Abstract
The aim of this study was to investigate the effect of interferon (IFN)-α on recruitment of platelets and monocytes within the murine small intestinal venular endothelium. Monocytes were isolated from bone marrow of C57B6 mice. Platelets were collected from murine blood. Rolling and adhesion to submucosal microvessels in the small intestine were examined under an intravital fluorescence microscope after injection of fluorescein-labelled monocytes or platelets. In some mice, IFN-α (5 × 105U/kg) was administered intraperitoneally. After treatment with an antibody against P-selectin, changes in monocyte and platelet migration were also investigated. Changes in monocyte migration under the condition of thrombocytopenia were also investigated. Platelets and monocytes interacted with murine intestinal microvessels, although only few platelets and monocytes showed migration behaviour. Intraperitoneal injection of IFN-α enhanced the migration of both platelets and monocytes in the intestinal microvessels. Pretreatment with anti-P-selectin attenuated the increase in migration of platelets and monocytes induced by administration of IFN-α. Thrombocytopenia decreased the rolling ratio of monocytes, suggesting that the effect of IFN-α on migration was P-selectin-dependent, derived from both the endothelium of microvessels and platelets. The results of this study suggest that IFN-α acts as a potent proinflammatory agent via its stimulatory effect on the endothelium–platelet–monocyte interaction in intestinal microvessels by a P-selectin-dependent mechanism.
Keywords: ileum, inflammation, interferon, mice, monocytes, platelets
Introduction
Interferon (IFN)-α has several biological actions, including activation of macrophages, decrease in division of tumour cells and anti-viral activity. IFN-α is used for treatment of diseases with diverse pathogeneses and manifestations, including chronic viral hepatitis B or C, chronic myelogenous leukaemia, hairy cell leukaemia, Kaposi's sarcoma, laryngeal and genital papillomas and various angiogenic diseases [1]. IFN-α is a multi-functional immunomodulatory cytokine and its mechanism is thought to be mediated by regulating and down-modulating T helper type 2 (Th2) cytokines [2,3]. IFN-α has been shown to be effective for treatment of ulcerative colitis (UC) [4,5]. However, some prospective studies have shown that IFN-α was not effective for treatment of inflammatory bowel disease (IBD), and induction and exacerbation of UC or Crohn's disease (CD) by IFN-α during treatment of chronic hepatitis C have also been reported. Thus, the immunological effect of IFN-α on IBD is still a controversial issue. Various side effects and toxicities of IFN-α have been observed in all clinical studies to date. Retinal haemorrhage [6] and myocardial infarction [7,8] have been reported as adverse effects. Guyer et al. reported cases of retinal ischaemic retinopathy, characterized by cotton-wool spot formation, capillary non-perfusion, arteriolar occlusion and haemorrhage, associated with the use of IFN-α. In addition, we have reported previously that IFN-α increased leucocyte–endothelial interaction in the mesenteric microcirculation of rats in vivo, suggesting that IFN-α has a proinflammatory effect by increasing leucocyte recruitment. We have reported previously in vivo behaviour of monocyte migration in the murine intestinal mucosa [9], and that blockade of monocyte migration to the intestine ameliorated inflammation in experimental chronic ileitis [10]. Recently, we have shown that platelets contribute to the inflammatory condition in which monocytes are involved via platelet–monocyte interaction in lipopolysaccharide (LPS)-induced acute ileitis [11]. In addition, we demonstrated that control of platelet recruitment ameliorates chronic murine ileitis by decreasing monocyte migration [12]. Because thrombocytopenia is usually seen in patients treated with IFN, we hypothesized that IFN enhances platelet–endothelial interaction, evoking a proinflammatory effect of monocytes by increasing monocyte recruitment to the intestinal mucosa. The objective of this study was to assess the influence of IFN-α on microcirculation in the small intestine, focusing on platelet and monocyte interactions with the venular endothelium.
