Abstract
Several amyloidogenic proteins including insulin, β-lactoglobulin and albumin, form spherulites in vitro under non-physiological conditions. These micrometer-sized, roughly spherical structures are composed of ordered arrays of amyloid fibrils in radial arrangements which, characteristically, show a typical Maltese cross pattern of light extinction under the polarizing microscope. The physiological significance of amyloid spherulites is unknown though in Alzheimer’s disease senile plaques composed primarily of β sheets of Aβ42 have, very occasionally, been shown to give a Maltese cross pattern of light extinction under crossed polarisers. Herein we describe the first observation of the formation in vitro of spherulites of Aβ42. They were formed under near-physiological conditions in which the β sheet conformation of preformed aggregates of Aβ42 had been abolished following the addition of an excess of copper. Incubation of these preparations at 37°C for up to 9 months resulted in the formation of globular structures, 5 – 20 μm in diameter, which exhibited a Maltese cross pattern of light extinction typical of spherulites. Near-identical spherulitic structures were also observed in abundance in 30 μm thick sections of Alzheimer’s disease brain tissue. Synchrotron x-ray fluorescence showed that the location of these spherulites in AD tissue coincided with locally elevated concentrations of tissue copper. The formation in vitro of spherulites of Aβ42 which morphologically appeared analogous to spherulitic structures observed in vivo strongly supports the hypothesis that spherulites and senile plaques in AD tissue are one and the same structures and that their ultimate formation may involve copper.
INTRODUCTION
The first observations in vitro of the self-assembly of amyloid fibrils into spherulites [1-3] prompted further studies both into their mode of formation [4-8] and their physiological significance. The possibility of the latter was suggested by the occurrence of spherulitic structures in various tissue preparations associated with neurodegenerative conditions [9,10]. However, to-date none of the amyloidogenic proteins which have been shown to form spherulites in vitro under non-physiological conditions have also been shown to form similar structures in vivo. Indeed, none of the amyloidogenic peptides which have been implicated in human disease, for example Aβ in Alzheimer’s disease, α synuclein in Parkinson’s disease or amylin in diabetes, have been shown to form spherulites in vitro or in vivo. We looked to bridge this gap for Aβ42, the amyloidogenic peptide which is found in the core of senile plaques in AD and the deposition of which forms the central tenet of the Amyloid Cascade Hypothesis of neuronal disruption and loss in AD [11].
MATERIALS AND METHODS
In vitro preparation and observation of spherulites of Aβ42
Aβ42 was purchased as the lyophilised salt (Bachem, Saffron Walden, UK) and dissolved in 0.01mM NaOH at 1 mg/mL (ca 200μM). All peptide assays were prepared in modified Krebs-Henseleit (KH) medium (NaCl – 123.5mM; KCl – 4.8mM; MgSO4 – 1.2mM; CaCl2 – 1.4mM; glucose – 11.0mM) buffered with 100mM PIPES at pH 7.40 and including 0.05% w/v sodium azide to inhibit microbial growth (all chemicals from Sigma, Poole, UK). Cu(II) was added as a certified stock (ca 1g/L) in 2% HNO3 (PE Life Sciences, Beaconsfield, UK). Images of spherulites were obtained by optical, polarising and fluorescence microscopy using an Olympus BX50 microscope with a ColourView III digital camera using Cell D software.
Preparation of brain tissue for light microscopy and synchrotron X-ray fluorescence
Hippocampal tissue obtained from the brain of a confirmed case of Alzheimer’s disease in 90 year old woman and which had been stored frozen at −80°C for 4 years was cryosectioned at 30 μm thickness with a sapphire blade, and mounted on acid-washed glass slides. Sections were stored frozen, and air-dried prior to staining. For imaging by synchrotron microfocus X-ray fluorescence (μXRF), slide cover-slips were removed overnight in xylene, and replaced with a Kapton ® window. Synchrotron X-ray microfocus fluorescence mapping was performed at the I18 Diamond synchrotron beamline [12] as described elsewhere [13], mapping with a 10 keV incident beam and 4μm in-plane resolution to obtain element-specific maps in regions of interest. Energy dispersive X-ray peaks were integrated using PyMCA [14] to extract copper and iron from the complete fluorescence spectra for regions of interest. The metal maps were adjusted to match the higher resolution of the optical microscopy by linear interpolation and then overlaid with the corresponding light microscopy images using a transformation matrix obtained using custom Matlab®-based code and six to ten common registration features in each image.
