Abstract
Double cord blood transplantation (DCBT) with two matched or partially matched cord blood units has been implemented successfully to circumvent the limitations of graft cell dose associated with single CBT. After DCBT, sustained haematopoiesis is derived almost exclusively from only one of the donated units. None the less, we previously observed two of six evaluable DCBT patients still having mixed donor–donor chimerism at 28 and 45 months post-transplantation, respectively. In the present study we utilize flow cytometry techniques to perform the first thorough analysis of phenotype and functionality of cord blood units in patients with mixed donor–donor chimerism. Our results suggest that the two stable cord blood units are different phenotypically and functionally: one unit shows more naive T cells, lower T cell cytokine production and higher frequencies of natural killer cells, the other shows higher frequencies of well-differentiated and functional lymphocytes. Additionally, in comparison with control patients having a single prevailing cord blood unit, the patients with donor–donor chimerism exhibit less overall T cell cytokine production and a smaller fraction of memory T cells. Furthermore, our results indicate that human leucocyte antigen-C match of donor units may partly explain the development of a donor–donor mixed chimerism.
Keywords: allogeneic, cord blood transplantation, HLA-C, mixed chimerism, SCT
Introduction
Umbilical cord blood is an alternative stem cell source in allogeneic stem cell transplantations (allo-HCT) when no human leucocyte antigen (HLA)-identical donor is accessible [1–3]. Due to its rapid availability and naive cell content, cord blood is used increasingly in allo-HCT and in several studies has proved comparable in outcome to both bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT) regarding relapse, transplantation-related mortality and disease-free survival [4,5].
Immunologically naive cord blood stem cells have an increased potential for proliferation, creating lower cell dose requirements and enabling engraftment with up to two major HLA-mismatches [6]. Despite these advantages, the limited cell dose of a cord blood unit may result in delayed engraftment and poorer immune reconstitution compared to BMT and PBSCT, which in turn increases susceptibility to infections and graft failure [4,7]. Limited cell dose has, however, been circumvented successfully by using double cord blood transplantation (DCBT), where two matched or partially matched cord blood units are co-transplanted [8–11].
Stable mixed donor–recipient chimerism has been shown to be common after allo-HCT with a reduced intensity conditioning (RIC) regimen [12]. In contrast, long-term stable mixed donor–donor chimerism of two cord blood units is a very rare event. With few exceptions, previous chimerism results in DCBT report that sustained haematopoiesis in a vast majority of the patients is derived from only one of the donated units [10,11].
We have assessed previously the chimeric pattern in T, B and myeloid cells using polymerase chain reaction (PCR)-based chimerism analysis in seven patients after DCBT [13]. Three of six evaluable patients showed donor–donor mixed chimerism in all cell lineages at 90 days after DCBT. Unexpectedly, two of these three patients still had mixed donor–donor chimerism at 28 and 45 months after DCBT, respectively.
Besides our report, there are four described cases of mixed donor–donor chimerism at day 60 or more post-transplantation [11,14–16]. Only the paper by Yen et al. reports stable mixed chimerism for more than 12 months.
Chimerism studies are based on the unit distribution of T, B and myeloid cells or total DNA from whole blood, giving no data on differences in phenotype or functionality of the discrete cord blood units.
In the present study we utilize flow cytometry techniques on blood samples for the first thorough analysis of phenotype and functionality of cord blood units in patients with stable donor–donor chimerism. In addition, we compare the phenotype and functionality of these units to cells from cord blood-transplanted individuals with only one prevailing unit. Factors inducing donor–donor mixed chimerism are so far unidentified. The usage of high-dose anti-thymocyte globulin (ATG) has been associated with delayed development of donor chimerism after allo-HCT [17] and was further proposed to induce tolerance and mixed donor–donor chimerism after cord blood transplantation [13].
T cell reconstitution after cord blood transplantation is severely delayed, creating a favourable niche for natural killer (NK) cell expansion [18]. NK cell function is regulated partly by inhibitory and activating killer cell immunoglobulin-like receptors (KIRs) that recognize certain HLA-C molecules, and a match of HLA-C antigens could therefore induce tolerance between cord blood units [19]. Hence, we typed the donor cord blood units for HLA-C retrospectively in order to study this possible tolerance-inducing factor.
Our results suggest that the two stable cord blood units are different phenotypically and functionally. One unit displays more naive T cells, lower cytokine production and higher frequencies of NK cells, while the other presents with higher frequencies of well-differentiated and functional lymphocytes of memory phenotype. Furthermore, in comparison to control patients with a single prevailing cord blood unit, the patients with donor–donor chimerism exhibit less overall T cell cytokine production and a smaller fraction of memory T cells. Interestingly, our results indicate that a perfect HLA-C match of donor units may partly explain the development of a donor–donor mixed chimerism.
Materials and methods
Patient characteristics
Between June 2004 and May 2008 seven patients received double cord blood grafts at the Centre for Allogenic Stem cell Transplantation (CAST), Karolinska University Hospital, Huddinge. Three of the seven patients showed mixed donor–donor chimerism in all cell lineages 90 days post-transplantation, and two of these three still had mixed donor–donor chimerism at long-term follow-up (28 and 45 months, respectively).
Both patients received myeloablative pretreatment, one of them (ID: 1119) conditioned with cyclophosphamide (Cy) at 60 mg/kg on 2 consecutive days (-5 and -4 or -4 and -3) combined with fractionated total body irradiation (FTBI) 3 Gy for 4 consecutive days, and the other (ID: 1173) with a combination of busulphan (Bu) (4 mg/kg/day × 4) and Cy at 60 mg/kg on 2 consecutive days [20]. Three of the patients in the control group received the same conditioning regimen as patient 1173, and one of them was conditioned as patient 1119.