Materials and methods
Animals
Male C57B6 mice, 8–10 weeks old (Clea Japan, Tokyo Japan), were maintained on standard laboratory chow (SLC, Tokyo, Japan) and in specific pathogen-free conditions. The care and use of laboratory animals were in accordance with the guidelines of the animal facility in National Defense Medical College (NDMC). This study protocol was approved by Animal Ethical Committee of NDMC (no. 08103).
Isolation of monocytes and plateles and labelling with carboxyfluorescein diacetate succinimidyl ester (CFSDE)
Monocytes were isolated from the bone marrow of murine thigh and labelled as described previously[11,12]. Briefly, bone marrow cells were obtained from thigh bone of C57B6 mice and monocytes were isolated by magnetic activated cell sorting (MACS; Miltenyi Biotec, Auburn, CA, USA) with beads-conjugated anti-rabbit CD11b polyclonal antibody (Miltenyi Biotec). The purity of monocytes and uniformity of the isolation procedure were compared between batches by a fluorescence-activated cell sorter (FACSCalibur; Becton-Dickinson, Mountain View, CA, USA) using rabbit anti-mouse CD14 polyclonal antibody (Santa Cruz Biotec, Santa Cruz, CA, USA) and confirmed that approximately 94% of CD11b+ cells from each batch expressed CD14. Platelets were isolated from blood of donor mice, as described previously (H26, H27 [13,14]). Blood from the mice was collected from the heart and platelets were isolated by centrifugation at 600 g with 0·1 ml acid citrate dextrose buffer. The expression of P-selectin on platelets was compared between batches by FACS using rat anti-mouse P-selectin (RB40.34; BD PharMingen, San Diego, CA, USA) and confirmed that expression of P-selectin did not differ between batches. CFDSE (Molecular Probes, Eugene, OR, USA) was dissolved in dimethylsulphoxide at 15·6 mM, divided into small aliquots (each 300 µl), and stored in a cuvette sealed with argon gas at −20°C until experimental use. Monocytes (approximately 2 × 107) in 1·5 ml of phosphate-buffered saline (PBS) were incubated with CFDSE solution for 10 min at 4°C and washed with PBS. Platelets (approximately 1 × 108) were incubated with CFDSE solution for 10 min at 4°C and washed with PBS.
Animal preparation for intravital observation
For migration studies, mice were anaesthetized with 50 mg/kg pentobarbital sodium, and the abdomen of each mouse was opened with a midline incision. An ileal segment 1–3 cm in length was selected for observation. The intestine was kept warm and moist by continuous superfusion with PBS warmed to 37°C. PBS was injected into the selected segment using a 30-gauge needle. The behaviour of monocytes and platelets in submucosal venules was observed from the serosal side using an intravital microscope. The behaviour of CFDSE-labelled monocytes and platelets was visualized on a monitor through a silicon-intensified target image tube (SIT) system, using a previously described method, and recorded on a digital hard disk recorder [4]. Microcirculation was observed by fluorescence microscope (BX51WI; Olympus, Tokyo, Japan) equipped with a contrast-enhancing unit (C-2400-08; Hamamatsu Photonics, Shizuoka, Japan) and × 10 ultraviolet-fluorite objective lens (Uplan Fl; Olympus, Tokyo, Japan).
Administration of IFN-α and monoclonal antibodies
IFN-α (5 × 105 U/Kg) was administered intraperitoneally 2 h before CFDSE-labelled monocytes or platelets were injected via a cervical vein. In some experiments, 2 mg/kg of rat anti-mouse P-selectin (RB40.34; BD PharMingen, San Diego, CA, USA) was administered via a cervical vein 5 min prior to CFDSE-labelled monocyte administration [9–11,15]. To induce thrombocytopenia, mice were injected intraperitoneally with GPIbα (CD42b) monoclonal antibody (EMFRET Analytics, Würzburg, Germany) with 80 µg in 200 µl PBS 24 h before the initiation of the experiment [11]. This treatment induced severe thrombocytopenia.
