Abstract
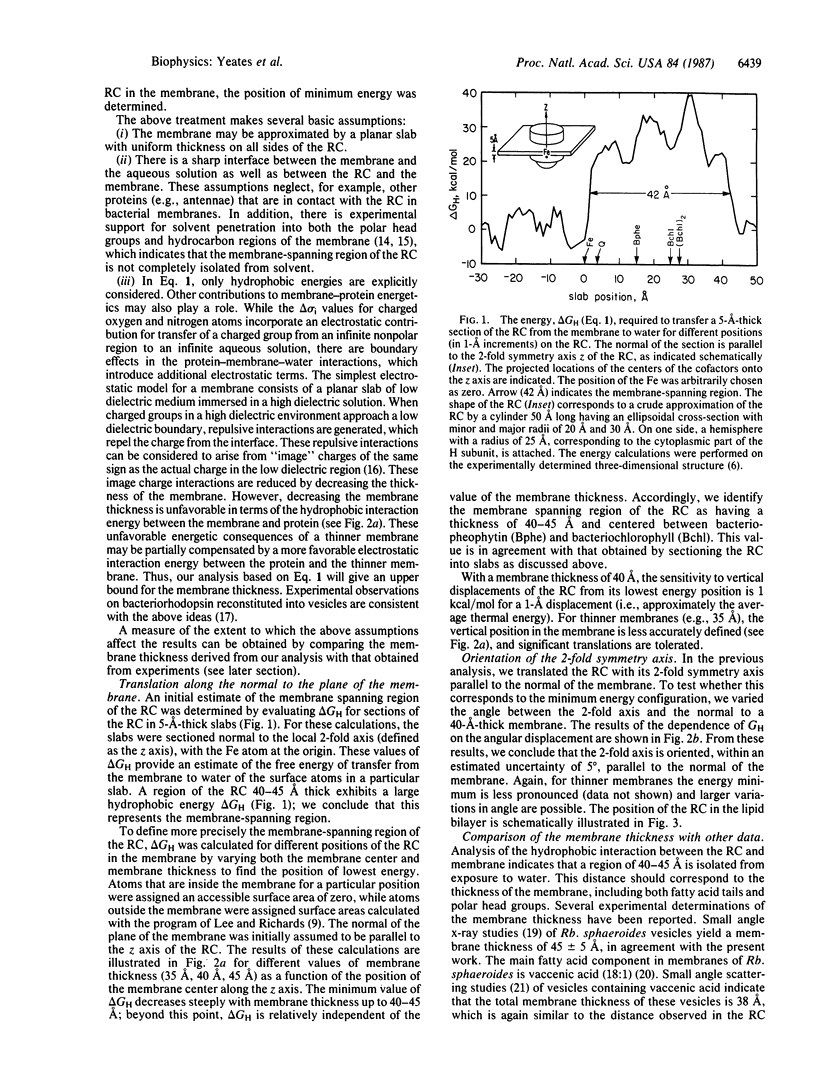
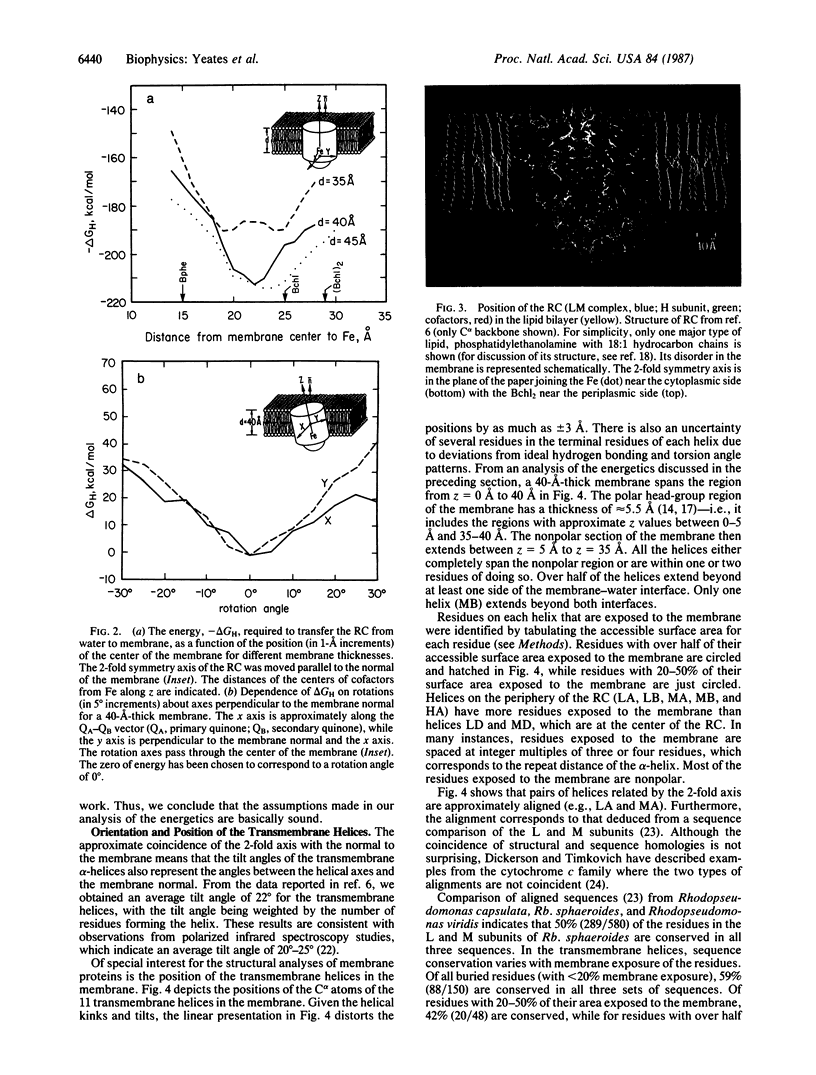
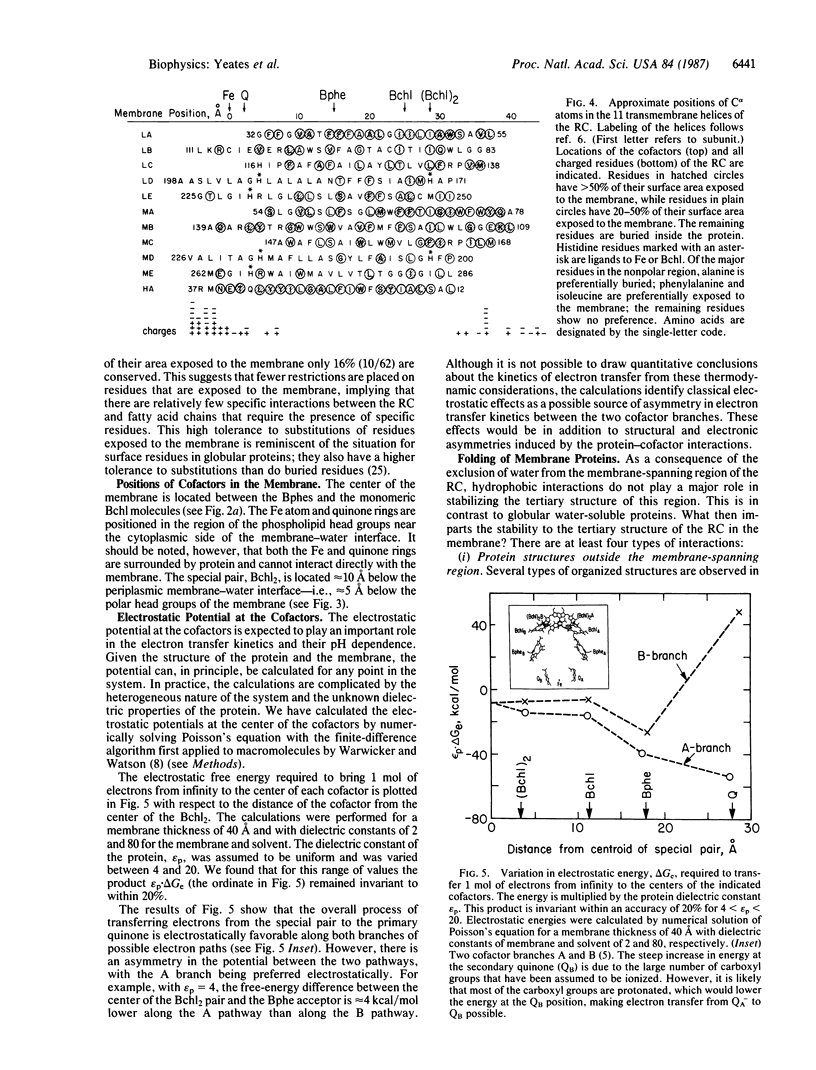
The energetics of membrane-protein interactions are analyzed with the three-dimensional model of the photosynthetic reaction center (RC) from Rhodobacter sphaeroides. The position of the RC in the membrane and the thickness of the membrane were obtained by minimizing the hydrophobic energy with the energy function of Eisenberg and McLachlan. The 2-fold symmetry axis that relates the L and M subunits is, within the accuracy of 5 degrees, parallel to the normal of the membrane. The thickness of the membrane is estimated to be 40-45 A. Residues that are exposed to the membrane are relatively poorly conserved in the sequences of homologous RC proteins. The surface area of the RC is comparable to the surface areas of water-soluble proteins of similar molecular weight. The volumes of interior atoms in the RC are also similar to those of water-soluble proteins, indicating the same compact packing for both types of proteins. The electrostatic potential of the cofactors was calculated. The results show an asymmetry in the potential between the two possible pathways of electron transfer, with the A branch being preferred electrostatically.