Patients and control group all received 5 days (days -5 to -1) of treatment with anti-thymocyte globulin (ATG) (Thymoglobuline; Genzyme, Cambridge, MN, USA), at a total dose of 8 mg/kg [21].
All patients and donors were typed using PCR-sequence-specific primers (SSP) typing for both HLA classes I and II antigens (Table 1) [22]. Both patients and control group received cyclosporin A (CyA) combined with prednisolone for graft-versus-host disease (GVHD) prophylaxis as described previously [23].
Table 1.
Human leucocyte antigen (HLA) typing.
| HLA-A |
HLA-B |
HLA-C |
HLA-DRB1 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Donor 1 | Donor 2 | Rec | Donor 1 | Donor 2 | Rec | Donor 1 | Donor 2 | Rec | Donor 1 | Donor 2 | Rec | Donor–donor match |
| 1171 | 0201/3101 | 3101/3201 | 3101/3201 | 4001/5101 | 3501/4001 | 1503/4001 | 03xx/15xx | 03xx/04xx | 0202/0304 | 0101/1302 | 0101/1301 | 0101/1302 | 5/8 |
| 1174 | 2402/6801 | 2402/6801 | 2402/6801 | 3503/1501 | 3503/1501 | 3503/5601 | 0303/0401 | 0313/0423 | 0102/0401 | 0401/0801 | 0404/0801 | 0401/0801 | 8/8 |
| 1275 | 02xx/6803 | 02xx/6803 | 0203/6801 | 3905/3906 | 3901/3905 | 3543/3905 | 07xx/07xx | 01xx/07xx | 0102/0702 | 0802/0407 | 0407/0802 | 0404/0802 | 7/8 |
| 1033 | 0101/6801 | 0101/6801 | 0101/6801 | 0801/4402 | 0801/4405 | 4002/4402 | 05xx/07xx | 02xx/07xx | 0202/0704 | 1501/0401 | 0401/1501 | 0404/1501 | 7/8 |
| 1119 | 0201/0301 | 0201/6801 | 0201/1101 | 1801/4402 | 0801/4402 | 4402/5501 | 05xx/07xx | 05xx/07xx | 0303/0501 | 0301/04xx | 0301/04xx | 0301/0328 | 6/8 |
| 1173 | 0201/3001 | 02xx/3001 | 0203/3001 | 1302/5201 | 1302/52xx | 1302/1512 | 1202/0602 | 12xx/06xx | 04xx/06xx | 12xx/1502 | 1104/1502 | 1202/1502 | 7/8 |
Neither of the two patients was diagnosed with acute or chronic GVHD, according to the criteria defined previously [24,25]. For detailed patient and donor characterization, see [13]. Supportive care has been described in detail previously [26]. Patient 1173 was diagnosed with leukaemia relapse 28 months after DCBT.
Inclusion criteria for the control group were single or double cord blood-transplanted patients with one single cord blood unit prevailing, with a follow-up time of at least 12 months. Median time post-transplantation in the flow cytometry control group (n = 4) was 21 months (range 13–131 months) compared to 34·5 months (range 26–43) in the two patients included in this study. Median age in the control group was 33 years (range 19–57 years) compared to 28·5 years (range 24–33 years) for the two patients.
Characteristics for the control group included in the HLA-C typing assay are described elsewhere [13].
This study was approved by the regional ethics committee in Stockholm (DNR 425/97).
Chimerism analysis
PCR amplification of short tandem repeats (STRs) was used to evaluate various degrees of donor and recipient chimerism [27]. CD3+, CD19+ and CD33+ cells were enriched from blood samples using immunomagnetic beads (Dynal, Oslo, Norway), as described previously [28].
For patient 1173, CD3-CD56+ and CD3-CD14+ cells were also enriched from blood samples using immunomagnetic CD3 beads (Dynal) for negative selection of non-T cells, and subsequent incubation with anti-CD56 and anti-CD14 microbeads, according to the manufacturer's protocol (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Visual separation of the two cord blood units in patient 1119
Because the two cord blood units of patient 1119 were mismatched at HLA-A (see Table 1), biotin-conjugated anti-HLA-A3 antibodies and subsequent staining with peridinin chlorophyll (PerCP)-labelled streptavidin enabled a flow cytometric-based discrimination of the units in this patient.
Antibodies and reagents
Fluorescein isothiocyanate (FITC), phycoerythrin (PE) and allophycocyanin (APC)-anti-CD8 (RPA-T8), FITC, PE, V450, PE-Cy5 and APC-anti-CD3 (UCHT1), PerCP-anti-CD3 (SK7), APC-anti-CD4 (RPA-T4), FITC-anti-CD45RO (UCHL1), APC-anti-CD27 (L128), FITC and PE-anti-CD19 (HIB19), PE-anti-CD16 (Leu-11c), FITC and PE-Cy5-anti-CD25 (M-A251), FITC and APC-anti-CD45RO (UCHL1), FITC and APC-anti-CD56 (MCAM16·2), FITC-anti-CD107a (H4A3), PerCP-anti-CD14 (MϕP9), FITC-anti-CD14 (M5E2), APC-Cy7-anti-CD8 (SK1), PerCP and PE-labelled streptavidin anti-biotin, PE-anti-interferon (IFN)-γ (4S.B3), APC-anti-tumour necrosis factor (TNF)-α (MAb11), APC-anti-interleukin (IL)-2 (5344·111), PE-anti-IL-10 (JES3-9D7), FITC-anti-TCRVβ23 (AHUT7) and FITC-anti-T cell receptor (TCR)Vγ9 (B3) were purchased from BD Biosciences (Franklin Lakes, NJ, USA). FITC-anti-Vβ1 (BL37·2), FITC-anti-Vβ2 (MPB2D5), FITC-anti-TCRVβ3 (CH92), FITC-anti-TCRVβ7·1 (ZOE), FITC-anti-TCRVβ8 (56C5·2), FITC-anti-Vβ11 (C21), FITC-anti-Vβ12 (VER2·32·1), FITC-anti-Vβ13·1 (IMMU 222), FITC-anti-TCRVβ14 (CAS1·1.3), FITC-anti-TCRVβ16 (TAMAYA1·2), FITC-anti-Vβ17 (E17·5F3·15·13), FITC-anti-TCRVβ20 (ELL1·4), FITC-anti-Vβ22 (IMMU 546) and PE-anti-CD85j (HP-F1) were purchased from Beckman Coulter (Fullerton, CA, USA), and PE-anti-CCR7 (150503) was from R&D Systems, Inc. (Minneapolis, MN). FITC-anti-Vβ5 (MEM-262) and biotin-labelled anti-HLA class I A3 (4i153) were purchased from Abcam (Cambridge, UK) and FITC-anti-forkhead box P3 (FoxP3) (236A/E7) and PE-anti-IL-17A (eBio64DEC17) were from eBioscience, Inc. (San Diego, CA, USA).
