Abstract
The success of pregnancy depends upon regulatory mechanisms that allow the fetus to survive and develop to term in the uterus, despite maternal immune cells' awareness of paternal alloantigens. At least some of these specific mechanisms are mediated by the effect of fetal trophoblast cells. In the present study we examine the effect of human placental cytotrophoblast cells (CTCs) on the maturation of dendritic cells (DCs) in vitro. For that purpose, CTCs were isolated from samples of placentae at 5–11 weeks of gestation and co-cultured with peripheral blood monocytes under conditions inducing DC maturation. CTC were shown to alter the morphology, phenotype and functional properties of DCs. As a result, a significant portion of cells acquire fibroblast-like morphology and some of the cells retain the expression of CD14. DCs matured in the presence of CTCs do not differ from usual DCs in terms of CD80, CD83 and CD86 expression, as well as the ability to induce allogenic lymphocytes proliferation. However, CTCs reduce significantly the ability of DCs to stimulate interferon-γ production and the loss of CD62L by T cells. The results obtained indicate that DCs may be involved in pregnancy-associated changes of cytokine production and T cell migration.
Keywords: cytokines/interleukins, dendritic cells, reproductive immunology, T cells
Introduction
Maternal acceptance of the fetus expressing paternal alloantigens during pregnancy is a unique immunological phenomenon [1]. It is known that the maternal immune system reacts to paternal antigens; however, during normal pregnancy specific mechanisms limit the destructive alloimmune response and allow the fetus to survive and develop to term. These mechanisms include modulation of uterine natural killer (NK) cell functions [2–4], induction of regulatory T cells [5–8], inhibition of effector T lymphocytes through tryptophan catabolism [9,10] and clonal deletion of immune cells recognizing paternal antigens [11,12]. The local balance between T helper type 1 (Th1) and Th2–Th3 cytokines within the uterus and fetal–placental unit also contributes to the success of pregnancy [13–16].
At least some of these specific mechanisms are mediated by the effect of fetal trophoblast cells. Different subtypes of these cells produce hormones and anti-inflammatory cytokines [17], express non-classical human leucocyte antigen (HLA) molecules (HLA-E, HLA-F and HLA-G) [18,19] and other protective molecules: CD200 [20], PDL1 (B7-H1), PDL2 (B7-DC) [21], FasL [22] and complement regulatory proteins [23].
Direct contact between maternal immune system cells and fetal trophoblast cells occurs within uterine decidua. This highly specialized mucous membrane includes trophoblast of anchoring chorionic villi, cytotrophoblast cell columns branching off it and invasive extravillous cytotrophoblast cells [24]. The composition of maternal decidual leucocytes includes dendritic cells (DCs), along with NK cells, macrophages and T lymphocytes [25–30]. DCs are known to be antigen-presenting cells with a unique ability to recruit naive T lymphocytes into a primary immune response and to induce their maturation into effector and memory T cells [31,32]. Although the common functions of DCs are antigen processing and T lymphocyte activation, they differ in surface markers, migratory patterns and cytokine output. These differences can determine the fate of T cells activated and, ultimately, the strength of immune response and the balance between Th1 and Th2 responses.
The data on decidual DCs are presented in reviews [16,33,34]. Based on the data on antigen-presenting cells in human decidua, Ulrike Kammerer et al. [33] identified at least two populations of myeloid DCs with lin-CD11c+CD205+CD83- or CD83+ characteristics, accounting in sum for around 1–2% of all decidual leucocytes, and a large population of immature decidual macrophages that can be subdivided into classical CD68+CD163+ macrophages or immature CD14+CD4+CD209+CD83- DCs, capable of maturing into classical DCs. It should be noted that the quantity of typical myeloid DCs in decidua declines by about half during the first trimester of pregnancy compared to the endometrium of non-pregnant uterus. At the same time, the concentration of immature CD14+CD4+CD209+CD83- DCs increases substantially during pregnancy. In addition, a small population of lin- decidual DCs has been described recently that includes three different subpopulations: BDCA-1+ or BDCA-3+ myeloid DCs and BDCA-2+CD123+ plasmacytoid DCs, which account for 0·2–0·5% of decidual mononuclear cells [35].
To date, limited experimental evidence has been obtained suggesting the possible involvement of DCs in the local balance between Th1 and Th2–Th3 cytokines during pregnancy [16]. It has been shown that murine decidua during most of the normal gestation is characterized by a reduced level of CD8A+ DCs which produce interleukin (IL)-12, which is critical for the stimulation of Th1 [36]. At the same time, the quantity of IL-10 producing DCs increases. Uterine DCs from mice with a high abortion rate display increased IL-12 : IL-10 ratios compared to mice with a normal pregnancy [37]. Human endometrium and decidua include mainly immature myeloid DCs capable of maturing into IL-12 producents [27,28,36]. This seems to contradict the assumption that resident uterine DCs participate in the local balance between Th1 and Th2–Th3 cytokines during pregnancy. At the same time, one study suggests that IL-12 production by human decidua myeloid DCs is relatively weak compared to that of peripheral blood DCs [27]. In this work we have tested the hypothesis that human DCs participate in the cytokine shift during pregnancy. For that purpose, we have studied the effect of cytotrophoblast cells (CTCs) on the morphology, phenotype and functional properties of monocyte-derived DCs in vitro.
