of the newly born cells according to their birth date and to individual memory abilities (Drapeau etal., 2007). In AU rats, learning increases the survival of cells generated at least 1 week before the learning episode, whereas it decreases survival of cells produced during the early phase of learning. In AI rats, cell survival was not influenced by learning. These data indicate that learning, and not training, decreased the survival of adult-born cells, and that spatial memory abilities critically depend on dynamic regulation of adult-born cell survival. Moreover, in contrast to what has been observed in young rats, the late phase of learning (nor the earliest phase) did not increase the proliferation of the cells produced concurrently with this phase. However, given that aging might delay the process leading to neurogenesis, we hypothesized that the learning-induced increase in cell proliferation may also be delayed in the old brain. To this end, cell proliferation was examined 9–14 days after the completion of spatial training. Unexpectedly, cell proliferation was negatively correlated to memory ability: cell proliferation was higher in rats unable to master the task in comparison to animals displaying preserved spatial memory. This surprising result, explained in terms of enhanced swimming activity, is consistent with a previous study (Bizon etal., 2004). In this case, the number of surviving cells born 1 week after testing, which were 3 weeks old at the time of the sacrifice, was higher in AI rats compared to AU rats. These interindividual differences in survival likely come from initial differences in cell proliferation. Thus, an aberrant delayed rebound in cell proliferation may also participate in memory impairment.
The homeostatic regulation of cell survival observed in AU rats (and in young rats) is consistent with the selective stabilization theory, according to which only a particular set of contacts will be selected among many others, thereby sculpting the precise circuits that are crucial for a given function (Changeux etal., 1976). The mechanisms underlying this selective stabilization process are unknown, but it might be hypothesized that only the adult-born cells which are successfully connected, both in terms of efferent output and afferent input, are the ones rescued by activity-dependent stimuli generated in the course of learning. The other fundamental question which remains to be answered is what is the function of these surviving neurons?
Biological mechanism involved in age-related decline in spatial memory and neurogenesis in old rats
We have studied the role of the HPA axis in the existence of such phenotypic interindividual variations. Indeed, its up-regulation is involved in the appearance of age-related disorders (Landfield etal., 1978, 1981; Issa etal., 1990; Sapolsky, 1992). Furthermore, corticosterone inhibits neurogenesis in the aged brain (see pp. 8–9). Thus, the hypothesis at work was that excessive levels of corticosterone throughout the life of the animal would favor the emergence of memory deficits (pathological aging) by reducing neurogenesis. In favor of this hypothesis, we found that the magnitude of HPA axis activity in old animals was correlated with their level of hippocampal neurogenesis and memory ability. Indeed, animals with the heaviest adrenal glands, indicative of chronic HPA axis hyperactivity, exhibited the worst performance in the water maze and the lowest number of proliferating cells or 3-week-old surviving cells (Montaron etal., 2006). This indicates that hyperactivity of the HPA axis produces spatial memory deficits by decreasing hippocampal neurogenesis. In order to strengthen this hypothesis, the secretion of corticosterone was reduced from midlife onward by adrenalectomy, and its effect on neurogenesis and spatial memory ability in old rats was analyzed. It was found that lowering corticosterone secretion from midlife onward reduced the decline in neurogenesis observed in old rats and prevented age-related memory disorders (Montaron etal., 2006). These results demonstrate that exposure to high levels of corticosterone throughout life is responsible for age-related memory disorders and the age-related decline in neurogenesis. It remains to be determined whether other biological factors described to modulate neurogenesis in the aged brain (see Table 3) could be useful in preventing or curing the development of memory disorders during the course of aging.
Table 3.
