Abstract
CD200R1 is a member of the immunoglobulin supergene family that is thought to play an inhibitory role in immunity. Previous studies have established the anti-arthritic effect of CD200Fc, an agonist of CD200R1. However, the physiological role played by CD200R1 in arthritis remains to be established. The aims of this study are to assess the contribution of endogenous CD200R1 in regulating the severity of arthritis and to determine its role in shaping the immune response to type II collagen within the context of collagen-induced arthritis, an animal model of rheumatoid arthritis. Arthritis was induced in DBA/1 mice by immunization with type II collagen and the kinetics of expression of CD200R1 and CD200 were monitored by quantitative reverse transcription–polymerase chain reaction. Next, a comparison was made between CD200R1−/− and wild-type mice in terms of the progression of collagen-induced arthritis, as well as the B and T cell responses to type II collagen. The expression of both CD200R1 and CD200 was increased after immunization and reached maximal levels at the height of the inflammatory response. In addition, the severity of arthritis was increased significantly in CD200R1−/− mice compared to wild-type mice. However, little or no differences were observed between CD200R1−/− and wild-type mice in terms of the T or B cell responses to type II collagen. It was concluded that the CD200R1/CD200 pathway is up-regulated in arthritis and plays a significant physiological role in regulating the severity of disease. In contrast, CD200R1 plays a minimal role in shaping the immune response to collagen in this model.
Keywords: CD200, collagen-induced arthritis, immunoregulation, rheumatoid arthritis
Introduction
The treatment of rheumatoid arthritis (RA) has been revolutionized by the advent of neutralizing or blocking biological therapies, including anti-tumour necrosis factor (TNF) monoclonal antibody (mAb), TNF receptor (TNFR)-immunoglobulin (Ig), anti-CD20 mAb, anti-IL-6R mAb and cytotoxic T lymphocyte-associated (CTLA)4-Ig. Recently, we and others reported on a novel form of biological therapy, CD200-Fc, that acts as an agonist for the inhibitory receptor, CD200R1. Thus, treatment with CD200-Fc from the time of immunization with type II collagen blocked subsequent development of collagen-induced arthritis (CIA) in DBA/1 mice [1]. Subsequently, we showed that CD200-Fc reduced dose-dependently the clinical and histological severity of established CIA with a similar degree of efficacy to TNFR-Ig [2].
These data are supported by the observation that genetic ablation of CD200 increases susceptibility to allergic autoimmune encephalomyelitis (EAE) and CIA [3,4] and enhances inflammatory responses to influenza infection [5], suggesting a physiological role for CD200 in controlling immune-mediated pathologies. However, in addition to CD200R1, at least four members of the CD200R family have been identified: CD200R2 (or CD200RLc), CD200R3 (CD200RLb), CD200R4 (CD200RLa) and CD200R5 [6,7], and it is not entirely clear whether CD200 also binds R2–R5 or whether other ligands for these homologues exist. One study has suggested that CD200 binds exclusively to CD200R1 [8], whereas another study has suggested binding to other CD200R receptors [7].
The aims of this study are to assess the physiological role played by CD200R1 in arthritis by measuring its expression during the course of CIA and by analysing the progression of arthritis in CD200R1−/− mice, compared to wild-type controls. In addition, we have compared the T and B cell responses to type II collagen in CD200R1−/−versus wild-type mice in order to establish whether CD200R1 plays a role in shaping the adaptive arm of the immune response. Our findings show clearly that CD200R1 plays a physiological role in limiting the severity of arthritis, but has minimal influence on the immune response to type II collagen.
Materials and methods
Mice
DBA/1 mice were purchased from Harlan (Bicester, UK). CD200R1−/− mice [9] were generously provided by Professor R. Gorczynski (Toronto, Canada). CD200R1−/− mice were bred on a C57BL/6J background and were housed alongside wild-type C57BL/6J mice purchased from Charles River (Margate, UK). All mice were maintained at 21°C ± 2°C on a 12-h light/dark cycle with food and water ad libitum. Male mice at 8–12 weeks of age were used for all experiments. All animal experiments were approved by the local Ethical Review Process Committee and the UK Home Office.
Induction of CIA
DBA/1 mice were immunized at 8–12 weeks of age subcutaneously at the base of the tail with 200 µg of bovine type II collagen emulsified in Freund's complete adjuvant (CFA; BD, Oxford, UK). CD200R1−/− and wild-type C57BL/6J mice were immunized with chicken type II collagen at 10–14 weeks of age. The development of clinical arthritis was assessed in a blinded fashion using a semi-quantitative scoring system for each paw, where 0 = normal, 1 = slight swelling and/or erythema in the ankle/wrist, 2 = pronounced oedematous swelling and 3 = joint rigidity [10]. Each limb was graded, giving a maximum score of 12 per mouse. In addition, the thickness of each affected hind-paw was monitored using calipers. Three separate experiments were performed and the results were pooled.