Analysis of monocyte and platelet dynamics
The number of adherent monocytes was counted off-line by digital video disk images. Monocytes adhering to vascular walls with or without occasional movements were defined as rolling monocytes. In these rolling monocytes, those which were adhering to vascular walls without movement and remaining stationary for a period of more than 30 s were defined as adherent monocytes. We counted the numbers of total influx, rolling monocytes and adherent monocytes using a ×10 objective lens for 30 min after the injection. The rolling rate was defined as the ratio of rolling monocytes divided by total monocytes and the adhering number was defined as count number of adherent monocytes in 1 mm2. Those platelets adhering to vascular walls without movement and remaining stationary for a period of more than 30 s were defined as adherent platelets. We counted the number of adherent platelets in a 1 mm2 field. In another set of experiments platelets were labelled with CFDSE and monocytes were labelled with rhodamine G. These platelets and monocytes were infused together into recipient mice and the interaction between platelets and monocytes was observed simultaneously by 3CCD camera (VB-7010; Keyence, Osaka, Japan), ×10 ultraviolet-fluorite objective lens (Uplan Fl; Olympus, Tokyo, Japan) and fluorescence mirror unit (UIS2; Olympus, Tokyo, Japan).
Statistics
All results are expressed as means ± standard errors (s.e.) of four or five mice. For comparison of rolling and adhesion, the mean values were evaluated statistically by a non-parametric Mann–Whitney U-test. Statistical significance was defined as P < 0·05.
Results
Interaction of platelets and monocytes with intestinal microvessels
Figure 1a shows in vivo images of fluorescence-labelled platelets in intestinal submucosal microvessels in the control condition obtained using an intravital fluorescein microscope. In the control uninflamed intestine, only a few platelets showed the characteristic rolling behaviour on the surface of microvessels.
Fig. 1.
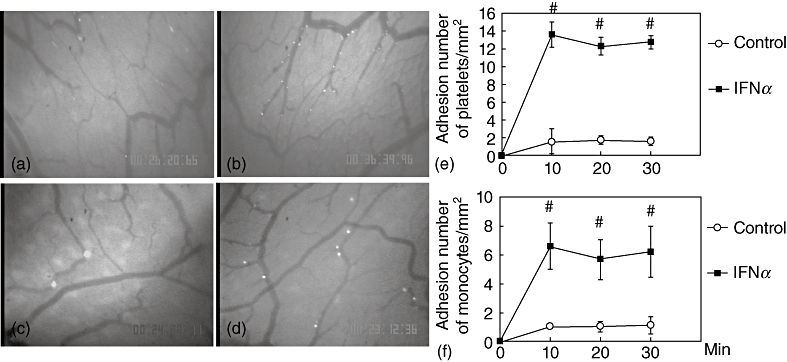
Effect of interferon (IFN)-α on migration of platelets and monocytes. Representative photos of in vivo observation of platelets migration in intestinal microvessels (a,b). In control mice, a small number of labelled platelets adhered to submucosal venules (a). In IFN-α-treated mice, the number of adherent platelets increased significantly (b). Representative photos of in vivo observation of monocyte migration in intestinal microvessels (c,d). In control mice, a small number of labelled monocytes adhered to submucosal venules (c). In IFN-α-treated mice, the number of adherent monocytes increased significantly (d). Time–course of platelets adhesion in the small intestine submucosal venules. IFN-α increased platelets adhesion significantly (e). Time–course of monocytes adhesion in the small intestine submucosal venules. IFN-α increased monocytes adhesion significantly (f). ○: Control;  : IFN-α treated group; #P < 0·05 versus control.
: IFN-α treated group; #P < 0·05 versus control.
Figure 1b shows in vivo images of platelets in IFN-α-treated intestinal submucosal microvessels. The number of rolling platelets increased significantly in IFN-α treated mice. The microvessel diameters were not changed by IFN-α treatment. Figure 1e shows the platelet adhesion time–course to the small intestine microvessels. IFN-α treatment increased the number of adherent platelets significantly (approximately 12–14/mm2) compared with the control group.