Aging of the neurogenic microenvironment and its effects on neurogenesis
| Evolution with aging | Effect on neurogenesis in aged brain | |||
|---|---|---|---|---|
| Corticosteroids | Increase basal levelProlonged stress-induced secretion | (Sapolsky, 1992) | Acute ADX: increase of cell proliferation in the DG | (Cameron & McKay, 1999) |
| Increased expression of GR by precursors | (Garcia etal., 2004a) | Long-term ADX: increase of cell proliferation in the DG | ||
| Expression of MR by precursors | Inverse correlation between adrenal glands’ weight and proliferation or number of new neurons in the DG | (Montaron etal., 1999) (Montaron etal., 2006) (Montaron etal., 2006) | ||
| Neurosteroids | Acute Preg-S icv infusion: increase of cell proliferation in the DG | (Mayo etal., 2003) | ||
| Glutamate | NMDA-R antagonist ip injection: increase of the number of radial glia-like cells, proliferating cells and new neurons in the DG | (Nacher etal., 2003) | ||
| EGF signaling | Decrease of EGF-R expression in the SVZ (non-studied in DG) | (Enwere etal., 2004) | HB-EGF icv infusion: (3 days): increase of cell proliferation in the DG and the SVZ | (Jin etal., 2003a) (Enwere etal., 2004) |
| Decrease of TGFα expression in the SVZ (non-studied in DG) | EGF icv infusion (3 days): increase of cell proliferation in the SVZ | |||
| IGF-I | Decrease of IGF-I concentration Decrease of IGF-I receptor expression | (Sonntag etal., 1997) (Sonntag etal., 1999) (Lai etal., 2000) (Shetty etal., 2005) | IGF-I icv infusion (14 days): increase of cell proliferation in the DG | (Lichtenwalner etal., 2001) |
| FGF-2 | Decrease of hippocampal concentration of FGF-2 Decrease of FGFR-2 in the DG, the SVZ, the RMS, and the OB | (Shetty etal., 2005) (Chadashvili & Peterson, 2006) | FGF-2 icv infusion (3 days): strong increase of cell proliferation in the aged DG and the SVZ | (Jin etal., 2003a) |
| FGF-2 icv infusion (2 weeks): increase of both cell proliferation and dendritic growth in middle-aged DG | (Rai etal., 2007) | |||
| Vasculature and VEGF | Decrease of cerebral microvasculature (especially marked in DG) | (Riddle etal., 2003) (Hattiangady & Shetty, 2008) | ? | |
| Decrease of microvascular plasticity | (Sonntag etal., 1997) | |||
| Increase of the distance between precursors and blood vesselsReduced VEGF synthesis | (Shetty etal., 2005) (Hattiangady & Shetty, 2008) | |||
| Cell cycle regulators | Increase of p16INK4a expression (undetectable in young animals) | (Molofsky etal., 2006) | Bmi-1 KO mice′: premature senescence of NSC and decrease of proliferation in SVZ, the phenotype is rescued by p16INK4a or p19Arf deletion | (Molofsky etal., 2005) (Bruggeman etal., 2005) |
| P16INK4a KO mice: proliferation is increased in SVZ but not DG of aged mice | (Molofsky etal., 2006) | |||
DG, dentate gyrus; EGF, epidermal growth factor; FGF, fibroblast growth factor; IGF-I, insulin-like growth factor-I; icv, intracerebroventricular; OB, olfactory bulb; Preg-S, pregnenolone sulfate; RMS, rostral migratory stream; SVZ, subventricular zone; VEGF, vascular endothelial growth factor.
Predictive model of aging
The next question that we addressed was whether these interindividual variations in the functional expression of neurogenesis could be predicted early in life. We took advantage of natural individual differences in the activity of the HPA axis in young adult animals, which are associated with a behavioral reactivity trait, and determined whether they could predict the extent of age-induced memory impairments. Indeed, rats that are high-behavioral responders to stress (HRs) exhibit prolonged corticosterone secretion in response to stress when they are young, premature aging of the HPA axis, and an increased propensity to develop age-related memory deficits in comparison to rats that are low-behavioral responders to novelty (LRs) (Dellu etal., 1994, 1996). By comparing these two groups of animals that spontaneously differ in their behavioral reactivity to novelty, it was found that cell proliferation, cell survival, and consequently neurogenesis were higher in LRs in comparison to HRs (Lemaire etal., 1999). Because behavioral and neuroendocrinological reactivity in youth has been demonstrated to be predictive of spatial memory impairments later in life, these individual differences in neurogenesis may account, at least in part, for individual differences in memory abilities in old age. In other words, the subjects starting off with impaired neurogenesis (HRs) are predisposed to the development of age-related memory disorders. A longitudinal study, however, is necessary to verify this prediction. This raises the issue of whether boosting neurogenesis in the young HRs by exposing them to enriched lifestyle conditions (see below) will prevent the appearance of memory deficits when they reach senescence.