Cell surface and intracellular staining for flow cytometry
Blood from cord blood-transplanted patients were separated using density gradient centrifugation (Lymphoprep, Fresenius Kabi, Oslo, Norway) before washing in phosphate-buffered saline (PBS).
Cell surface staining was performed as described previously [29]. Briefly, cells were incubated with indicated antibodies and isotype-matched controls diluted in PBS supplemented with 1% fetal calf serum (FCS) (staining buffer) for 30 min at 4°C and washed before fluorescence activated cell sorter (FACS) analysis.
For intracellular staining, cells were first surface-stained, then fixed, permeabilized and stained with indicated antibodies according to the standard protocol for the BD Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA, USA).
Stained cells were acquired on a BD FACSCalibur or Dako CyAn ADP using CellQuest software (Becton Dickinson Labware, Franklin Lakes, NJ, USA) and Summit software (Dako Colorado, Inc., Fort Collins, CO, USA), respectively. Data were analysed using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Cytokine production and CD107a up-regulation assays
For assessing T cell function, PBMCs were resuspended in complete medium with GolgiStop® (BD Biosciences) and incubated with 250 nM ionomycin calcium salt and 50 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, Schnelldorf, Germany) for 8 h at 37°C in 5% CO2. Alternatively, for NK cell function, PBMCs were resuspended in complete medium with GolgiStop® and incubated with 50 ng/ml PMA or K562 cells at a ratio of 1:1 for 4 h at 37°C in 5% CO2. After incubation, cells were harvested, washed with PBS and stained for FACS analysis.
Statistical analysis
Data were analysed and displayed using Prism (GraphPad, San Diego, CA, USA) and Excel (Microsoft Corp, Redmond, WA, USA) software. Data are presented as absolute % or mean % ± standard error of the mean (s.e.m.), if not stated otherwise.
Results
Stable donor–donor chimerism in patient 1119 and 1173
Chimerism analyses are performed routinely to monitor engraftment, relapse and possible rejections after allo-HCT [27]. Donor- and recipient-derived haematopoiesis were measured as relative percentages of CD3+, CD19+ and CD33+ cells in peripheral blood, as described in Material and methods. Figure 1a shows CD33+ CD19+ and CD3+ cells in patients 1119 and 1173, indicating a fluctuating but constant double donor–donor chimerism over time in both patients (28 and 45 months, respectively). The following analyses were performed with blood samples from months 25 to 26 (1173) and 42, 43 and 55 (1119) post-transplantation, respectively.
Fig. 1.
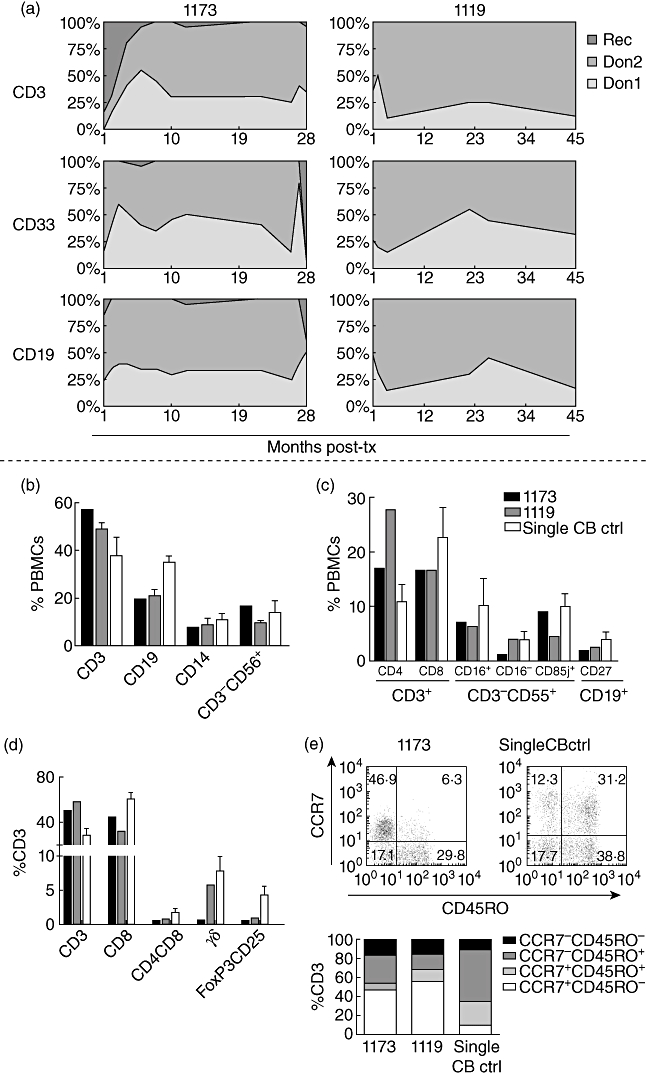
Cord blood-transplanted patients with a stable donor–donor chimerism have reduced frequencies of regulatory-, γδ- and CD4+CD8+ memory T cells, as well as a more naive T cell phenotype compared to patients with only one donor prevailing. (a) Chimerism analysis of CD33+, CD19+ and CD3+ cells from patients 1119 and 1173, showing a stable double donor–donor chimerism over time (28 and 45 months, respectively). The following analyses were performed with blood samples from months 25 to 26 (1173), and 42, 43 and 55 (1119) post-transplantation, respectively. (b,c) Flow cytometry analysis of different cell populations indicated on the x-axis, comparing patients 1119 and 1173 and single cord blood controls. Data are presented as % of peripheral blood mononuclear cells (PBMCs) for 1173, and mean % of PBMCs for 1119 (n ≥ 2) and the single cord blood controls (n = 4). (d) % of CD3+ T cell subpopulations indicated on the x-axis. Data are presented as % of CD3+ cells for 1173, and mean % of CD3+ cells for 1119 (n ≥ 2) and the single cord blood controls (n = 4). (e) T cell maturation profile (from least to most mature: CCR7+CD45RO-; CCR7+CD45RO+; CCR7-CD45RO+; CCR7-CD45RO-) for 1119 and 1173 compared to single cord blood controls, as indicated on the x-axis. Data are presented as % of CD3 cells for 1173, and mean % of CD3+ cells for 1119 (n = 3) and the single cord blood controls (n = 4).