Materials and methods
Antibodies and reagents
Fluorescent labelled monoclonal antibodies to CD83 and CD62L were purchased from BD Biosciences (San Diego, CA, USA), antibodies to CD80 and CD86 were from Beckman Coulter Corp. (Marseille, France) and antibodies to CD3, CD14 and HLA-DR were from Immunosorbent (Moscow, Russia). Monoclonal antibodies to monomorphic determinant of HLA class I antigens were purchased from MedBioSpectr (Moscow, Russia). Monoclonal antibodies OV-TL 12/30 to cytokeratin-7 and LSAB2 system-horseradish peroxidase (HRP) for immunohistochemical staining were purchased from DakoCytomation, Inc. (Carpinteria, CA, USA). Recombinant human IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) were purchased from Invitrogen (Carlsbad, CA, USA). Lipopolysaccharide (LPS) from Salmonella typhi (LPS) was received from L. A. Tarasevich State Institute of Standardization and Control of Biomedical Preparations (Moscow, Russia). We used Dulbecco's modified Eagle's medium (DMEM) (Sigma Chemical Co., St Louis, MO, USA) with 10% heat-inactivated fetal calf serum (FCS) (Biolot, St Petersburg, Russia), 0·584 g/1 of l-glutamine, 110 mg/l of sodium pyruvate, 3·9 mg/l of 2-β-mercaptoethanol and 10 mg/1 of gentamicin (Sigma).
Isolation of CTCs
Written informed consent was obtained from all donors of blood and placenta samples. CTCs were isolated from placenta samples obtained from women with normal pregnancy (5–11 weeks of gestation) during elective abortions. Chorionic villi samples were dissected carefully, washed extensively to remove blood and cut into fine pieces. The material was trypsinized in 0·25% trypsin-ethylenediamine tetraacetic acid (EDTA) (Gibco, San Francisco, CA, USA) for 35 min at 37°C. The suspension was filtered through a cell dissociation sieve. Cells were washed by centrifugation, resuspended and overlaid on Percoll 15–25–30–40% (Pharmacia, Stockholm, Sweden). After centrifugation at 460 g for 45 min, cells from 30% Percoll layer were collected, washed twice and resuspended in DMEM with FCS. The cells were plated in 24-well plates (Costar, Bedford, MA, USA) at a concentration of 3 × 105 cells/ml. Cell cultures were cultured at 37°C in atmosphere containing 5% of CO2. The purity of each trophoblast preparation was verified using immunohistochemical staining of cytokeratin-7, and then cultures with 95%+ purity were used in further experiments. In separated experiments, the expression of HLA-DR and monomorphic determinant of HLA class I antigens on CTCs was examined by flow cytometry analysis.
Generation of monocyte-derived DCs
Peripheral blood mononuclear cells (PBMCs) from healthy pregnant women were isolated by sedimentation through Hystopaque-1077 (Sigma), washed twice and plated in 24-well plates (Costar) at 5 × 106 cells per well. After 3 h, the non-adherent cells were removed and adherent cells were cultured in DMEM with FCS. The next day (day 1) adherent cells were collected, counted and plated with or without trophoblast cells. CTCs were added directly or separated in transwell inserts (Greiner Bio-One, Frickenhausen, Germany). The ratio of adherent PBMCs to CTCs was 4 : 1. In separated experiments adherent PBMCs were plated with 25% of CTC-conditioned medium (CM, medium from 2-day CTC cultures, plated at 5 × 105 cells per ml). IL-4 (20 ng/ml) and GM-CSF (100 ng/ml) were added to cultures of adherent PBMCs and control cultures of trophoblast cells on days 1 and 4. On day 7 supernatants were collected for IL-1β, IL-6 and IL-10 measurement by enzyme-linked immunosorbent assay (ELISA) and cells were stimulated with LPS at a concentration of 1 µg/ml. After 24 or 48 h supernatants were collected for IL-1β, IL-6, IL-10 and IL-12p70 measurement and cells were harvested for flow cytometry analysis or mixed lymphocyte culture (MLC).
MLC
Mature DCs were assayed for their ability to stimulate allogenic lymphocytes in MLC. The stimulating cells were: (i) DCs, developed in the absence of CTCs; (ii) DCs, developed in the direct presence of CTCs (DC–CTCs); (iii) DCs, developed in the absence of CTCs; and mixed with CTCs at a ratio of 4 : 1 only at the point of administration to MLC. In separate experiments DCs developed with CTCs in transwell systems and CM-treated DCs were used. The responding cells were allogenic lymphocytes (non-adherent PBMCs from healthy pregnant women) at a concentration of 1 × 106/ml. DCs were co-cultured with lymphocytes in 96-well plates in the following ratios: 1 : 10, 1 : 25, 1 : 50 and 1 : 100. Control cultures were DCs and DC–CTCs without lymphocytes, unstimulated lymphocytes and lymphocytes mixed with CTCs or with unstimulated monocytes in corresponding quantities. In transwell experiments additional controls were lymphocytes mixed with DCs obtained in presence of decidual fibroblasts in transwell inserts. After 3 days of culture, supernatants were collected for interferon (IFN)-γ and IL-4 measurement by ELISA, and cells were pulsed with 0·5 µCi [3H]-thymidine for 18 h. Radionuclide incorporation into the DNA was measured further by β-scintillation counting. The results were expressed as radioactivity (count per min; cpm) per well. Assays were performed in triplicate. In separate experiments cells from MLC were harvested for flow cytometry analysis.