Risk factor of pathological aging: early deleterious life events
The interindividual differences that we observed may result from both genetic and environmental influences that predispose the individual (vulnerable phenotype) to develop memory disorders. Given the role of stress and corticosterone in pathological aging, a particular emphasis was given to early environmental experiences such as prenatal stress. Prenatal stress is known to significantly affect the development of the brain and the organization of behavior. In particular, prenatal stress impairs memory processes, but the underlying mechanisms are unknown.
We tested the hypothesis that prenatal-stress-induced memory deficits are related to impaired neurogenesis. By comparing juvenile (28-day-old), adult (3-month-old), middle-aged (10-month-old), and old (22-month-old) animals, it was found that prenatal stress cut cell proliferation in half over a lifetime. In fact, there was a premature decline of cell proliferation with increasing age (Lemaire etal., 2000). Cell survival and cell phenotype, in contrast, were not influenced by prenatal stress, at least when examined in adulthood (Lemaire etal., 2000, 2006). As a consequence, the number of adult-born neurons was reduced in prenatally stressed rats. Increased activity of the HPA axis following prenatal stress could explain the curtailed neurogenesis. Indeed, prenatal stress increases HPA activity as assessed by adrenal mass (Lemaire etal., 2000), and this effect is blocked by postnatal handling, a manipulation that counteracts the down-regulation of neurogenesis induced by prenatal stress (Lemaire etal., 2006). These results do not exclude the possibility that other mechanisms which remain to be elucidated mediate the deleterious effect of prenatal stress on neurogenesis.
Structural hippocampal defects resulting from prenatal stress were associated with impairment in spatial memory and in learning-induced regulation of some aspects of neurogenesis. When tested at 4 months of age, a significant difference in the rate of acquisition was observed between control and prenatally stressed rats. The latter did not reach an asymptotic level of performance. This impairment was associated with a disruption of learning-induced cell proliferation. These results are in agreement with the observation that, in young and aged rats, cell proliferation is increased only when the task is mastered (Döbrössy etal., 2003).
In summary, early life stress has long-lasting, deleterious effects on hippocampal neurogenesis, producing impairment in hippocampal-related spatial tasks and blocking the learning-induced increase in cell proliferation. More importantly, the neurobiological consequences of prenatal stress on hippocampal neurogenesis can be reversed with a form of postnatal environmental stimulation, therefore suggesting that pathological aging could be prevented.
Preventing pathological aging: positive life events
An intellectually and a physically active life is known to protect from memory impairment during the course of ‘normal’ aging or in neurodegenerative disorders (Laurin etal., 2001; Vaillant & Mukamal, 2001; Le Carret etal., 2005; Hattori etal., 2007). Therefore, it is of importance to determine whether age-related decline in memory and neurogenesis can be reversed by stimulating life events.
Enriched environment
Exposure of young adult rodents to a complex enriched environment increases hippocampal neurogenesis by promoting neuron survival (Kempermann etal., 1997; Nilsson etal., 1999; Auvergne etal., 2002; Brown etal., 2003; Ueda etal., 2005; Rossi etal., 2006; Hattori etal., 2007; Tashiro etal., 2007) and, in some cases, by increasing cell proliferation (Kempermann etal., 1998a). At the same time, these conditions improve some aspects of spatial learning (Kempermann etal., 1997; Nilsson etal., 1999).
In the aging brain, short-term (40-day-long) (Kempermann etal., 1998b) or long-term (10 months starting at 10 months old) (Kempermann etal., 2002) exposures to an enriched environment improve neurogenesis in senescent subjects. This effect has been associated with changes in cell survival, neuronal differentiation (Kempermann etal., 1998b), and/or cell proliferation, although this latter effect did not reach statistical significance (Kempermann etal., 2002). From a functional point of view, a slight improvement in spatial performances was observed when animals were tested in the water maze after only 40 days of enriched environment (Kempermann etal., 1998b), while a longer exposure, from 10 months to 20 months of age, yielded a stronger improvement of enriched mice scores (Kempermann etal., 2002).
Physical activity
Voluntary access to a running wheel has been shown to be one of the components that may lead to an increase in neurogenesis when living in an enriched environment. However, besides promoting the survival of the newborn cells and their neuronal differentiation, exercise has been shown to also increase cell proliferation in young adults (van Praag etal., 1999a,b).