Patients 1119 and 1173 have reduced frequencies of γδ-, regulatory- and CD4+CD8+ memory T cells compared to patients with only one donor unit prevailing
The immune reconstitution is important for the clinical outcome post-SC. Therefore, the immune cell composition of the patients with donor–donor chimerism was compared to the composition in individuals with a single donor chimerism after SCT.
Flow cytometry analysis revealed that the frequencies of T, B, NK cells and monocytes (CD3+, CD19+, CD3-CD56+ and CD14+, respectively) did not differ markedly between donor–donor mixed chimerism patients and single cord blood controls (n = 4) (Fig. 1b). The same comparability was displayed for subtypes such as the ‘dim’ and ‘bright’ NK cells (CD3-CD56+CD16+/−), LIR-1+ NK cells (CD3-CD56+CD85j+), and memory B cells (CD19+CD27+), although the CD4+/CD8+ ratios were somewhat inversed (Fig. 1c and d).
Interestingly, the frequencies of the TCR-γδ T cells (0·6% and 5·7 ± 1·7%, n = 3 versus 7·9 ± 2·0%, n = 4), regulatory FoxP3+CD25+ T cells (0·4% and 0·9%, n = 2 versus 4·3 ± 1·2%, n = 4) and the CD4+CD8+ memory T cells (0·4% and 0·7%, n = 2 versus 1·2 ± 0·6%, n = 4) were lower in the donor–donor mixed chimerism patients compared to the control group (Fig. 1d).
Patients 1119 and 1173 have a more naive T cell phenotype compared to patients with only one donor prevailing
Expression of cell surface markers CD45RO and CCR7 has been utilized to characterize different subsets of CD3+ T cells [30,31]: naive CCR7+CD45RO- cells, central memory CCR7+CD45RO+ cells, effector memory CCR7-CD45RO+ cells and terminally differentiated CCR7-CD45RO- cells. The T cell maturation profile for the donor–donor mixed chimerism patients displayed a more naive phenotype, with noticeably higher frequencies of naive CCR7+CD45RO- cells (46·9% and 55·7%, n = 2 versus 9·8 ± 5·8%, n = 4) and fewer CCR7-CD45RO+ effector memory cells (29·8% and 16·6% versus 54·3 ± 10·5%) compared to single cord blood controls (Fig. 1e).
The stable donor–donor chimerism is composed of a predominating major unit with higher frequencies of γδ- and CD4+CD8+ memory T cells, and a smaller minor unit with higher amounts of NK cells and naive T cells
To investigate further the immunological properties of donor–donor chimerism, a comparison of the cord blood units within each of the patients was also performed, in order to distinguish possible individual phenotypes. The chimerism analyses indicated a fluctuation of the two units over time (Fig. 1a). At the time of the present study, flow cytometric typing assay with HLA-A3 antibodies for patient 1119 and PCR-based chimerism analysis of cell subpopulations for patient 1173 (as described in Materials and methods) showed that both patients 1119 (76·5% ± 6·1 versus 23·4 ± 6·1%, n = 3) and 1173 (53·4% and 40·9%) had a major and a minor unit (Fig. 2a and b).
Fig. 2.