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the cofactors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5730–5734. doi: 10.1073/pnas.84.16.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney J. L. Volume occupation, environment and accessibility in proteins. The problem of the protein surface. J Mol Biol. 1975 Aug 25;96(4):721–732. doi: 10.1016/0022-2836(75)90148-5. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983 May 15;166(2):203–210. doi: 10.1016/s0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Pape E. H., Menke W., Weick D., Hosemann R. Small angle x-ray scattering of the thylakoid membranes of Rhodopseudomonas speroides in aqueous suspensions. Biophys J. 1974 Mar;14(3):221–232. doi: 10.1016/s0006-3495(74)85909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C. H. Building models of globular protein molecules from their amino acid sequences. I. Theory. J Mol Biol. 1982 Feb 15;155(1):53–62. doi: 10.1016/0022-2836(82)90491-0. [DOI] [PubMed] [Google Scholar]
- Rashin A. A., Iofin M., Honig B. Internal cavities and buried waters in globular proteins. Biochemistry. 1986 Jun 17;25(12):3619–3625. doi: 10.1021/bi00360a021. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The interpretation of protein structures: total volume, group volume distributions and packing density. J Mol Biol. 1974 Jan 5;82(1):1–14. doi: 10.1016/0022-2836(74)90570-1. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Levy R. M., Salemme F. R. alpha-Helix dipole model and electrostatic stabilization of 4-alpha-helical proteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4545–4549. doi: 10.1073/pnas.79.15.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Depth of water penetration into lipid bilayers. Methods Enzymol. 1986;127:511–521. doi: 10.1016/0076-6879(86)27041-x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sprang S., Yang D., Fletterick R. J. Solvent accessibility properties of complex proteins. Nature. 1979 Jul 26;280(5720):333–335. doi: 10.1038/280333a0. [DOI] [PubMed] [Google Scholar]
- Teller D. C. Accessible area, packing volumes and interaction surfaces of globular proteins. Nature. 1976 Apr 22;260(5553):729–731. doi: 10.1038/260729a0. [DOI] [PubMed] [Google Scholar]
- Wada A. The alpha-helix as an electric macro-dipole. Adv Biophys. 1976:1–63. [PubMed] [Google Scholar]
- Warwicker J., Watson H. C. Calculation of the electric potential in the active site cleft due to alpha-helix dipoles. J Mol Biol. 1982 Jun 5;157(4):671–679. doi: 10.1016/0022-2836(82)90505-8. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986 Dec;1(4):312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]