In 19-month-old mice, 45 days of unlimited access to a running wheel restores the subsequent number of surviving newly born cells to a level corresponding to 3 months old, non-running mice (van Praag etal., 2005). Furthermore, the percentage of cells differentiating into a neuronal phenotype shows a 2.5-fold increase. The pro-neurogenic results of exercise were also observed at both 10 months and 20 months of age after an ‘acute’ shorter exposition to the running wheel (Kronenberg etal., 2006). Interestingly, whereas in young animals a longer exposure to the running wheel suppresses the proliferative effect, exercise from 3 months to 9 months of age significantly reduces the age-dependent decline in cell proliferation, even if it does not maintain net neurogenesis at levels corresponding to a younger age (Kronenberg etal., 2006). In 14-month-old rats, a smaller yet significant enhancement of cell proliferation is also observed after short-term exposure to a treadmill (Kim etal., 2004). As expected, experience-induced increases in neurogenesis were associated with an improvement in spatial learning abilities (van Praag etal., 2005). Recently, it has been shown that maternal running and swimming cause increases in the cell genesis (Bick-Sander etal., 2006; Lee etal., 2006; Kim etal., 2007) and short-term memory (Lee etal., 2006; Kim etal., 2007) of the offspring. These data suggest that such ‘prenatal exercise’ may favor successful aging.
Altogether, these results show that positive life events such as exposure to a variety of new (and changing) stimuli throughout life may improve memory function by increasing neurogenesis during the course of aging. Recently, it has been shown that social isolation leading to an HPA axis hyperactivity precludes the positive effects of exercise on neurogenesis (Stranahan etal., 2006). This suggests that the existence of interindividual differences should be taken into account in the future as they may buffer or preclude the effects of positive life events on the aging brain.
Conclusion
Altogether, the results shown strongly suggest that hippocampal neurogenesis is involved in the aging of memory functions (see Fig. 3). Aging is not inescapable, and wide interindividual differences are observed among aged subjects. These differences in the risk of developing age-related memory disorders can be predicted earlier in life. Low hippocampal plasticity may render animals more vulnerable to aging processes. On the other hand, subjects starting off with a high level of neurogenesis may be resistant to the development of age-related memory disorders. These different phenotypic orientations may result from early deleterious or positive life events, which will shape the developmental trajectory of the subjects within a genetic envelope. For example, prenatal stress affects neurogenesis in pathological ways throughout life and precipitates age-related memory impairments. On the contrary, positive life events such as perinatal enrichment may prevent the appearance of an age-related decline in neurogenesis and memory abilities. Given that hippocampal neurogenesis plays a pivotal role in environmentally induced vulnerability to the development of pathological aging, a better understanding of the mechanisms that regulate neurogenesis during aging is required. Indeed, the challenge for future research will be to develop therapeutic strategies aimed at first stimulating plasticity in the aged brain and preventing the alteration of memory function that may appear in some individuals.
Fig. 3.
Conceptual framework highlighting the role played by hippocampal neurogenesis in environmentally induced vulnerability to the development of pathological aging. In aged rats, interindividual differences in the risk of developing age-related memory disorders are associated with individual differences in hippocampal neurogenesis. These interindividual differences can be predicted earlier in life. Animals with a low level of neurogenesis [i.e. high-behavioral responders to stress (HRs)] may not be able to adapt to environmental demands and, thus, may be more vulnerable to aging processes. On the other hand, the low-behavioral responders to novelty (LRs) starting off with a high level of neurogenesis would be resilient to the development of age-related memory disorders. These different phenotypes may result from early environmental experiences. For example, stressful events during the pre- and/or the postnatal period may lead to an HR phenotype and vulnerability to the aging processes. On the contrary, positive life events during development might favor an LR phenotype, resistant to the appearance of memory disorders.