Figure 1c shows in vivo images of fluorescence-labelled monocytes in intestinal submucosal microvessels in the control condition obtained by using an intravital fluorescein microscope. In the control uninflamed intestine, only a few monocytes showed characteristic rolling behaviour on the microvessel surface and the monocyte rolling ratio was approximately 21% (Table 1). Figure 1f shows the time–course of monocyte adhesion to the small intestine microvessels. Under the IFN-α treatment condition, the number of adherent monocytes also increased significantly (Fig. 1d,f). The monocyte rolling ratio also increased significantly to approximately 42% (Table 1). The increase in rolling fluorescein-labelled monocytes was observed 10 min after infusion and continued during the observation period.
Table 1.
The rolling ratio of fluorescence-labelled monocytes in submucosal venules of small intestine.
| Rolling ratio (%) | |
|---|---|
| Control | 21 ± 2 |
| IFN-α | 42 ± 2# |
| IFN-α + anti-P-selectin | 21 ± 3* |
| IFN-α + anti-platelet | 30 ± 2* |
Effect of interferon (IFN)-α and monoclonal antibodies. Rolling monocytes were counted for 30 min after infusion of 5,6-carboxy-succinimidyl fluorescein-ester (CFSE)-labelled monocytes. Rolling ratio was expressed as percentage of total number of monocytes appearing.
P < 0·05 versus control;
P < 0·05 versus IFN-α-treated group.
Effect of pretreatment with anti-adhesion molecules on IFN-α-induced platelet adhesion
To investigate the mechanism by which platelet adhesion to intestinal microvessels is enhanced by IFN-α, the effect of an anti-P-selectin antibody was investigated. Fig. 2a shows the effects of pretreatment with anti-P-selectin monoclonal antibody (mAb) on enhanced platelet migration. As shown in this image, the number of adherent platelets was decreased significantly by pretreatment with anti-P-selectin mAb. The time–course of the effect by anti-P-selectin on platelet adhesion is shown in Fig. 2b. Enhanced platelet adhesion by IFN-α was decreased significantly by pretreatment with anti-P-selectin mAb and reached basal levels, showing that IFN-α-induced platelet adhesion to intestinal microvessels was dependent on P-selectin.
Fig. 2.
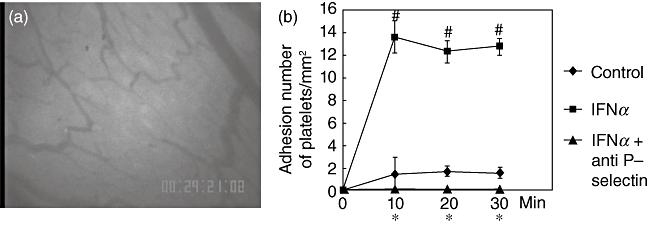
Effect of the anti-P-selectin antibody treatment on enhanced platelet migration which was induced by interferon (IFN)-α treatment. Representative photographs of in vivo observation of platelet migration in intestinal microvessels. The anti-P-selectin antibody treatment to IFN-α-treated animals blocked migration behaviour of platelets (a). Time–course of platelets adhesion in the small intestine submucosal venules. IFN-α increased platelets adhesion significantly. Anti P-selectin antibody attenuated the increase of migration of platelets induced by IFN-α. (b). ○: Control;  : IFN-α-treated group; ▴: IFN-α+ anti-P-selectin antibody-treated group. #P < 0·05 versus control; *P < 0·05 versus IFN-α-treated group.
: IFN-α-treated group; ▴: IFN-α+ anti-P-selectin antibody-treated group. #P < 0·05 versus control; *P < 0·05 versus IFN-α-treated group.