Results and Discussion
The formation of spherulites of Aβ42 in vitro
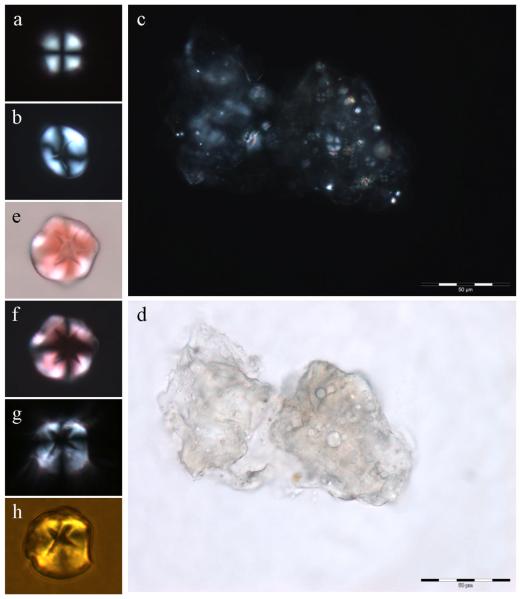
Spherulites of the proteins insulin, β lactoglobulin and human serum albumin all form spontaneously in solution at either acidic pH or high temperature, conditions which favour the denaturation of the protein [1-3]. We postulated that spherulites of Aβ42 would form under near-physiological conditions in saturated preparations of peptide which were aged for many months at 37°C. The idea was that prolonged ageing at body temperature would substitute for the denaturants, acidic pH or high temperature, used previously to stimulate spherulite formation in other proteins. Monomeric Aβ42 was diluted into modified Krebs-Henseleit (KH) medium at pH 7.40 to a concentration of 40 μM and thereafter incubated at 37°C for 7 days. After 7 days the peptide was dialysed against further volumes of KH medium before being diluted with the same medium to give multiple replicates of a peptide concentration of ca 8 μM. Thioflavin T fluorescence was used to demonstrate the presence of significant quantities of β sheets of Aβ42 fibrils in each of these replicates before they were all placed in an incubator at 37°C. At this time Cu(II) was added to one set of replicates to a 10-fold excess. We had recently demonstrated that under such conditions Cu(II) abolished the β sheet conformation of preformed aggregates of Aβ42 [15]. The latter effect was confirmed in these preparations using ThT fluorescence and TEM and thereafter all peptide solutions were allowed to age for 6 months without agitation at 37°C. After this time all Aβ42 only preparations were highly ThT-positive while those which additionally included excess Cu(II) showed almost no ThT fluorescence. When Aβ42 only solutions were viewed under the polarising microscope there were no spherulites in any of the replicate solutions. Light microscopy demonstrated in these solutuions the presence of significant clusters of fibrillar deposits of amyloid which in the presence of Congo red gave apple-green birefringence under polarised light but, importantly, no evidence of any spherulitic structures. When the ThT-negative Aβ42 + Cu(II) solutions were viewed under the polarising microscope we identified spherulites by their typical Maltese cross pattern of light extinction (Fig. 1a,b). They were few and far between, approximately one spherulite for each 10-20 μL of solution, and though sometimes found in small groups they were mainly observed as isolated globular structures of 5-20 μm in diameter. They were clearly ‘soft’ structures which were easily compressed and they were quite fragile being broken up by vortexing of solutions. The smaller spherulites had no obvious central core while the cores of many larger spherulites were irregular (Fig. 1a,b) and it is not yet clear whether the irregularity is due to the presence of a mineral-like substance at their core or is simply an anomaly caused by rupture or tearing in the central region of larger spherulites. In support of the former suggestion when a fully hydrated core-less spherulite of ca 15 μm diameter was allowed to dry-out on the microscope slide over a 30 minute period the core of the spherulite was observed to expand considerably though without the appearance of any core anomaly. Further research will be required to resolve this conundrum. One significant group of perhaps 50 or more spherulites of varying sizes was observed occluded within a matrix of amorphous Aβ42 (Fig. 1c,d). Spherulites formed in vitro bound Congo red but, as previously shown for spherulites of α-L-Iduronidase [4] did not show apple-green birefringence under polarised light (Fig. 1e,f). They also bound the β sheet-specific fluor thioflavin T which could be observed by fluorescence microscopy (Fig. 1g,h). Thioflavin T has been used previously to suggest that spherulites of human serum albumin are composed of amyloid fibrils [3]. The anomaly in the core of the larger spherulites was not labelled with either Congo red or ThT and, if it is not an artifact, is assumed, as in observations of other amyloid spherulitic structures [1,3], to be non-proteinaceous or non β sheet Aβ42.