Flow cytometry analysis
DCs were stained with phycoerythrin (PE)-conjugated antibodies to CD14, CD83, CD86, HLA-DR and fluorescein isothiocyanate (FITC)-conjugated antibodies to CD80 and HLA-DR. The samples were analysed with FacsCanto II (BD Biosciences, Franklin Lakes, NJ, USA). DCs were gated according to forward scatter/side scatter (FSC/SSC) profiles. T cells from MLC were stained with FITC-conjugated antibodies to CD3 and phycoerythrin (PE)-conjugated antibodies to CD62L. T cells were gated according to FSC/SSC profiles and CD3 expression sequentially.
ELISA
Cytokine levels were measured by ELISA. Commercial kits for IL-1β, IL-6, IL-4 and IFN-γ measurement were purchased from Vector-Best (Novosibirsk, Russia); for IL-10, from Cytokine (St Petersburg, Russia) and for IL-12p70, from Biosource (Carlsbad, CA, USA). All measurements were performed according to the manufacturer's instructions.
Results
To investigate the effects of CTCs on DC maturation and functions we induced the development of DCs from peripheral blood monocytes both in the presence and in the absence of CTCs in vitro. CTCs were isolated from placenta samples at 5–11 weeks of gestation, as described in Materials and methods. The purity of each trophoblast preparation was verified by cytokeratin-7 staining. The CTCs obtained were cytokeratin-7+ and did not express HLA-DR and monomorphic determinant of HLA class I (data not shown).
Effect of CTCs on DC morphology and phenotype
Immature DCs were obtained by cultivation of monocytes with IL-4 and GM-CSF for 6 days. DC maturation was induced by administration of LPS. Cultures without CTCs showed morphological changes typical of DC development in vitro. These cultures consisted of large rounded and oval cells with thin processes (Fig. 1a). The cells' adhesiveness decreased and they formed cell clusters (Fig. 1b). Cultures of DCs and DC–CTCs displayed no visible differences in morphology during the first 4 days. Significant changes in DC–CTC cultures occurred on days 5–7. Cell clusters in these cultures disintegrated and most of the cells acquired spindle-shaped fibroblast-like morphology (Fig. 1c,d). As a result, DC–CTC cultures acquired mixed morphology, with both fibroblastoid and round cells present, whereas DC cultures maintained their original morphology with round cells. Cultures of DCs separated from CTCs by a transwell system also contained fibroblast-like cells (Fig. 1f). Control cultures of CTCs did not contain fibroblast-like cells (Fig. 1e).
Fig. 1.
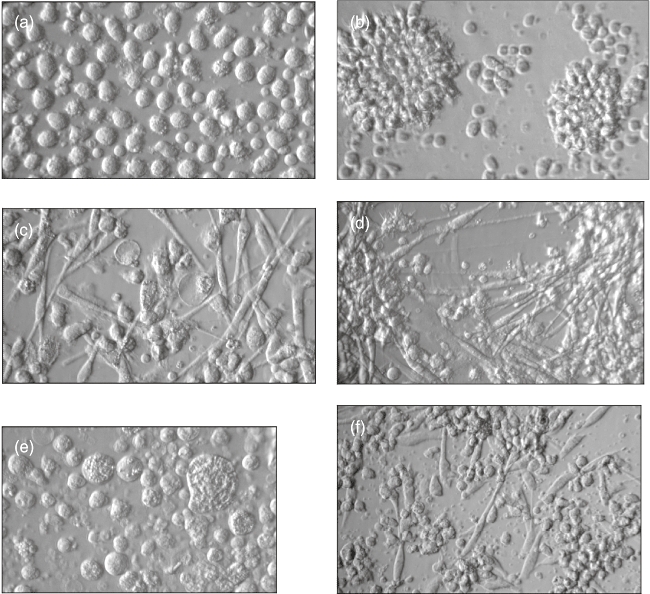
Morphology of dendritic cells (DCs) (a,b), DCs with cytotrophoblast cells (CTCs) (c,d), CTCs (e) and DCs separated from CTCs by a transwell system (f). Cells were cultured in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days and then for 1 more day with lipopolysaccharide (LPS). Hoffman modulation contrast, magnification: 360× (a,c,e) and 250× (b,d,f).
Mature DCs developed in the absence of CTCs had a typical phenotype. They were CD14-HLA-DR+CD86+ (Fig. 2b,f,g). More than 50% of cells expressed CD80 and more than 30% expressed CD83 (Fig. 2d–g). Cells from control cultures of unstimulated monocytes retained CD14 expression (Fig. 2a). Direct presence of CTCs during DC development resulted in the preservation of CD14 on part of the cells (Fig. 2c,g). Also, a small but statistically significant decrease of HLA-DR expression level was observed on DC–CTCs (Fig. 2g,h). There were no differences in CD80, CD83 and CD86 expression between DCs and DC–CTCs (Fig. 2d–g).