Effect of pretreatment with anti-adhesion molecules on IFN-α-induced monocyte migration
We have reported previously that platelets induced monocyte recruitment to the vascular endothelium by interaction between P-selectin on platelets and PSGl-1 on monocytes [11]. To clarify the platelet involvement in monocyte migration to intestinal microvessels, we examined further whether controlling platelet adhesion could attenuate monocyte migration to intestinal microvessels. For this purpose, the interaction of monocytes with microvessels was observed after treatment with anti-P-selectin mAb, which successfully attenuated IFN-α-induced platelet adhesion to the venular endothelium. As shown in Fig. 3a, IFN-α-induced enhancement of monocyte migration was attenuated by anti-P-selectin mAb. As shown in Fig. 3b, pretreatment with anti-P-selectin antibody attenuated significantly IFN-α-induced monocyte adhesion to intestinal microvessels. Table 1 shows the effects of pretreatment with these mAbs on the monocyte rolling ratio. Anti-P-selectin attenuated significantly the monocyte rolling ratio induced by IFN-α from approximately 42% to 21%. These findings suggest that platelet-derived P-selectin and its ligand on monocytes are also involved in this mechanism.
Fig. 3.
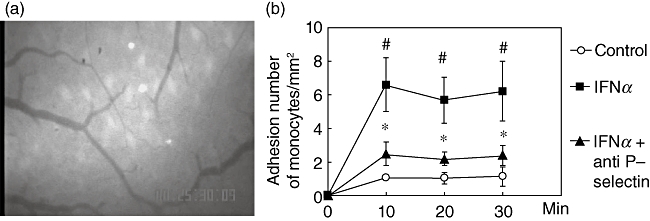
Effect of the anti-P-selectin antibody treatment on enhanced monocyte migration which was induced by interferon (IFN)-α treatment. Representative photographs of in vivo observation of monocyte migration in intestinal microvessels. The anti-P-selectin antibody treatment to IFN-α-treated animals blocked monocyte migration behaviour (a). Time–course of monocyte adhesion in the submucosal venules of small intestine. IFN-α increased monocyte adhesion significantly. Anti P-selectin antibody attenuated the increase of migration of monocytes induced by IFN-α. (b). ○: Control;  : IFN-α treated group; ▴: IFN-α+ anti P-selectin antibody-treated group; #P < 0·05 versus control; *P < 0·05 versus IFN-α-treated group.
: IFN-α treated group; ▴: IFN-α+ anti P-selectin antibody-treated group; #P < 0·05 versus control; *P < 0·05 versus IFN-α-treated group.
Effect of thrombocytopenia on IFN-α-induced monocyte migration
To clarify further the involvement of platelets in monocyte migration to intestinal microvessels, we examined whether a decrease in the number of platelets could attenuate monocyte migration to intestinal microvessels. For this purpose, the interaction of monocytes with microvessels was observed after treatment with anti-platelet mAb (GPIbα antibody). Treatment with anti-platelet mAb (GPIbα antibody) dramatically decreased the number of platelets from 105 ± 10 × 104 to 3·8 ± 1·2 × 104. As shown in Fig. 4, IFN-α-induced enhancement of monocyte migration was attenuated by anti-platelet mAb. Table 1 shows the effects of pretreatment with these mAbs on the monocyte rolling ratio. Induction of thrombocytopenia by anti-platelet mAb administration decreased the monocyte rolling ratio significantly from approximately 42% to approximately 30%. These findings suggest that platelets are involved in the mechanism of IFN-α-induced monocyte adhesion.
Fig. 4.
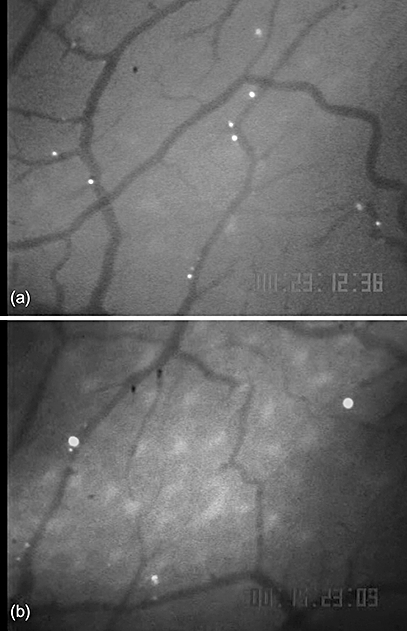
Effect of thrombocytepenia by anti-platelet antibody treatment on enhanced monocyte migration which was induced by interferon (IFN)-α treatment. Representative photographs of in vivo observation of monocyte migration in intestinal microvessels. In IFN-α-treated mice, the number of adherent monocytes increased significantly (a). The anti-platelet antibody treatment to IFN-α-treated animals attenuated the number of monocyte migration (b).