Acknowledgments
We are very grateful to Dr E. Pastrana (Columbia University) for her critical reading of the manuscript. Elodie Drapeau was supported by David and Lucile Packard Fellowship in Science and Engineering awarded to Dr F. Doetsch (#DLP 2004-27666). DNA was supported by the French National Institute of Health and Medical Research (INSERM) and University of Bordeaux 2.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous DN, Wojtowicz JM. Adult neurogenesis. New York: Cold Spring Harbor Laboratory Press; 2008. Neurogenesis and Hippocampal Memory System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alonso G. Proliferation of progenitor cells in the adult rat brain correlates with the presence of vimentin-expressing astrocytes. Glia. 2001;34:253–266. doi: 10.1002/glia.1059. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–372. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res. Dev. Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm. Metab. Res. 1999;31:120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- Auvergne R, Lere C, El Bahh B, Arthaud S, Lespinet V, Rougier A, Le Gal LS. Delayed kindling epileptogenesis and increased neurogenesis in adult rats housed in an enriched environment. Brain Res. 2002;954:277–285. doi: 10.1016/s0006-8993(02)03355-3. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Neurosteroids: a new brain function. J. Steroid Biochem. Mol. Biol. 1997;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Bick-Sander A, Steiner B, Wolf SA, Babu H, Kempermann G. Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc. Natl. Acad. Sci. USA. 2006;103:3852–3857. doi: 10.1073/pnas.0502644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur. J. Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Bondy CA. Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J. Neurosci. 1991;11:3442–3455. doi: 10.1523/JNEUROSCI.11-11-03442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T, Puche AC. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, van Lohuizen M. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19:1438–1443. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Baram TZ, Bender RA. Hippocampal neurogenesis is not enhanced by lifelong reduction of glucocorticoid levels. Hippocampus. 2005;15:491–501. doi: 10.1002/hipo.20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-d-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J. Functional integration of adult-born neurons. Curr. Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J. Comp. Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Benedetti L, Bourgeois JP, Brisson A, Cartaud J, Devaux P, Grunhagen H, Moreau M, Popot JL, Sobel A, Weber M. Some structural properties of the cholinergic receptor protein in its membrane environmental relevant to its function as a pharmacological receptor. Cold Spring Harb. Symp. Quant. Biol. 1976;40:211–230. doi: 10.1101/sqb.1976.040.01.023. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cuppini R, Bucherelli C, Ambrogini P, Ciuffoli S, Orsini L, Ferri P, Baldi E. Age-related naturally occuring depression of hippocampal neurogenesis does not affect trace fear conditioning. Hippocampus. 2006;16:141–148. doi: 10.1002/hipo.20140. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Le Moal M, Simon H. Reactivity to novelty during youth as a predictive factor of cognitive impairment in the elderly: a longitudinal study in rats. Brain Res. 1994;653:51–56. doi: 10.1016/0006-8993(94)90371-9. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, Simon H. Behavioral reactivity to novelty during youth as a predictive factor of stress-induced corticosterone secretion in the elderly – a life-span study in rats. Psychoneuroendocrinology. 1996;21:441–453. doi: 10.1016/0306-4530(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Dobrossy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. USA. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J. Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: a multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J. Neurosci. Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- van Eekelen JA, Rots NY, Sutanto W, Oitzl MS, De Kloet ER. Brain corticosteroid receptor gene expression and neuroendocrine dynamics during aging. J. Steroid Biochem. Mol. Biol. 1991;40:679–683. doi: 10.1016/0960-0760(91)90290-l. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Morschl E, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Role of astroglia and insulin-like growth factor-I in gonadal hormone-dependent synaptic plasticity. Brain Res. Bull. 1997;44:525–531. doi: 10.1016/s0361-9230(97)00238-4. [DOI] [PubMed] [Google Scholar]