Fig. 2.
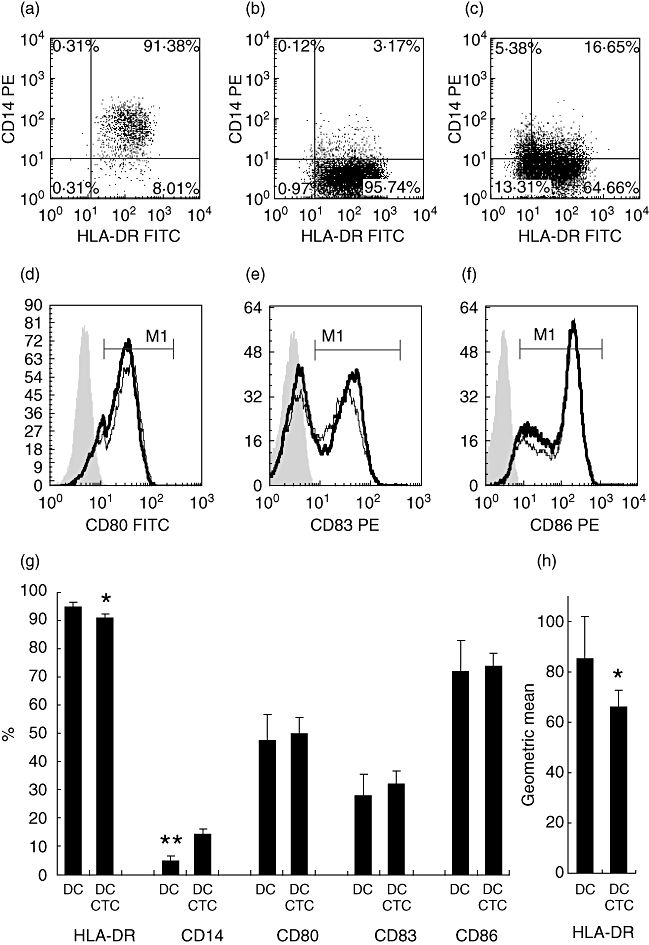
Expression of membrane antigens on mature dendritic cells (DCs) and DCs developed with cytotrophoblast cells (DC–CTCs). Immature DCs were obtained by cultivation of monocytes with or without CTCs in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days. DC maturation was induced by the administration of lipopolysaccharide (LPS) for 48 h. Then cells were harvested and analysed by flow cytometry after staining with human leucocyte antigen D-related (HLA-DR), CD14, CD80, CD83 and CD86. DCs were gated according to forward scatter/side-scatter (FSC/SSC) profiles. Representative data on CD14 and HLA-DR expression on unstimulated monocytes (a), DCs (b) and DC–CTCs (c). An example of expression of CD80 (d), CD83 (e) and CD86 (f) on DCs (thin line) and DC–CTCs (thick line). Filled histograms represent isotypic controls. Average percentage of antigen-bearing DCs and DC–CTCs is shown on histogram (g) (n = 17). Mean fluorescence intensity of HLA-DR staining is shown on histogram (h) (n = 17). Cell types and analysed antigens are indicated below histograms. Statistical differences between DC and DC–CTCs in paired t-tests are designated *P < 0·05 and **P < 0·0001.
Cytokine production by DCs and DC–CTCs
IL-1β, IL-6, IL-10 and IL-12p70 were determined by ELISA in culture supernatants of DCs developed in the presence or absence of CTCs. Both immature and mature (LPS-stimulated) DCs produced IL-1β, IL-6 and IL-10 (Fig. 3). Trophoblast cells had no effect on the production of these interleukins by DCs. CTCs from control cultures did not produce these interleukins. We did not observe any detectable production of IL-12p70 either in DC or DC–CTC cultures under the stimulation conditions used.
Fig. 3.
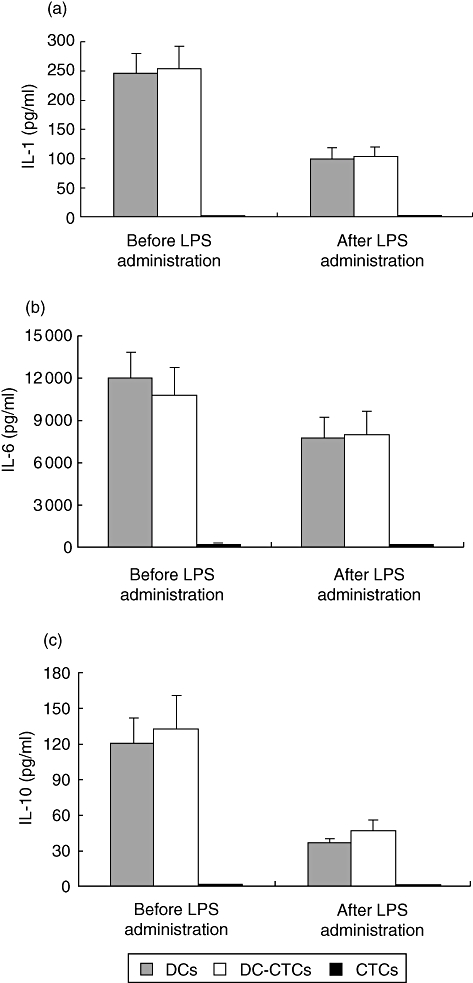
Concentrations of interleukin (IL)-1β (a), IL-6 (b) and IL-10 (c) in supernatants from dendritic cell (DC), DC–cytotrophoblast cell (CTC) and CTC cultures. Cells were cultured in the presence of IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days, then cultural medium was harvested for enzyme-linked immunosorbent assay (ELISA) of cytokines. Fresh medium with lipopolysaccharide (LPS) was added to cultures and 1 day later supernatants were collected for ELISA again.