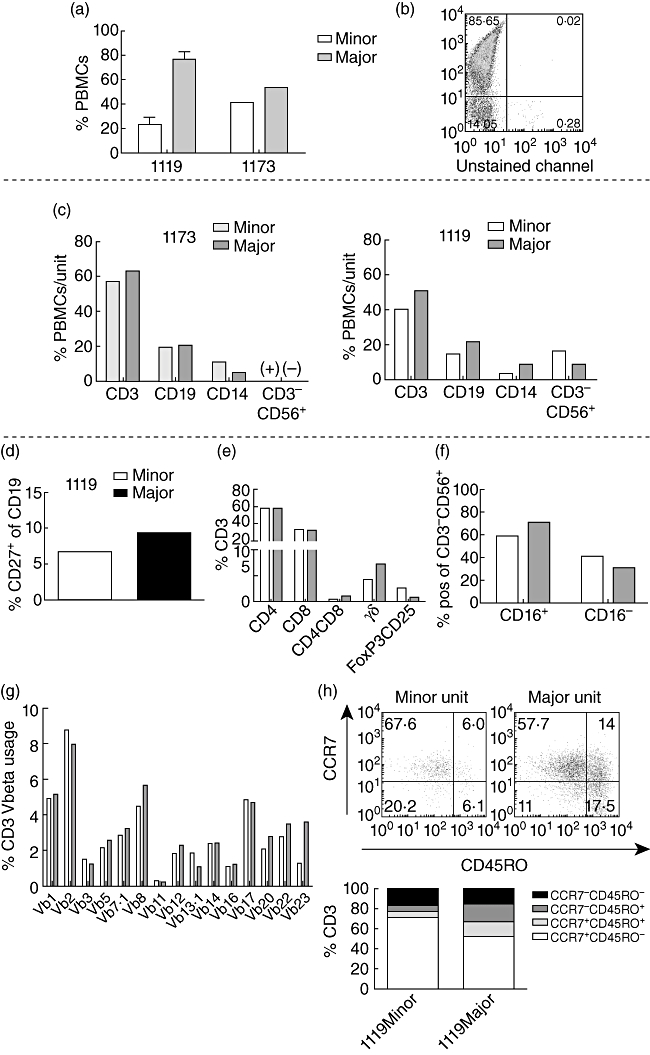
The stable donor–fonor chimerism is composed of a predominating major unit with higher frequencies of γδ- and CD4+CD8+ memory T cells, and a smaller minor unit with higher amounts of natural killer (NK) cells and more naive T cells. Analyses were performed with blood samples from months 25 to 26 (patient 1173) and 42, 43 and 55 (patient 1119) post-transplantation, respectively. (a,b) Mean % (1119) and % (1173) of peripheral blood mononuclear cells (PBMCs) from the major and minor cord blood units in both patients at the occasions of the analyses. (b) For patient 1119, flow cytometric typing assay with human leucocyte antigen (HLA)-A3 antibodies were utilized to separate the two HLA-A mismatched units. (c) An extension of the analysis in (a) for both patients. Frequencies of cell populations indicated on the x-axes are displayed as mean % (1119, n = 3) or % (1173) of PBMCs within each unit (major and minor). The CD3-CD56+ populations are shown as (+) or (−) in patient 1173 due to low signal strength. (d–h) Flow cytometric typing assay for patient 1119 only (n ≥ 2). (d) Mean % memory CD27+ cells of CD19+ B cells from each unit (major and minor). (e) Mean % of CD3+ T cell subpopulations indicated on the x-axis. (f) Mean % of CD16+/− cells within the CD3-CD56+ natural killer (NK) cell population in each unit. (g) Flow cytometry typing assay for T cell receptor (TCR) Vβ usage. Data are presented as % CD3+ cells positive for different Vβ indicated on the x-axis. (h) T cell maturation profile (from least mature to most mature: CCR7+CD45RO-; CCR7+CD45RO+; CCR7-CD45RO+; CCR7-CD45RO-) comparing the two units. Data are presented as mean % of CD3+ cells.
No common trend could be observed regarding the frequencies of CD3+ (39·8 ± 7·4% versus 51·0 ± 6·0%, n = 3 and 57·0% versus 62·8%), CD19+ (14·3% versus 21·3%, n = 2 and 19·6% versus 20·8%) and CD14+ (3·1% versus 8·2%, n = 2 and 11·1% versus 5·2%) cells between the units in both patients (Fig. 2c).
However, the minor unit in both patients displayed a higher prevalence of CD3-CD56+ NK cells [16·5% versus 8·7%, n = 2 in 1119 (+) versus (−) in patient 1173 due to low signal strength] (Fig. 2c).
The patient with acute myeloid leukaemia (AML), 1173, was unable to be included in further analyses due to a later relapse (seen as an increase of CD33+ cells of recipient origin in Fig. 1a). Hence, the following flow cytometry typing assays were performed on patient 1119 only.
The minor unit presented with lower frequencies of CD19+CD27+ memory B cells (6·7% versus 9·3%, n = 2), CD4+CD8+ memory T cells (0·4% versus 0·9%, n = 2), TCR-γδ T cells (4·1 ± 1·5% versus 7·1 ± 2·0%, n = 3) but higher frequency of regulatory FoxP3+CD25+ T cells (2·5% versus 0·7%, n = 2) (Fig. 2d and e). The frequencies of CD3-CD56+CD16-‘bright’ NK cells were comparable (41·4% versus 30·0%, n = 2) (Fig. 2f).
TCRVbeta usage distribution of the two cord blood units does not differ markedly
In order to study the TCR repertoire of the two cord blood units, flow cytometry typing assay using 15 different Vβ antibodies was performed. The analysis indicated comparable polyclonal patterns between the two units (Fig. 2g).
The minor unit has a more naive T cell profile
Differences in the T cell maturation profile of the two cord blood units was investigated by flow cytometry of cell surface markers CCR7 and CD45RO. The minor unit displayed lower frequencies of both CCR7+CD45RO+ central memory (5·1% versus 13·2%, n = 2) and CCR7-CD45RO+ effector memory T cells (6·3% versus 18·4%), and higher frequencies of naive CCR7+CD45RO- T cells (71·7% versus 52·7%), compared to the major unit (Fig. 2h).
Patient T cells 1119 produce less IFN-γ, TNF-α and IL-2 compared to single cord blood controls
Study of the functionality of different cell subsets is equally important as study of frequencies, both to compare patient 1119 with single cord blood controls, but also to study whether the two units within patient 1119 function in a similar manner.
PBMCs were stimulated with ionomycin and PMA and incubated for 8 h at 37°C, then stained for different cytokines. A representative dot plot of CD3+ T cell cytokine production is shown in the upper part of Fig. 3a. The frequency of T cells producing IFN-γ (3·4 ± 1·7% versus 13 ± 4·1%), TNF-α (2·7 ± 1·1% versus 16·2 ± 5·1%) and IL-2 (0·4 ± 0·2% versus 3·6 ± 1·2%) was lower in patient 1119 (n = 3), while T cells producing IL-17 (0·8 ± 0·4% versus 1·4 ± 0·5%) was comparable to T cells of single cord blood controls (n = 4) (Fig. 3a).