Fig. 1.
Spherulites of Aβ42 formed under near-physiological conditions in vitro. a, spherulite 10-12 μm in diameter with no core; b, spherulite 15-20 μm in diameter with clear core; c, large group of spherulites occluded within an amorphous matrix of Aβ42; d, the same group viewed by optical microscopy; e, spherulite 15-20 μm in diameter stained with Congo red; f, the same spherulite under the polarising microscope; g, spherulite 15-20 μm in diameter stained with ThT viewed by optical microscopy and crossed polarisers; h, the same spherulite stained with ThT viewed by fluorescence microscopy.
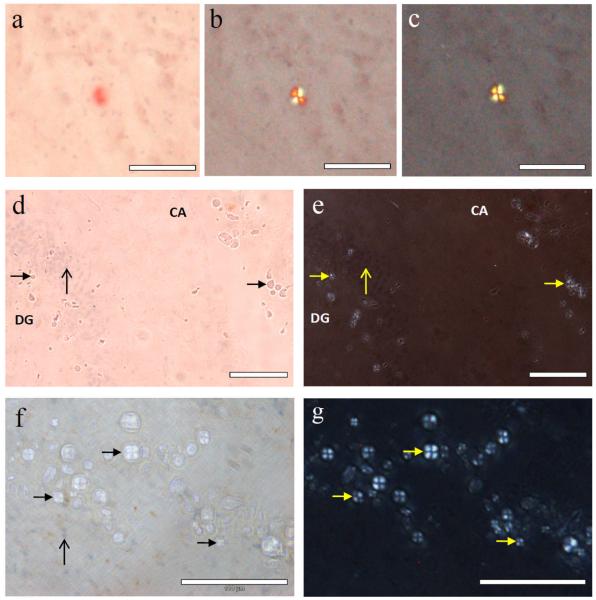
The observation of spherulites in AD tissue
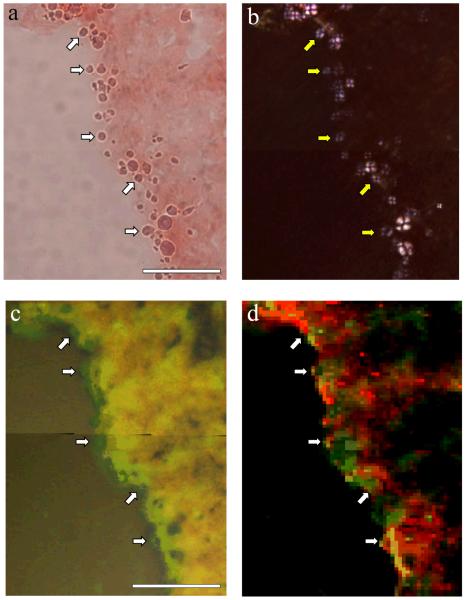
We have obtained the first images of spherulites of Aβ42 formed in vitro and it is clearly important to determine if similar structures are found in AD brain tissue. Hippocampal tissue was obtained from a female AD case and sectioned at −20°C to give 30 μm thick cross sections through Ammon’s horn (CA) including the dentate gyrus (DG). Congo red staining revealed numerous ghost tangles associated with pyramidal neurones and occasional amyloid deposits consistent with senile plaques. Both tangles and plaques stained intense red-orange and gave apple-green birefringence under polarised light while only plaques showed a Maltese cross pattern of light extinction which is characteristic of spherulites (Fig. 2a,b,c). Many more spherulitic structures, approximately 5 – 20 μm in diameter, were identified in the vicinity of the granule layer of the DG and in an adjacent area between the DG and the pyramidal layer of the CA by their Maltese cross pattern of light extinction. However, these spherulites, unlike senile plaques, were not positively stained by haematoxylin nor did they show a strong affinity for Congo red or give apple-green birefringence (Fig. 2d,e,f,g). Spherulites were clearly visible under crossed polarisers in both unstained tissue and in tissue stained with thioflavin T and they were easily identified in tissue as globular structures by optical microscopy. Identical spherulites were similarly distributed and abundant in formalin-fixed hippocampal tissue taken from the opposing hemisphere in the same AD case. There have been several previous reports of inclusions in aged and AD brain, for example, corpora amylacea and non-Congophilic granules, which though similar to the spherulites identified herein are also significantly different based upon their physical properties, their individual reactions to various histological stains and whether or not they show a Maltese cross pattern of light extinction under the polarising microscope [16-17]. In an attempt to