Discussion
This study provided in vivo evidence of the induction of interaction between monocytes, platelets and endothelium on microvessels of the murine small intestine by administration of IFN-α.
Accumulating evidence indicates recruitment of monocytes on the vessel wall either through a direct interaction with endothelial cells in the absence of platelets (monocytes–endothelium interaction) or indirectly by binding with platelets through PSGl-1–P-selectin interaction (monocytes–platelets interaction [16]. We have reported previously that inhibition of platelet migration attenuated monocyte migration induced by LPS in intestinal microvessels [11], suggesting that platelets on the inflamed endothelium of microvessels play an important role in monocyte migration. In addition, we reported that inhibition of platelet migration ameliorated murine ileitis by blocking the recruitment of monocytes to the inflamed intestinal mucosa.
In this study, we found that the rolling and adhesion of platelets and monocytes were increased significantly after IFN-α intraperitoneal administration, and thrombocytopenia induced by anti-platelet antibody (GPIbα antibody) decreased the monocyte rolling ratio. Collectively, these findings suggest that interaction between platelets and monocytes are involved in enhanced monocyte recruitment by treatment of IFN-α. In this study, anti-platelet mAb induced an approximately 96% decrease in the number of platelets. However, the increased rolling by IFN-α treatment was attenuated partially by this treatment. This suggests that monocytes also recruit on the vessel wall through a direct interaction with endothelial cells in the absence of platelets (monocytes–endothelium interaction). Consistent with this, anti-P selectin antibody treatment, which blocks both endothelium-derived P-selectin and platelet-derived P-selectin, completely blocked monocyte recruitment. Collectively, these findings suggest that the effect of IFN-α on monocyte migration was P-selectin-dependent, derived not only from platelets but also from the endothelium of microvessels. In our preliminary study, we investigated the surface expression of P-selectin on platelets and that of PSGl-1 on monocytes after incubation with IFN-αin vitro by flow cytometry. Expression of both surface adhesion molecules remained unchanged after IFN-α treatment, consistent with results of a previous study [17]. It was also shown in that study [17] that IFN-α activates the vascular endothelium, as assessed by von Willebrand factor antigen levels [17]. Taken together, the results suggest that IFN-α initially induces an increase in the expression of P-selectin on the vascular endothelium, resulting in the enhancement of rolling platelets and monocytes. Some monocytes interacted with platelets and they displayed rolling behaviour efficiently.
IFNs are used to treat a variety of diseases, including renal cell carcinoma, viral hepatitis and other malignant diseases. The doses of IFN-α used for treatment of viral hepatitis and renal cell carcinoma are 3–6 × 106 IU/m2[18] and 6–24 × 106 IU/m2[19], respectively. Previous clinical studies have revealed that IFN-α at a dose of more than 36 × 106IU induces severe toxicity and significantly alters the patient performance [20]. It is noteworthy that the IFN-α dosage used in the present study was equivalent to that used in humans. In our preliminary experiment, we investigated whether increased monocyte migration induced by IFN-α treatment injured the vascular endothelium. Albumin leakage from venules to the extravascular area, however, was not increased significantly by IFN-α treatment (data not shown). Thus, in the healthy endothelium, some protective mechanisms of the host might overcome the effect of aberrant monocyte migration induced by IFN-α. However, it is generally accepted that damaged vascular endothelium under inflamed conditions increases surface expression of adhesion molecules. Thus, it is possible that IFN-α has a proinflammatory effect under pathlogical conditions through increasing monocyte migration.