Fig. 3.
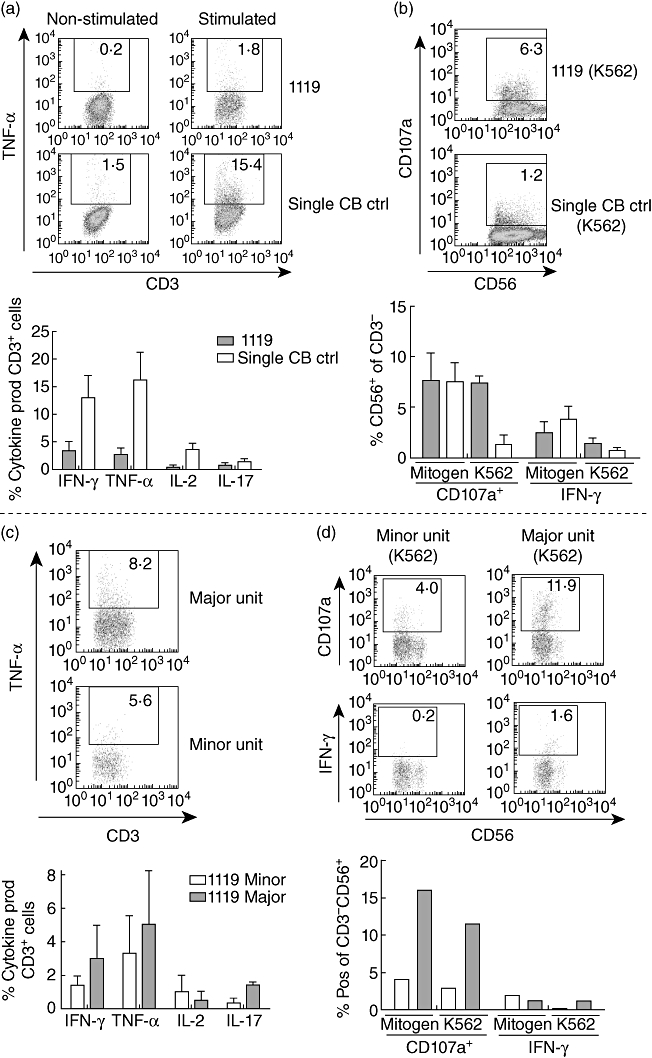
T cells but not natural killer (NK) cells of patient 1119 produce fewer cytokines compared to single cord blood controls, and the minor unit produces fewer cytokines than the major, with the exception of interleukin (IL)-2. Analyses were performed with blood samples from months 42, 43 and 55 post-transplantation for patient 1119. Peripheral blood mononuclear cells (PBMCs) were stimulated with (a,c) ionomycin and phorbol 12-myristate 13-acetate (PMA) and incubated for 8 h, or (b,d) mitogen or K562 for 4 h, at 37°C and stained with indicated antibodies. (a) The cytokine production by T cells in response to ionomycin and PMA of patient 1119 (n = 3) compared to single cord blood controls (n = 4). (b) Interferon (IFN)-γ production and CD107a up-regulation in NK cells of patient 1119 (n = 3) compared to single cord blood controls (n = 4). (c) Comparison of cytokine production by T cells of the two units in patient 1119 (n = 2). (d) IFN-γ production and CD107a up-regulation in NK cells of the two units in patient 1119 (n = 2).
The NK cells of patient 1119 are equally responsive to single cord blood controls when stimulated with mitogen, but not with K562 target cells
PBMCs were stimulated with PMA or K562 cells for 4 h at 37°C and then stained for FACS analysis. A representative dot plot of CD107a up-regulation upon K562 stimulation in CD3-CD56+ NK cells is shown in Fig. 3b. The frequencies of NK cells with up-regulated CD107a (7·6 ± 2·7% versus 7·5 ± 1·9%) and IFN-γ production (2·5 ± 1·1% versus 3·8 ± 1·3%) were comparable between patient 1119 (n = 3) and single cord blood controls (n = 4) when stimulated with PMA. However, when stimulated with K562 cells that lack major histocompatibility complex (MHC) class I molecules, a higher percentage of NK cells in patient 1119 up-regulated CD107a compared to the NK cells of single cord blood controls (7·4 ± 0·6% versus 1·3 ± 1·0%). This trend of higher responsiveness in the NK cells of patient 1119 was not as pronounced when looking at IFN-γ production (1·3 ± 0·6% versus 0·7 ± 0·4%) (Fig. 3b).
Minor unit T cells produce fewer cytokines than the major unit, with the exception of IL-2
T cell cytokine production in response to ionomycin and PMA was also investigated for the two cord blood units (Fig. 3c, n = 2). The T cells of the major unit tended to produce more IFN-γ (3·0% versus 1·4%), TNF-α (5·0% versus 3·4%) and IL-17 (1·4% versus 0·3%). In contrast, the minor unit responded with slightly higher frequencies of IL-2-producing cells (1·0% versus 0·5%).
Major unit NK cells are more responsive than those of the minor unit
NK cell function of the two units in patient 1119 was characterized by CD107a up-regulation and IFN-γ production in response to stimulation with mitogen or K562 cells. The majority of NK cells which up-regulated CD107a upon 4 h of stimulation with mitogen (15·9% versus 4·1%, n = 2) or K562 (11·5% versus 2·8%, n = 2) were found in the major unit. This trend could also be seen regarding IFN-γ production upon stimulation with K562 cells (1·2% versus 0·1%), while no difference was seen when stimulated with mitogen (Fig. 3d). A representative dot plot is shown of NK cell response to K562 cells in both the major and minor unit.