link the spherulites of Aβ42 that we observed in vitro to the spherulitic structures we found in vivo the possible role played by Cu(II) in their formation in vitro was investigated in AD tissue using synchrotron μXRF. Copper maps were made for regions surrounding the DG granule layer in which many spherulites had been identified by optical and polarising microscopy (Fig. 3a,b). Overlay of copper maps (Fig. 3c) and light microscopy images of these regions indicated the presence of locally elevated concentrations of copper in those regions which were most densely populated with spherulites. Iron maps of the same regions showed different intensity distributions to copper (Fig. 3d) and confirmed that the observed copper distributions were not purely a function of sample thickness or scattering.
Fig. 2.
Spherulites observed in Alzheimer’s hippocampal tissue stained with Congo red and haematoxylin. a, Congo-red-stained spherulite giving apple-green birefringence under progressively crossed polarisers (a-c), typical of the senile plaques observed in Alzheimer’s disease; d, spherulite structures (solid arrow-heads) in the same tissue section without either the strong affinity for Congo red or e, apple-green birefringence under crossed polarisers. In all sections the unstained spherulites were distributed in the granule cell layer (vertical arrow) of the dentate gyrus (DG) and in a surrounding band in Ammon’s horn (CA) within the pyramidal cell layer; f, hippocampal section with a lighter Congo red stain, haematoxylin-positive cells (vertical arrow) and the spherulites which also notably lack affinity for haematoxylin (solid arrow-heads) shown under partially crossed polarisers; g, same region under fully crossed polarisers. Scale bar in each image is 100 μm.
Fig. 3.
Synchrotron map of copper distribution in a spherulite-rich region of Alzheimer’s hippocampus. a, tissue region with a high population of spherulites on the edge of an internal tear between the pyramidal cell layer and the DG, shown with optical microscopy under partially crossed polarisers; b, the same region with polarising microscopy, where heavy staining of the section with Congo red produced a slight pink tint in the Maltese cross signature; c, map of copper intensity (green) for the same region, obtained with synchrotron X-ray microfocus fluorescence, overlaid on the corresponding optical image obtained at the synchrotron; d, map showing both copper intensity (green) and iron intensity (red), indicating that variations in copper intensity are not simply due to scattering effects or sample thickness. A subset of spherulites is indicated with arrows in each image to assist with registration. The scale bar is 100 μm.