- Gage FH, Chen KS, Buzsaki G, Armstrong D. Experimental approaches to age-related cognitive impairments. Neurobiol. Aging. 1988;9:645–655. doi: 10.1016/s0197-4580(88)80129-5. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD, Kodsi MH, McKinney M, Southerland S, Vella-Rountree L, Lewis MH. Markers for biogenic amines in the aged rat brain: relationship to decline in spatial learning ability. Neurobiol. Aging. 1990;11:507–514. doi: 10.1016/0197-4580(90)90111-c. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004a;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004b;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr. Opin. Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Experiencing VEGF. Nat. Genet. 2004;36:792–793. doi: 10.1038/ng0804-792. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging. 2008;29:129–177. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Hashimoto R, Miyakawa T, Yamanaka H, Maeno H, Wada K, Kunugi H. Enriched environments influence depression-related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav. Brain Res. 2007;180:69–76. doi: 10.1016/j.bbr.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol. Aging. 2004a;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur. J. Neurosci. 2004b;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur. J. Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Bittman EL. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm. Behav. 2002;41:343–350. doi: 10.1006/hbeh.2002.1767. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hijman R, Baare WF, van Eekelen S, van Ree JM. Odor discrimination and task duration in young and older adults. Chem. Senses. 2000;25:461–464. doi: 10.1093/chemse/25.4.461. [DOI] [PubMed] [Google Scholar]
- Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic–pituitary–adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur. J. Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun Y, Xie L, Jin L, Nishi E, Klagsbrun M, Greenberg DA. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J. Neurosci. 2002a;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2002b;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003a;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Xie L, Childs J, Sun Y, Mao XO, Logvinova A, Greenberg DA. Cerebral neurogenesis is induced by intranasal administration of growth factors. Ann. Neurol. 2003b;53:405–409. doi: 10.1002/ana.10506. [DOI] [PubMed] [Google Scholar]
- Kaneda H, Maeshima K, Goto N, Kobayakawa T, Ayabe-Kanamura S, Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem. Senses. 2000;25:331–337. doi: 10.1093/chemse/25.3.331. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur. J. Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr. Biol. 1998a;8:939–942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 1998b;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, Lee SJ, Lee HH, Yoon JH, Jeong IG, Kim CJ. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci. Lett. 2004;355:152–154. doi: 10.1016/j.neulet.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee SH, Kim SS, Yoo JH, Kim CJ. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int. J. Dev. Neurosci. 2007;25:243–249. doi: 10.1016/j.ijdevneu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Hibberd CJ, Gluckman PD, Seckl JR. Reduced expression of insulin-like growth factor 1 messenger RNA in the hippocampus of aged rats. Neurosci. Lett. 2000;288:66–70. doi: 10.1016/s0304-3940(00)01170-8. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal–pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lazar LM, Blum M. Regional distribution and developmental expression of epidermal growth factor and transforming growth factor-alpha mRNA in mouse brain by a quantitative nuclease protection assay. J. Neurosci. 1992;12:1688–1697. doi: 10.1523/JNEUROSCI.12-05-01688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Carret N, Auriacombe S, Letenneur L, Bergua V, Dartigues JF, Fabrigoule C. Influence of education on the pattern of cognitive deterioration in AD patients: the cognitive reserve hypothesis. Brain Cogn. 2005;57:120–126. doi: 10.1016/j.bandc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HK, Lee TH, Shin MC, Lee MH, Shin MS, Park S, Baek S, Kim CJ. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006;28:147–154. doi: 10.1016/j.braindev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur. J. Neurosci. 1999;11:4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol. Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J. Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J. Neurosci. 2005;25:10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczynski TJ. GABAergic deafferentation hypothesis of brain aging and Alzheimer's disease revisited. Brain Res. Bull. 1998;45:341–379. doi: 10.1016/s0361-9230(97)00347-x. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol. Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W, George O, Darbra S, Bouyer JJ, Vallee M, Darnaudery M, Pallares M, Lemaire-Mayo V, Le Moal M, Piazza PV, Abrous N. Individual differences in cognitive aging: implication of pregnenolone sulfate. Prog. Neurobiol. 2003;71:43–48. doi: 10.1016/j.pneurobio.2003.09.006. [DOI] [PubMed] [Google Scholar]
- McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci. Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology. 1992;55:204–213. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp. Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J. Comp. Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaron MF, Petry KG, Rodriguez JJ, Marinelli M, Aurousseau C, Rougon G, Le Moal M, Abrous DN. Adrenalectomy increases neurogenesis but not PSA-NCAM expression in aged dentate gyrus. Eur. J. Neurosci. 1999;11:1479–1485. doi: 10.1046/j.1460-9568.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur. J. Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Keating RF, Moskal JR. Basic fibroblast growth factor and epidermal growth factor exert differential trophic effects on CNS neurons. J. Neurosci. Res. 1988;21:71–79. doi: 10.1002/jnr.490210111. [DOI] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur. J. Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol. Aging. 2003;24:273–284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Saito H, Matsuki N. Basic fibroblast growth factor and brain-derived neurotrophic factor promote survival and neuronal circuit formation in organotypic hippocampal culture. Jpn. J. Pharmacol. 1997;75:319–326. doi: 10.1254/jjp.75.319. [DOI] [PubMed] [Google Scholar]