The effect of CTCs on T cell stimulatory capacity of DCs
Allogenic MLC was used to analyse T cell stimulatory capacity of DCs and DC–CTCs. Mature DCs demonstrated a strong ability to stimulate allogenic lymphocyte proliferation and IFN-γ production in a dose-dependent manner. The immediate presence of CTCs during DCs development had absolutely no effect on DC-induced T cell proliferation (Fig. 4a,c). However, DCs developed in the immediate presence of CTCs demonstrated a strong decline in IFN-γ-stimulating capacity (Fig. 4b,d). The IFN-γ concentration in supernatants from DC–CTC-stimulated MLCs was 2·88 ± 0·54 times lower than in supernatants from DC-stimulated MLCs (P < 0·00001 in paired t-tests, n = 42). CTCs added to lymphocytes had no effect on their proliferation and IFN-γ production. Simultaneous administration of DCs and CTCs (in a ratio of 4 : 1) to MLC resulted in a weak decrease of IFN-γ production compared to DCs (Fig. 4d). However, IFN-γ production in these cultures was significantly higher than in DC–CTC-stimulated MLCs (P < 0·005). Control cultures of DCs and DC–CTCs without lymphocytes did not produce IFN-γ (< 1·7 pg/ml).
Fig. 4.
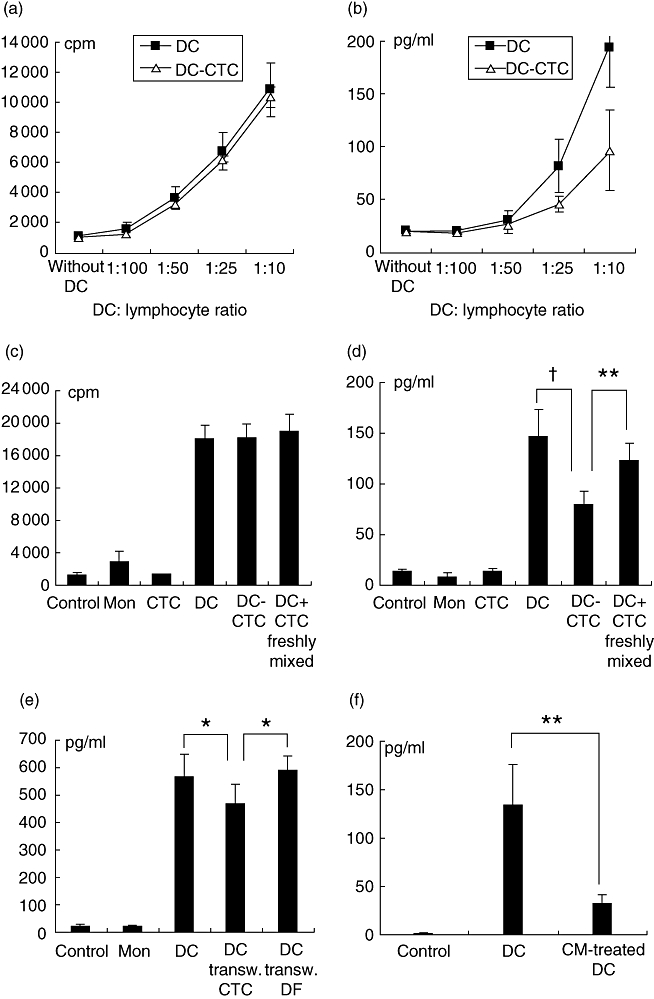
The effect of cytotrophoblast cells (CTCs) on T cell stimulatory ability of dendritic cells (DCs). Allogenic lymphocytes were stimulated by DCs, DC–CTCs and DCs mixed with CTCs at the point of administration to MLC (DC + CTC freshly mixed) (a–d). In separated experiments allogenic lymphocytes were stimulated by DCs obtained in the presence of CTCs in transwell inserts (DC transw. CTC) (e) and conditioned medium (CM)-treated DCs (f). Control cultures were unstimulated lymphocytes (control) and lymphocytes mixed with CTCs or with unstimulated monocytes (mon). In transwell experiments additional controls were lymphocytes mixed with DCs obtained in the presence of decidual fibroblasts in transwell inserts (DC transw. DF). Proliferation (a) and interferon (IFN)-γ production (b) in mixed leucocyte cultures (MLCs), stimulated by different quantities of DCs and DC–CTCs. IFN-γ production (d,e,f) and proliferation (c) in MLCs with different stimulation conditions (DC : lymphocyte ratio = 1 : 10). Stimulators are indicated below histograms. †P < 0·000001, **P < 0·005, *P < 0·05 in paired t-tests.