Spherulites of Aβ42 formed in vitro and senile plaques are analagous structures
We have made the first observation of spherulites of Aβ42 formed in vitro. They appear to share many of the properties of other amyloid-based spherulites formed in vitro except they were formed under near-physiological conditions and, perhaps, the prerequisite for Cu(II) in their formation. We have shown recently [15] that Cu(II) abolishes the β sheet structure of preformed fibrils of Aβ42 and this Cu(II)-induced reorganisation of these fibrils may be the actual prerequisite for spherulite formation. We have consistently not observed spherulites in preparations of Aβ42 which were highly thioflavin T positive and shown by TEM to include many diffuse classical amyloid fibrils. Our research suggests that Cu(II) binding to β sheets of Aβ42 catalyses the reorganisation of these polymers into the spherulitic structures we observed in in vitro preparations. A similar role for Cu(II) has been postulated for fibrillogenesis in β-2-microglobulin [18]. What can be the significance of the spherulites observed in AD tissue and why have such numerous and large structures not been commented upon previously? We first came across such in similarly thick sections of hippocampal tissue which had been cut for synchrotron work [15]. We are now wondering if the usual practice of sectioning tissue at 5 μm thickness for AD histology is responsible for this discrepancy in the observation of spherulites in AD brain tissue. For example, under polarised light, as is used routinely in the AD field to confirm apple-green birefringence of amyloid deposits, the phase difference between the two polarisations will be smaller in thinner sections of tissue thus potentially rendering the characteristic Maltese cross signature less obvious. In the rare previous example of a senile plaque being shown to exhibit a Maltese cross pattern of light extinction the tissue section was reported as 10 μm thickness [9]. In addition, and bearing in mind the ‘soft’ nature of spherulites formed in vitro, tissue sectioning might damage or ‘burst’ spherulites allowing their contents to spill out to leave various ‘ghosts’ of spherulitic origin some of which are routinely identified as senile or neuritic plaques? The latter might explain why some spherulites in AD tissue (those that have been damaged) gave apple-green birefringence under polarised light while many did not (Fig. 2). The inference here being that the arrangement of amyloid fibrils in intact spherulites (in vitro or in vivo) does not give apple-green birefringence. Senile or neuritic plaques are specific structures which are found in both the aged and the AD brain [19]. They are not simply deposits of Aβ in brain tissue. Senile plaques have been shown both herein and once elsewhere [9] to give a Maltese cross pattern of light extinction under the polarising microscope. Our research raises the intriguing possibility that spherulites and senile plaques are ostensibly one and the same and that their formation in vitro and in vivo requires Cu(II).
Acknowledgements
Thanks to Ms Mary Finnegan and Dr Paul Quinn for assistance with the synchrotron measurements; thanks to Dr Albina Mikhailova and Dr Vijay Antharam for preparing and sectioning the tissues. The tissue used in this study was provided by the Human Brain Tissue Bank at University of Florida.
This work was carried out with the support of Diamond Light Source. Support was received in part from EPSRC EP/D066654/1 and NIH R21NS060304.