HLA-C typing
NK cell function is regulated partly by inhibitory and activating killer cell immunoglobulin-like receptors (KIRs) that recognize certain HLA-C molecules [19]. Hence, HLA-C match might induce tolerance between immune systems.
The donor units of 1119 were both HLA-C 05/07, and the units of patient 1173 were both 12/06 (Table 1). Cord blood units of patients after DCBT with one single unit prevailing were used as control and were not matched for HLA-C, with the exception of patient 1174, where both units were HLA-C 03/04.
Discussion
Double cord blood transplantations has been demonstrated to be comparable to single cord blood transplantation regarding outcome [32], and despite an overall higher incidence of infections and delayed CD8+ T cell recovery after single umbilical cord blood transplantation (UCBT), non-relapse mortality and overall survival have been shown to be similar to that reported for other haematopoietic stem cell sources [4,33,34].
Stable mixed donor–donor chimerism after double cord blood transplantation is a very rare event, with the great majority of transplantations resulting in only one single unit prevailing [10,11]. The dose of CD3+ cells in the graft as well as an immune rejection mediated by CD8+ T cells in the winning unit have been suggested to predict which of the two cord blood units in a double transplantation eventually prevails [10,35]. Conversely, a study by Brunstein et al. indicated that neither total nucleated, CD34+ or CD3+ cell doses, HLA-matching, nucleated cell viability, ABO typing, gender match nor order of unit infusion is predictive of which unit will prevail [11]. In other words, to date, there is no solid predictive factor for unit dominance, and hence no ready explanation for the co-existence of both units. We have proposed previously that high-dose ATG after DCBT might induce tolerance and mixed donor–donor chimerism [13]. The present study extends this hypothesis by suggesting that HLA-C match and possible NK cell tolerance between units in combination with high-dose ATG increases the likelihood of stable donor–donor chimerism.
When NK cells are confronted with allogeneic targets which do not express inhibiting ligands, NK cell alloreactivity may occur. Thus, a match of HLA-C might produce a more tolerant relationship between the two immune systems after DCBT.
HLA-C/KIR mismatch between donor and recipient has been shown to be beneficial for graft versus leukaemia effect and hence to control relapse in haploidentical allo-HCT [36], although there are some contradictory reports regarding unrelated HLA-mismatched allo-HCT [37,38]. Concerning UCBT, HLA-C/KIR mismatch has been associated with less relapse as well as better overall survival [39]. Moreover, a lower relapse risk has also been reported for DCBT in comparison to single UCBT, regardless of HLA-C [40].
Here we show that the donor units of both patients with stable donor–donor chimerism were matched at HLA-C, strengthening our hypothesis of tolerance induction (Table 1). We could not observe an HLA-C match in three of the DCBT patients with a single unit prevailing. A plausible reason why the fourth control patient, 1174, despite having HLA-C matched cord blood units, did not show a stable mixed chimerism is an extremely high inter-unit difference in CD34+ numbers (22 to 1) and TNC (3 to 1) in favour of the prevailing unit [13].
Interestingly, the functional assays showed that the NK cells of patient 1119 were at least as responsive as the NK cells of the single cord blood controls when stimulated with mitogen. This finding proposes that even though the NK cells of the two units in patient 1119 seem to tolerate each other, the potential of an NK cell response per se is not impaired. When studying the response against functional targets such as K562 cells, the NK cells of patient 1119 show an even greater response than single cord blood controls, possibly demonstrating the importance of NK cell function in these patients.
After UCBT, the reconstitution of T cells is delayed, allowing NK cells to expand [18,41]. This delay is amplified further by the high-dose ATG [17]. Using a high-dose ATG regimen in a DCBT setting, where in addition the two cord blood units are matched at the HLA-C locus, thus seems to produce an environment favourable for developing tolerance and mixed donor–donor chimerism.
In this study, an immune system with long-term mixed donor–donor chimerism appears to be composed of two units distinct in form and function: a minor unit with slightly higher frequencies of NK cells, and a predominant major unit with a phenotype approaching that of a single prevailing cord blood unit. When looking at the individual responsiveness of the two units there seems to be a higher capacity of NK cells and especially T cells of the major unit to respond to different stimuli, suggesting that the major unit is indeed more similar to a single chimerism immune system.
This study demonstrates that donor–donor chimerism does not seem to conduct any functional advantage with regard to cytokine production in comparison to the immune systems of cord blood-transplanted patients with a single prevailing cord blood unit. In addition, the donor–donor chimerism patient has a more naive T cell phenotype and presents with fewer CCR7-CD45RO+, γδ- and CD4+CD8+ memory T cells compared to single cord blood controls. While others have reported a positive correlation of regulatory T cells and increased frequency of mixed recipient–donor chimerism [42], the present study does not concur. It is of importance, however, to consider that the patients in this study have donor–donor chimerism and not recipient–donor, and additionally that these patients are studied years after transplantation. The importance of regulatory T cells for tolerance induction might be more significant during the early months of immune reconstitution. Finally, the low frequency of regulatory T cells in the patients with donor–donor chimerism is reflected by their higher frequencies of naive T cells. Regulatory T cells are antigen-experienced, and as seen in the T cell maturation profile (Fig. 1e), patients 1119 and 1173 have large proportions of antigen-inexperienced T cells.
In summary, the present study shows that the two units in a stable mixed donor–donor chimerism are functionally and phenotypically different, and that conjunctly they are more naive than single cord blood controls. Moreover, the likelihood of a stable donor–donor chimerism in DCBT may be increased when the donor units are matched at the HLA-C locus and the patient is treated with high-dose ATG.
Acknowledgments
This work was supported by the Children's Cancer Foundation (PROJ08/013), ALF Gothenburg, the Stockholm County Council, SSMF and Karolinska Institutet.
Disclosure
The authors report no potential conflicts of interest.