The difference in IFN-γ production between MLCs stimulated with DC–CTCs and DCs freshly mixed with CTCs at a ratio of 4 : 1 was not a consequence of the difference in CTC concentration. The preliminary assay of HLA-DR+ and HLA-DR- cells in DC and DC–CTC cultures showed a stable DC : CTC ratio during the entire DC development period. The concentration of HLA-DR- CTCs in DC–CTC cultures on day 7 was 19·47 ± 2·33% (n = 13).
CTC–CM decreases the IFN-γ-stimulating capacity of DCs (Fig. 4f). CM was obtained from 2-day CTC cultures, plated at optimal concentration without IL-4, GM-CSF and LPS. CM-treated DCs induced IFN-γ-production 4·45 ± 0·83 times lower than DCs (n = 9, P < 0·005). Average concentrations of IFN-γ in MLCs stimulated by DCs and CM-treated DCs were 133·67 ± 43·17 and 31·22 ± 9·63, respectively. Co-cultivation of DCs and CTCs separated by transwell inserts also leads to a decrease of IFN-γ-stimulating ability compared with DCs (Fig. 4e). The difference was small (1·34 ± 0·14 times), but statistically significant (P < 0·05).
None of the 30 lymphocyte cultures used in the experiment responded to DC and DC–CTC stimulation by any significant production of IL-4. Very weak IL-4 production was registered in all control MLCs, to which CTCs without DCs were added (mean concentration of IL-4 2·80 ± 0·49 pg/ml). Simultaneous addition of DCs and CTCs to MLC led to a small but reproducible and detectable production of IL-4 (6·20 ± 1·08 pg/ml).
Impact of DCs and DC–CTCs on CD62L expression by T cells
CD62L is a leucocyte homing receptor required for T cell trafficking to lymph nodes. It is known that CD62L is expressed on naive T cells and central memory T cells targeted to lymph nodes, whereas effector memory T cells targeted to inflamed tissue are CD62L-[38–42]. In order to examine DC-induced changes of T cell migration patterns, quantities of CD3+CD62L+ T cells and CD3+CD62L- T cells were determined. Mature DCs induced a statistically significant increase in the quantity of CD3+CD62L- T cells in MLC on day 4 (Fig. 5). The number of CD3+CD62L- T cells in DC-stimulated MLCs and in MLCs stimulated by simultaneous administration of DCs and CTCs was equal. DC–CTCs induced a smaller increase in the number of CD3+CD62L- cells compared to DCs. The difference was low, but statistically significant (P < 0·001 in paired t-tests, n = 12, Fig. 5d).
Fig. 5.
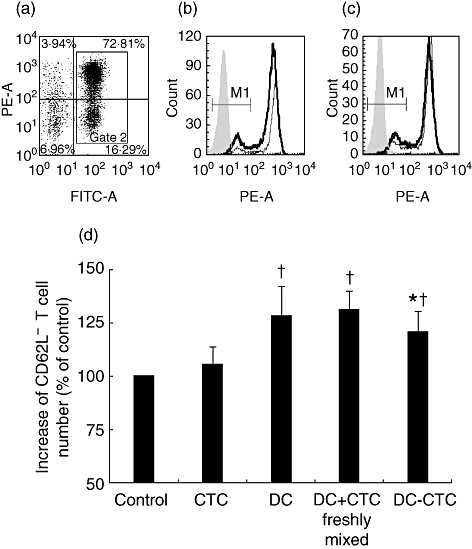
The impact of dendritic cells (DCs) and DC–cytotrophoblast cells (CTCs) on T cell expression of CD62L. Allogenic lymphocytes were stimulated with DCs, DC–CTCs and DCs mixed with CTCs at the point of administration in mixed leucocyte culture (MLC) (DC + CTC freshly mixed). Control cultures were unstimulated lymphocytes (control) and lymphocytes with CTCs. After 86 h of culture, cells were harvested and analysed by flow cytometry after staining with CD3-fluorescein isothiocyanate (FITC) and CD62L-phycoerythrin (PE). Lymphocytes were gated according to forward scatter/side scatter (FSC/SSC) profiles (not shown). (a) T cells were gated according to CD3 expression. (b) Expression of CD62L on unstimulated CD3+ T cells (thin line) and T cells stimulated by DCs (thick line). (c) Expression of CD62L on CD3+ T cells stimulated by DCs (thick line) and DC–CTCs (thin line). Filled histograms represent isotypic controls. (d) Increase in amount of CD62L- cells among CD3+ T cells stimulated by DCs, DC–CTCs and DCs freshly mixed with CTCs compared with unstimulated lymphocytes. Statistical differences in paired t-tests with control level are designated †P < 0·05; the difference between DC–CTCs and DCs and freshly mixed DCs and CTCs are designated *P < 0·001.