Cytokines play a key role to determine differentiation of T cells into Th1, Th2, T regulatory and Th17 cells. It is now generally accepted that the two forms of IBD are associated with distinct immune profiles, which are classified as a fairly typical Th1 response in CD and an atypical Th2 response in UC [21]. Based on these findings, in some studies IFN-α has been used for treatment of UC [4,5,22]. IFNs are cytokines possessing immunoregulatory properties and have been used to treat a number of chronic inflammatory disorders successfully, including chronic viral hepatitis B or C, chronic myelogenous leukaemia, hairy cell leukaemia, Kaposi's sarcoma, laryngeal and genital papillomas and various angiogenic diseases [1]. However, IFN-α is known to worsen several diseases linked to Th1-mediated pathophysiology such as psoriasis and arthritis [1]. Two studies showed that IFN-α was effective for treatment of UC, while another study showed that it was not effective. In addition, induction or exacerbation of UC during treatment of chronic hepatitis C by IFN-α has been reported [23–26]. Based on these reports, the effectiveness of IFN-α for treatment of UC as a Th2-linked disease remains unclear. Furthermore, for CD, only one clinical trial using IFN-α has been performed and the results showed no therapeutic effectiveness [27]. Although the mechanism of action of IFN-α remains to be elucidated, it is difficult to accept IFN-α as a drug for treatment of IBD [28].
In this study, adhesion of platelets increased significantly after administration of IFN-α, suggesting the promotion of P-selectin expression on the microvessel endothelium in the small intestine in a few hours. Although the mechanism of the increase in P-selectin expression remains unclear, IFN-α seems to have a proinflammatory effect in a normal immunological state in the small intestine. Although, as mentioned above, some clinical trials did not demonstrate the effectiveness of IFN-α, the present study suggests that IFN-α acts as a potent proinflammatory agent via its stimulatory effect on endothelium–platelet–monocyte interaction in intestinal microvessels, and the results of our study may provide basic information to explain why IFN-α has a proinflammatory role in the intestinal mucosa.
Disclosure
There is no personal or financial conflict of interest to disclose for any of the authors listed.
References
- 1.Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proc Natl Acad Sci USA. 1994;91:1198–205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schandene L, Del Prete GF, Cogan E, et al. Recombinant interferon-alpha selectively inhibits the production of interleukin-5 by human CD4+ T cells. J Clin Invest. 1996;97:309–15. doi: 10.1172/JCI118417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaser A, Molnar C, Tilg H. Differential regulation of interleukin 4 and interleukin 13 production by interferon alpha. Cytokine. 1998;10:75–81. doi: 10.1006/cyto.1997.0270. [DOI] [PubMed] [Google Scholar]
- 4.Sumer N, Palabiyikoglu M. Induction of remission by interferon-alpha in patients with chronic active ulcerative colitis. Eur J Gastroenterol Hepatol. 1995;7:597–602. [PubMed] [Google Scholar]
- 5.Madsen SM, Schlichting P, Davidsen B, et al. An open-labeled, randomized study comparing systemic interferon-alpha-2A and prednisolone enemas in the treatment of left-sided ulcerative colitis. Am J Gastroenterol. 2001;96:1807–15. doi: 10.1111/j.1572-0241.2001.03875.x. [DOI] [PubMed] [Google Scholar]
- 6.Guyer DR, Tiedeman J, Yannuzzi LA, et al. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111:350–6. doi: 10.1001/archopht.1993.01090030068041. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenblick M, Rosin A. Cardiotoxicity of interferon. A review of 44 cases. Chest. 1991;99:557–61. doi: 10.1378/chest.99.3.557. [DOI] [PubMed] [Google Scholar]
- 8.Grunberg SM, Kempf RA, Itri LM, Venturi CL, Boswell WD, Jr, Mitchell MS. Phase II study of recombinant alpha interferon in the treatment of advanced non-small cell lung carcinoma. Cancer Treat Rep. 1985;69:1031–2. [PubMed] [Google Scholar]