Quantitative reverse transcription–polymerase chain reaction (qRT–PCR)
Hind and front paws of DBA/1 mice were removed just above the ankle/wrist during the multiple time-points (14 days after collagen immunization and on days 1, 2, 3, 4, 5, 6, 7 and 10 of arthritis), snap-frozen and pulverized.
RNA isolation and reverse transcription PCR were performed as described previously [11]. Briefly, RNA was purified from 15–20 mg of joint tissue using the RNA Stat-60 (Tel-Test, Friendswood, TX, USA). For each sample, 500 ng of RNA was reverse-transcribed in a final volume of 50 µl. RNA transcripts were quantified by qRT–PCR using TaqMan chemistry and a Rotorgene 6000 real-time thermocycler (Corbett® Life Science, Sydney, Australia). Forward and reverse primers for CD200R1 and CD200, as well as fluorogenic TaqMan fluorescein/tetramethylrhodamine (FAM/TAMRA)-labelled hybridization probes were used (assays-on-demand; Applied Biosystems, Foster City, CA, USA). To control for sample cellularity, hypoxanthine guanine phosphoribosyl transferase (HPRT) forward and reverse primers and a TaqMan-labelled probe were included in separate PCRs. Each 20 µl of PCR mix also included 1 ×TaqMan universal PCR Master Mix with AmpliTaq Gold DNA polymerase, uracil-N-glycosylase (AmpErase), deoxyribonucleotide triphosphates (dNTPs) with 2′-deoxyuridine 5′-triphosphate (dUTP), and a passive reference to minimize background fluorescence fluctuations.
Thermal cycle conditions were 2 min at 50°C to allow activation of uracil-N-glycosylase, 10 min at 95°C to activate AmpliTaq polymerase, and 40 cycles of 95°C for 15 s at 60°C for 1 min. The threshold cycle (Ct) was determined for each sample using Rotorgene 6000 software. Tenfold dilutions of cDNA from interferon (IFN)-γ-stimulated bone marrow-derived dendritic cells or lipopolysaccharide (LPS)-stimulated RAW 264·7 cells were included in each run, and the standard curves were generated by linear regression using log (Ct) versus log (cell number). The cell equivalent (CE) number for synovial samples was calculated using the standard curve. All data are expressed as the ratio between the gene of interest CE and the HPRT CE, yielding relative expression units.
Measurement of anti-type II collagen IgG
Levels of anti-collagen IgG1 and IgG2a in serum obtained from mice at the end of the experiment (day 10 of arthritis) were measured by enzyme-linked immunosorbent assay, as described previously [12]. Briefly, 96-well plates (Nunc A/S, Roskilde, Denmark) were coated with 2 µg/ml of bovine type II collagen, blocked, and then incubated with serially diluted sera. Bound IgG was detected by incubation with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG1 or IgG2a (BD Pharmingen, San Diego, CA, USA), followed by substrate [tetramethylbenzidine (TMB); KPL, Gaithersburg, MD, USA]. Optical densities were measured at 450 nm. A standard pool of anti-collagen IgG was obtained by pooling sera from several mice with CIA. The levels of collagen-specific IgG1 and IgG2a in the standard serum were assigned arbitrarily a concentration of 1000 U/ml and test sera were then related to this value.
T cell proliferation and cytokine production
Two weeks after immunization with type II collagen, inguinal lymph nodes and spleens were harvested from CD200R1−/− and wild-type mice. Single-cell suspensions were prepared, resuspended in RPMI-1640 supplemented with fetal calf serum (10%), HEPES (10 mM), sodium pyruvate (1 mM), 2-mercaptoethanol (50mM), L-glutamine (1%) and 100 units/ml of penicillin/streptomycin (100 units/ml) and cultured for 64 h in triplicate in 96-well flat-bottomed plates (5 × 106 cells/ml) with 50 µg/ml bovine CII (500 µg/ml) or anti-CD3 mAb (clone 145-2C11; 0·1 ng/ml). After 48 h, supernatant was removed for measurement of cytokines and 1 µCi [3H]-thymidine (Amersham, Buckinghamshire, UK) was added for the last 16 h for measurement of thymidine incorporation. The results were expressed as counts per minute (cpm).
Cytokine production was measured using Bender Medsystems Th1/Th2 Multiplex beads according to the manufacturer's instructions (Bender Medsystems, Vienna, Austria). Briefly, supernatants were subjected to bead capture, and detection was performed by BD fluorescence activated cell sorter (FACS)CantoII using FACSDiva software (BD, Oxford, UK)
Statistical analysis
Statistical analyses were performed by t-test or analysis of variance (anova), followed by Bonferroni's multiple comparison test, where appropriate. P-values of 0·05 or less were considered statistically significant.