References
- 1.Knudtzon S. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood. 1974;43:357–61. [PubMed] [Google Scholar]
- 2.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12:34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–53. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109:1322–30. doi: 10.1182/blood-2006-04-020172. [DOI] [PubMed] [Google Scholar]
- 6.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 7.Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–60. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–5. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–9. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 11.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson J, Uzunel M, Brune M, et al. Mixed chimaerism is common at the time of acute graft-versus-host disease and disease response in patients receiving non-myeloablative conditioning and allogeneic stem cell transplantation. Br J Haematol. 2001;115:935–44. doi: 10.1046/j.1365-2141.2001.03174.x. [DOI] [PubMed] [Google Scholar]
- 13.Berglund S, Okas M, Gertow J, Uhlin M, Mattsson J. Stable mixed donor-donor chimerism after double cord blood transplantation. Int J Hematol. 2009;90:526–31. doi: 10.1007/s12185-009-0398-y. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 15.De Lima M, St John LS, Wieder ED, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–6. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 16.Yen HJ, Chiou TJ, Hung GY, et al. Long-term mixed full-donor chimerism with dominance reversion after a double-unit cord blood transplant. Eur J Haematol. 2008;80:366–7. doi: 10.1111/j.1600-0609.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 17.Remberger M, Mattsson J, Svahn BM, Ringden O. Using reduced intensity conditioning and HLA-identical sibling donors, antithymocyte globulin increases the risk of relapse, which can be overcome by a high stem cell dose. Bone Marrow Transplant. 2008;42:769–71. doi: 10.1038/bmt.2008.246. [DOI] [PubMed] [Google Scholar]
- 18.Velardi A, Ruggeri L, Mancusi A, et al. Clinical impact of natural killer cell reconstitution after allogeneic hematopoietic transplantation. Semin Immunopathol. 2008;30:489–503. doi: 10.1007/s00281-008-0136-1. [DOI] [PubMed] [Google Scholar]
- 19.Schellekens J, Rozemuller EH, Petersen EJ, van den Tweel JG, Verdonck LF, Tilanus MG. Patients benefit from the addition of KIR repertoire data to the donor selection procedure for unrelated haematopoietic stem cell transplantation. Mol Immunol. 2008;45:981–9. doi: 10.1016/j.molimm.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–201. [PubMed] [Google Scholar]
- 21.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–7. [PubMed] [Google Scholar]
- 22.Schaffer M, Aldener-Cannava A, Remberger M, Ringden O, Olerup O. Roles of HLA-B, HLA-C and HLA-DPA1 incompatibilities in the outcome of unrelated stem-cell transplantation. Tissue Antigens. 2003;62:243–50. doi: 10.1034/j.1399-0039.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 23.Ringden O, Okas M, Uhlin M, Uzunel M, Remberger M, Mattsson J. Unrelated cord blood and mismatched unrelated volunteer donor transplants, two alternatives in patients who lack an HLA-identical donor. Bone Marrow Transplant. 2008;42:643–8. doi: 10.1038/bmt.2008.239. [DOI] [PubMed] [Google Scholar]
- 24.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–14. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 26.Svahn BM, Remberger M, Myrback KE, et al. Home care during the pancytopenic phase after allogeneic hematopoietic stem cell transplantation is advantageous compared with hospital care. Blood. 2002;100:4317–24. doi: 10.1182/blood-2002-03-0801. [DOI] [PubMed] [Google Scholar]
- 27.Winiarski J, Mattsson J, Gustafsson A, et al. Engraftment and chimerism, particularly of T- and B-cells, in children undergoing allogeneic bone marrow transplantation. Pediatr Transplant. 1998;2:150–6. [PubMed] [Google Scholar]
- 28.Mattsson J, Uzunel M, Tammik L, Aschan J, Ringden O. Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia. 2001;15:1976–85. doi: 10.1038/sj.leu.2402311. [DOI] [PubMed] [Google Scholar]
- 29.Uhlin M, Masucci MG, Levitsky V. Regulation of lck degradation and refractory state in CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2005;102:9264–9. doi: 10.1073/pnas.0406333102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caron G, Duluc D, Fremaux I, et al. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–7. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 31.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–18. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradstock K, Hertzberg M, Kerridge I, et al. Single versus double unrelated umbilical cord blood units for allogeneic transplantation in adults with advanced hematological malignancies: a retrospective comparison of outcomes. Intern Med J. 2009;39:744–51. doi: 10.1111/j.1445-5994.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 33.van Burik JA, Brunstein CG. Infectious complications following unrelated cord blood transplantation. Vox Sang. 2007;92:289–96. doi: 10.1111/j.1423-0410.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–11. [PubMed] [Google Scholar]
- 35.Gutman JA, Turtle CJ, Manley TJ, et al. Single unit dominance following double unit umbilical cord blood transplantation coincides with a specific CD8+ T cell response against the non-engrafted unit. Blood. 2010;115:757–65. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 37.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–19. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 38.Schaffer M, Malmberg KJ, Ringden O, Ljunggren HG, Remberger M. Increased infection-related mortality in KIR-ligand-mismatched unrelated allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78:1081–5. doi: 10.1097/01.tp.0000137103.19717.86. [DOI] [PubMed] [Google Scholar]
- 39.Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft versus leukemia effect in recipients of two units. Blood. 2009;114:4293–9. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng J, Sun ZM, Liu HL, Geng LQ, Wang XB. [Reconstitution of NK cells and their receptors in patients with acute leukemia following unrelated cord blood stem cell transplantation] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:426–30. [PubMed] [Google Scholar]
- 42.Serafini G, Andreani M, Testi M, et al. Type 1 regulatory T cells are associated with persistent split erythroid/lymphoid chimerism after allogeneic hematopoietic stem cell transplantation for thalassemia. Haematologica. 2009;94:1415–26. doi: 10.3324/haematol.2008.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]