- Ni Dhuill CM, Fox GB, Pittock SJ, O’Connell AW, Murphy KJ, Regan CM. Polysialylated neural cell adhesion molecule expression in the dentate gyrus of the human hippocampal formation from infancy to old age. J. Neurosci. Res. 1999;55:99–106. doi: 10.1002/(SICI)1097-4547(19990101)55:1<99::AID-JNR11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J. Comp. Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Opanashuk LA, Mark RJ, Porter J, Damm D, Mattson MP, Seroogy KB. Heparin-binding epidermal growth factor-like growth factor in hippocampus: modulation of expression by seizures and anti-excitotoxic action. J. Neurosci. 1999;19:133–146. doi: 10.1523/JNEUROSCI.19-01-00133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Petit TD, Ivy O. Neuroplasticity, Learning and Memory, Neurology and Neurobiology. New York: A.R. Liss, Inc; 1988. [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA–NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur. J. Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb. Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res. Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, Le Moal M, Abrous DN. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur. J. Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Rotwein P, Burgess SK, Milbrandt JD, Krause JE. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc. Natl. Acad. Sci. USA. 1988;85:265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol. Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J. Comp. Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia G, Yague AG, Garcia-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Han VK, Lee DC. Regional expression of transforming growth factor-alpha mRNA in the rat central nervous system. Neurosci. Lett. 1991;125:241–245. doi: 10.1016/0304-3940(91)90039-v. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Lee DC, Guthrie KM, Gall CM. Cellular localization of transforming growth factor-alpha mRNA in rat forebrain. J. Neurochem. 1993;60:1777–1782. doi: 10.1111/j.1471-4159.1993.tb13403.x. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/Progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Simon M, Czeh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Trice JE. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp. Brain Res. 1988;72:399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Cain WS. Old-age deficits in the sense of smell as gauged by thresholds, magnitude matching, and odor identification. Psychol. Aging. 1987;2:36–42. doi: 10.1037//0882-7974.2.1.36. [DOI] [PubMed] [Google Scholar]
- Stoelzel CR, Stavnezer AJ, Denenberg VH, Ward M, Markus EJ. The effects of aging and dorsal hippocampal lesions: performance on spatial and nonspatial comparable versions of the water maze. Neurobiol. Learn. Mem. 2002;78:217–233. doi: 10.1006/nlme.2001.4054. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Winseck A, Vinsant S, Park OH, Kim H, Oppenheim RW. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J. Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Watanabe Y, Kato H, Araki T. Immunohistochemical changes related to ageing in the mouse hippocampus and subventricular zone. Mech. Ageing Dev. 2007;128:303–310. doi: 10.1016/j.mad.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J. Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J. Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Sakakibara S, Yoshimoto K. Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience. 2005;135:395–402. doi: 10.1016/j.neuroscience.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat. Neurosci. 1999;2:848. doi: 10.1038/12226. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Mukamal K. Successful aging. Am. J. Psychiatry. 2001;158:839–847. doi: 10.1176/appi.ajp.158.6.839. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Johe KK, Hazel TG, Collazo D, McKay RD. Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J. Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P, Cowan WM, Ueno N, Baird A, Guillemin R. Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc. Natl. Acad. Sci. USA. 1986;83:3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wati H, Kudo K, Qiao C, Kuroki T, Kanba S. A decreased survival of proliferated cells in the hippocampus is associated with a decline in spatial memory in aged rats. Neurosci Lett. 2006;399:171–174. doi: 10.1016/j.neulet.2006.01.056. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol. Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wilcox JN, Derynck R. Localization of cells synthesizing transforming growth factor-alpha mRNA in the mouse brain. J. Neurosci. 1988;8:1901–1904. doi: 10.1523/JNEUROSCI.08-06-01901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Cooper-Kuhn CM, Aigner R, Winkler J, Kuhn HG. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Kee N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 2006;1:1399–1405. doi: 10.1038/nprot.2006.224. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur. J. Neurosci. 2005;22:785–792. doi: 10.1111/j.1460-9568.2005.04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc. Natl. Acad. Sci. USA. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J. Neurosci. Res. 2006;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]