Discussion
The present study examines the effect of human placental CTCs on the maturation and functional properties of dendritic cells in vitro. The contact of trophoblast cells with maturing DCs in vivo is possible inside the uterine decidua. During pregnancy fetal trophoblast penetrates the endometrium and transforms it by interacting with various cells of the endometrium and producing steroid hormones and cytokines [24,43]. This results in the formation of decidua – a specialized mucous membrane which includes, along with other cells, a unique set of maternal immune system cells. Up to 2% of all leucocytes of the decidua are typical myeloid DCs and approximately 10% are macrophage-like immature DCs [33]. As is well established, mature DCs are able to stimulate effectively a naive T lymphocyte response to antigen and influence the balance between Th1 and Th2 responses. Immature DCs and DCs modulated by anti-inflammatory mediators do not trigger an immune response, but rather induce immunological tolerance through stimulation of regulatory T cells and elimination of antigen-specific immune T lymphocytes [44,45]. The ability of DCs to induce both immune response and tolerance suggests that these cells may be of particular relevance for maintaining pregnancy [16,33,34].
In the present study, culturing monocytes with CTCs under conditions stimulating DCs development resulted in formation of cells with a different morphology from that of regular monocyte-derived DCs. Most cells in DC cultures were of rounded shape and showed low adhesion to the surface of culture plates, while DC–CTC cultures contained a large number of fibroblast-like, spread cells. CTCs were not the source of fibroblast-like cells. The observed changes in DC morphology suggest that CTCs may have an effect on the adhesive properties of DCs and, perhaps, on the migration of DCs from decidua to draining lymphatic nodes. This assumption is consistent with the data from Collins et al. [46], who have shown recently that the migration ability of murine decidual DCs is inhibited and, despite continuing responsiveness to CCL21, DCs cannot reach draining lymph nodes even after strong stimulation with LPS.
The presence of CTCs during the development of DCs resulted in the retention of CD14 expression on some of the cells and a small decrease in the expression of HLA-DR. DC–CTCs did not differ from DCs in the expression of CD80, CD83 and CD86, as well as the ability to induce allogenic lymphocyte proliferation. However, the presence of CTCs during DCs development reduced significantly their ability to stimulate IFN-γ production in allogenic MLC. Based on experiments with CTC-conditioned medium and transwell systems we hypothesize that this effect is mediated at least partially by soluble factors. CM-treated DCs had lower IFN-γ-inducing ability compared with DCs developed in the presence of CTCs. In our opinion, this difference can be a consequence of down-regulation of CTC activity by DCs or DC maturation inductors (LPS, IL-4, GM-CSF).
The most likely reason for weakness of DC–CTC IFN-γ-stimulating capacity seems to be CTC-induced inhibition of IL-12p70 production. As noted in the Introduction, published data are available that IL-12 production by human and murine decidual DCs is weak [27,36]. However, based on the data obtained from our study, we were unable to determine the role of changes in IL-12 production in CTC-induced decrease of IFN-γ-stimulating ability of DCs because, like some other authors [47], we were unable to identify IL-12p70 production by DCs after LPS stimulation. We suggest that the production of this cytokine, critical for inducing IFN-γ secretion [36,48], begins under these stimulation conditions only in MLC, when DCs are stimulated with CD40L of T lymphocytes.
CTCs reduced DCs ability to induce the loss of CD62L molecule by T lymphocytes. This molecule is important for T cell migration to lymph nodes [41]. In our view, the retention of CD62L expression limits migration of DC-stimulated T cells to the pregnant uterus, and thus prevents the participation of these cells in local immune reactions dangerous for the fetus.
Thus, the results obtained suggest that DCs may be involved in the regulation of T lymphocytes migration and in maintaining the necessary cytokine production balance during pregnancy by reducing the secretion of IFN-γ– a key Th1 response cytokine.
Acknowledgments
The research is supported by the Russian Fund for Fundamental Research, project 07-04-00244. The authors thank Professor Alexander Yarilin for stimulating discussions and Andrei Mikheev for help in preparing the text.