- 9.Ishii N, Tsuzuki Y, Matsuzaki K, et al. Endotoxin stimulates monocyte–endothelial cell interactions in mouse intestinal Peyer's patches and villus mucosa. Clin Exp Immunol. 2004;135:226–32. doi: 10.1111/j.1365-2249.2003.02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue T, Tsuzuki Y, Matsuzaki K, et al. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol. 2005;77:287–95. doi: 10.1189/jlb.0204104. [DOI] [PubMed] [Google Scholar]
- 11.Higashiyama M, Hokari R, Matsunaga H, et al. P-selectin-dependent monocyte recruitment through platelet interaction in intestinal microvessels of LPS-treated mice. Microcirculation. 2008;15:441–50. doi: 10.1080/10739680701703551. [DOI] [PubMed] [Google Scholar]
- 12.Kamada N, Hisamatsu T, Okamoto S, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–8. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa M, Cooper D, Russell J, et al. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–82. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- 14.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet–endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–80. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga H, Hokari R, Higashiyama M, et al. Cilostazol, a specific PDE-3 inhibitor, ameliorates chronic ileitis via suppression of interaction of platelets with monocytes. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1077–84. doi: 10.1152/ajpgi.00240.2009. Epub 2009 Oct 8. [DOI] [PubMed] [Google Scholar]
- 16.Lim YC, Snapp K, Kansas GS, Camphausen R, Ding H, Luscinskas FW. Important contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-alpha-activated endothelium under flow in vitro. J Immunol. 1998;161:2501–8. [PubMed] [Google Scholar]
- 17.Sieghart W, Homoncik M, Jilma B, et al. Antiviral therapy decreases GpIIb/IIIa activation of platelets in patients with chronic hepatitis C. Thromb Haemost. 2006;95:260–6. doi: 10.1160/TH05-12-0781. [DOI] [PubMed] [Google Scholar]
- 18.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–5. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 19.Iacobelli S, Garufi C, Irtelli L, et al. A phase I study of recombinant interferon-alpha administered as a seven-day continuous venous infusion at circadian-rhythm modulated rate in patients with cancer. Am J Clin Oncol. 1995;18:27–31. doi: 10.1097/00000421-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Quesada JR, Talpaz M, Rios A, Kurzrock R, Gutterman JU. Clinical toxicity of interferons in cancer patients: a review. J Clin Oncol. 1986;4:234–43. doi: 10.1200/JCO.1986.4.2.234. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–12. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilg H, Vogelsang H, Ludwiczek O, et al. A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis. Gut. 2003;52:1728–33. doi: 10.1136/gut.52.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitoro A, Yoshikawa M, Yamamoto K, et al. Exacerbation of ulcerative colitis during alpha-interferon therapy for chronic hepatitis C. Intern Med. 1993;32:327–31. doi: 10.2169/internalmedicine.32.327. [DOI] [PubMed] [Google Scholar]
- 24.Mavrogiannis C, Papanikolaou IS, Elefsiniotis IS, Psilopoulos DI, Karameris A, Karvountzis G. Ulcerative colitis associated with interferon treatment for chronic hepatitis C. J Hepatol. 2001;34:964–5. doi: 10.1016/s0168-8278(01)00022-8. [DOI] [PubMed] [Google Scholar]
- 25.Khalil A, Lucidarme D, Desurmont P, Hamdan-Khalil R, Filoche B. [Crohn's disease associated with interferon and ribavirin treatment for chronic hepatitis C] Gastroenterol Clin Biol. 2005;29:193–6. doi: 10.1016/s0399-8320(05)80736-5. [DOI] [PubMed] [Google Scholar]
- 26.Sprenger R, Sagmeister M, Offner F. Acute ulcerative colitis during successful interferon/ribavirin treatment for chronic hepatitis. Gut. 2005;54:438–9. doi: 10.1136/gut.2004.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasche C, Reinisch W, Vogelsang H, et al. Prospective evaluation of interferon-alpha in treatment of chronic active Crohn's disease. Dig Dis Sci. 1995;40:800–4. doi: 10.1007/BF02064982. [DOI] [PubMed] [Google Scholar]
- 28.Seow CH, Benchimol EI, Griffiths AM, Steinhart AH. Type I interferons for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2008;(3):CD006790. doi: 10.1002/14651858.CD006790.pub2. [DOI] [PubMed] [Google Scholar]