Footnotes
Competing Interests
None
References
- 1.Krebs MRH, MacPhee CE, Miller AF, Dunlop IE, Dobson CM, Donald AM. The formation of spherulites by amyloid fibrils of bovine insulin. Proc. Natl. Acad. Sci. USA. 2004;101:14420–14424. doi: 10.1073/pnas.0405933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromley EHC, Krebs MRH, Donald AM. Aggregation across the length-scales in beta-lactoglobulin. Faraday Disc. 2005;128:13–27. doi: 10.1039/b403014a. [DOI] [PubMed] [Google Scholar]
- 3.Juárez J, Taboada P, Goy-López S, Cambón A, Madec M-B, Yeates SG, Mosquera V. Additional supra-self-assembly of human serum albumin under amyloid-like-forming solution conditions. J. Phys. Chem. B. 2009;113:12391–12399. doi: 10.1021/jp904167e. [DOI] [PubMed] [Google Scholar]
- 4.Ruth L, Eisenberg D, Neufeld EF. α-L-Iduronidase forms semi-crystalline spherulites with amyloid-like properties. Acta Cryst. 2000;D56:524–528. doi: 10.1107/s090744490000007x. [DOI] [PubMed] [Google Scholar]
- 5.Yagi N, Ohta N, Lida T, Inoue K. A microbeam X-ray diffraction study of insulin spherulites. J. Mol. Biol. 2006;362:327–333. doi: 10.1016/j.jmb.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Domike KR, Hardin E, Armstead DN, Donald AM. Investigating the inner structure of irregular β-lactoglobulin spherulites. Eur. Phys. J. E. 2009;29:173–182. doi: 10.1140/epje/i2009-10465-y. [DOI] [PubMed] [Google Scholar]
- 7.Yagi N, Ohta N, Matsuo T. Structure of amyloid fibrils of hen egg white lysozyme studied by microbeam X-ray diffraction. Int. J. Biol. Macromol. 2009;45:86–90. doi: 10.1016/j.ijbiomac.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Domike KR, Donald AM. Kinetics of spherulite formation and growth: Salt and protein concentration dependence on proteins β-lactoglobulin and insulin. Int. J. Biol. Macromol. 2009;44:301–310. doi: 10.1016/j.ijbiomac.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Jin L-W, Klayborn KA, Kurimoto M, Geday MA, Maezawa I, Sohraby F, Estrada M, Kaminksy W, Kahr B. Imaging linear birefringence and dichroism in cerebral amyloid pathologies. Proc. Natl. Acad. Sci. USA. 2003;100:15294–15298. doi: 10.1073/pnas.2534647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snow AD, Sekiguchi R, Nochlin D, Fraser P, Kimata K, Mizutani A, Arai M, Schreier WA, Morgan DG. An important role of heparan sulphate proteoglycan (Perlecan) in a model system for the deposition and persistence of fibrillar Aβ-amyloid in rat brain. Neuron. 1994;12:219–234. doi: 10.1016/0896-6273(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 12.Mosselmans JF, Quinn PD, Dent AJ, Cavill SA, Moreno SD, Peach A, Leicester PJ, Keylock SJ, Gregory SR, Atkinson KD, Rosell JR. I18 - the microfocus spectroscopy beamline at the Diamond Light Source. J. Synchrotron Rad. 2009;16:818–824. doi: 10.1107/S0909049509032282. [DOI] [PubMed] [Google Scholar]
- 13.Collingwood JF, Mikhaylova A, Davidson MR, Batich C, Streit WJ, Eslin T, Terry J, Barrea R, Underhill RS, Dobson J. High resolution X-ray absorption spectroscopy studies of metal compounds in neurodegenerative brain tissue. J. Phys. Conf. Ser. 2005;17:54–60. [Google Scholar]
- 14.Solé VA, Papillon E, Cotte M, Walter P, Susini J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B. 2007;62:63–68. [Google Scholar]
- 15.House E, Mold M, Collingwood JF, Baldwin A, Goodwin S, Exley C. Copper abolishes the β-sheet secondary structure of preformed amyloid fibrils of Aβ42. J. Alzh. Dis. 2009;18:811–817. doi: 10.3233/JAD-2009-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobin SA, Wisniewski HM, Kieras FJ, Iqbal K. Morphological and chemical characterization of a starch granule-like polyglucosan deposit isolated from human brain. Acta Neuropathol. (Berl.) 1981;55:47–52. doi: 10.1007/BF00691530. [DOI] [PubMed] [Google Scholar]
- 17.Kimura T, Fujise N, Ono T, Shono M, Yuzuriha T, Katsuragi S, Takamatsu J, Miyakawa T, Kitamura T. Identification of an aging-related spherical inclusion in the human brain. Pathol. Int. 2002;52:636–642. doi: 10.1046/j.1440-1827.2002.01402.x. [DOI] [PubMed] [Google Scholar]
- 18.Srikanth R, Mendoza VL, Bridgewater JD, Zhang G, Vachet RW. Copper binding to β-2-microglobulin and its pre-amyloid oligomers. Biochemistry. 2009;48:9871–9881. doi: 10.1021/bi901172y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince PG. Epidemiological pathway of dementia: Attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PloS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]