Disclosure
The authors declare no conflict of interest.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp. 1953;7:320–38. [Google Scholar]
- 2.Rukavina D, Rubesa G, Gudelj L, Haller H, Podack ER. Characteristic of perforin expressing lymphocytes within the first trimester deciduas of human pregnancy. Am J Reprod Immunol. 1995;33:394–404. doi: 10.1111/j.1600-0897.1995.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 3.Clark DA, Arck PC, Jalali R, et al. Psycho-neuro-cytokine/endocrine pathways in immunoregulation during pregnancy. Am J Reprod Immunol. 1996;35:330–37. doi: 10.1111/j.1600-0897.1996.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 4.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 5.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 6.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito S, Sasaki Y, Sakai M. CD4+CD25high regulatory T cells in human pregnancy. J Reprod Immunol. 2005;65:111–20. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen G, Mottonen M, Alanen A, Lassia O. Phenotypic characterization of regulatory T cells in the human deciduas. Clin Exp Immunol. 2004;136:373–78. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo Y, Boyd CAR, Spyropoulou I, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87–98. doi: 10.1016/j.jri.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JS, Vassmer D, Ferguson TA, Miller L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J Immunol. 1997;158:4122–28. [PubMed] [Google Scholar]
- 12.Tafuri A, Alfering J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–33. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 13.Dealtry GB, O'Farrell MK, Fernandez N. The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol. 2000;123:107–19. doi: 10.1159/000024441. [DOI] [PubMed] [Google Scholar]
- 14.Knackstedt MK, Zenclussen AC, Hertwig K, et al. Th1 cytokines and the prothrombinase fgl2 in stress-triggered and inflammatory abortion. Am J Reprod Immunol. 2003;49:210–20. doi: 10.1034/j.1600-0897.2003.01192.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaouat G, Ledee-Bataille N, Dubanchet S, et al. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 16.Blois SM, Kammerer U, Alba Soto C, et al. Dendritic cells: key to fetal tolerance? Biol Reprod. 2007;77:590–98. doi: 10.1095/biolreprod.107.060632. [DOI] [PubMed] [Google Scholar]
- 17.Tarrade A, Kuen RL, Malassiné A, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001;81:1199–211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 18.Hunt JS, Andrews GK, Wood JW. Normal trophoblasts resist induction of class I HLA. J Immunol. 1987;138:2481–7. [PubMed] [Google Scholar]
- 19.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G in immune tolerance in pregnancy. FASEB J. 2005;19:681–93. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 20.Clark DA, Keil A, Chen Z, et al. Placental trophoblast from successful human pregnancies expresses the tolerance signaling molecule, CD200 (OX-2) Am J Reprod Immunol. 2003;50:187–95. doi: 10.1034/j.1600-0897.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 21.Petroff MG, Chen L, Phillips TA, et al. B7 family molecules are favorably positioned at the human maternal–fetal interface. Biol Reprod. 2003;68:1496–504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 22.Mor G, Gutierrez LS, Eliza M, Kahyaoglu F, Arici A. Fas-fas ligand system-induced apoptosis in human placenta and gestational trophoblastic disease. Am J Reprod Immunol. 1998;40:89–94. doi: 10.1111/j.1600-0897.1998.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 23.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillières Clin Obstet Gynaecol. 1992;6:439–60. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 24.Aplin JD, Haigh T, Jones CJP, Church HJ, Vicovac L. Development of cytotrophoblast columns from explanted first-trimester humanplacental villi: role of fibronectin and integrin α5β1. Biol Reprod. 1999;60:828–38. doi: 10.1095/biolreprod60.4.828. [DOI] [PubMed] [Google Scholar]
- 25.Abraham S, Indrasingh I, Vettivel S, Chandi G. Gross morphology and ultrastructure of dendritic cells in the normal human decidua. Clin Anat. 2000;13:177–80. doi: 10.1002/(SICI)1098-2353(2000)13:3<177::AID-CA3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Kämmerer U, Schoppet M, McLellan AD, et al. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–69. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki S, Tsuda H, Sakai M, et al. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003;74:514–22. doi: 10.1189/jlb.1102566. [DOI] [PubMed] [Google Scholar]
- 28.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–46. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 29.Askelund K, Liddell HS, Zanderigo AM, et al. CD83(+)dendritic cells in the decidua of women with recurrent miscarriage and normal pregnancy. Placenta. 2004;25:140–45. doi: 10.1016/S0143-4004(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 30.Ivanova E, Kyurkchiev D, Altankova I, et al. CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol. 2005;53:199–205. doi: 10.1111/j.1600-0897.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 32.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 33.Kammerer U, Kruse A, Barrientos G, Arck PC, Blois SM. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest. 2008;37:499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- 34.Laskarin G, Kammerer U, Rukavina D, et al. Antigen-presenting cells and materno–fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol. 2007;58:255–67. doi: 10.1111/j.1600-0897.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 35.Ban YL, Kong BH, Qu X, Yang QF, Ma YY. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin Exp Immunol. 2008;151:399–406. doi: 10.1111/j.1365-2249.2007.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70:1018–23. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- 37.Blois SM, Tometten M, Kandil J, et al. Intracellular adhesion molecule-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto–maternal interface in murine pregnancies. J Immunol. 2005;174:1820–9. doi: 10.4049/jimmunol.174.4.1820. [DOI] [PubMed] [Google Scholar]
- 38.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 40.Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30:1823–9. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4+ and CD8+ T cells. J Exp Med. 1999;189:1765–76. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blander JM, Sant'Angelo DB, Metz D, et al. A pool of central memory-like CD4 T cells contains effector memory precursors. J Immunol. 2003;170:2940–8. doi: 10.4049/jimmunol.170.6.2940. [DOI] [PubMed] [Google Scholar]
- 43.Aplin JD. Implantation, trophoblast differentiation and hemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–92. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 44.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 45.Hubert P, Jacobs N, Caberg J-H, Boniver J, Delvenne P. The cross-talk between dendritic and regulatory T cells: good or evil? J Leuk Biol. 2007;82:787–94. doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- 46.Collins MK, Tay C-S, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–73. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geijtenbeek TBH, van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]