Results
CD200R1/CD200 expression during the course of CIA
We have shown previously by immunohistochemistry that CD200R1 is expressed abundantly in arthritic mouse joints and lymph nodes [2]. In the present study we examined the kinetics of expression of CD200R1, as well as its ligand, CD200, during the course of CIA. Mouse paws were examined by qRT–PCR before and after the development of CIA and results were compared with those of healthy unimmunized mice. The results show clearly that the expression of both CD200R1 and CD200 increases after onset of arthritis, reaching a peak at days 5–7 of arthritis, and declining at day 10 of arthritis (Fig. 1). CIA in DBA/1 mice is a relatively acute form of arthritis that reaches a peak of intensity at around day 7 of arthritis and is in decline by day 10. Hence we can conclude from this part of the study that the kinetics of expression of CD200R1 and CD200 mirrors that of the inflammatory response.
Fig. 1.
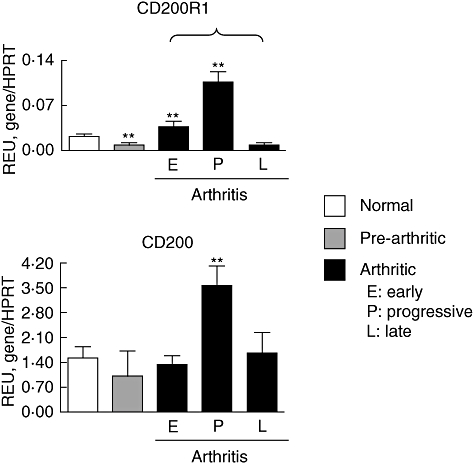
Kinetics of CD200R1/CD200 expression in collagen-induced arthritis (CIA). The expression of CD200R1 and CD200 in mouse paws was determined by quantitative reverse transcription–polymerase chain reaction. Pre-arthritic paws were taken on day 14 after immunization. E: early arthritis (days 1–4 of arthritis); P: progressive arthritis (days 5–7 of arthritis); L: late arthritis (day 10 of arthritis). Values are the mean ± standard error of the mean relative expression units (n = 6 for CD200R1−/− and wild-type mice per each time-point examined). Asterisks refer to differences when compared to normal mice. *P < 0·05; **P < 0·001.
Increased severity of arthritis in CD200R1−/− mice
We have demonstrated previously that treatment with CD200-Fc fusion protein, a ligand of CD200R1, reduces the severity of CIA in DBA/1 mice [2]. This shows that we can manipulate the CD200R1 pathway therapeutically, but does not address the question of whether CD200R1 plays a physiological role in reducing the intensity of inflammatory responses. Hence, the progression of arthritis in CD200R1−/− mice was compared to wild-type controls. Mice were immunized with chicken type II collagen using an established protocol and followed for a period of 50 days. The clinical severity of arthritis in CD200R1−/− mice was significantly greater than that of wild-type controls (Fig. 2), confirming that the CD200R1 plays a physiological role in limiting severity of arthritis.
Fig. 2.
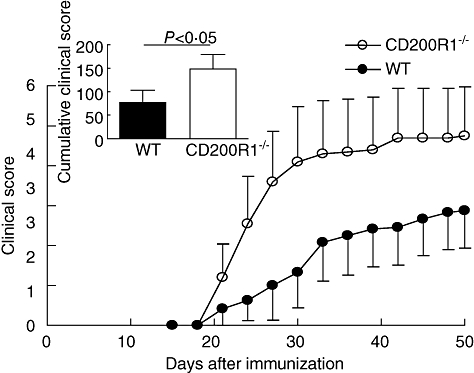
Increased severity of arthritis in CD200R1−/− mice. CD200R1−/− and wild-type mice were immunized with chicken type II chicken collagen in Freund's complete adjuvant (CFA) on day 0. Arthritis incidence was calculated based on three independent experiments and correspond to mean ± standard error of the mean (s.e.m.) (n = 24 and 29 for CD200R1−/− and wild-type controls, respectively). Inset shows cumulative clinical scores of arthritic mice up to day 50 post-immunization, as indicated by area under the curve. Data representative of three independent experiments.
Immune responses to type II collagen are normal in CD200R1−/− mice
Previous studies have shown that treatment with CD200-Fc reduces TNF and interleukin (IL)-1β mRNA expression in the joints of mice with CIA [2]. However, the focus of the present study was upon T cell cytokine production, which is best analysed in draining lymph node cell cultures. Hence, we carried out a more comprehensive analysis of the effect of CD200R1 on immune responsiveness by comparing levels of anti-type II collagen antibody as well as proliferation and cytokine production by inguinal lymph node cells from collagen-immunized CD200R1−/− mice and wild-type mice.
No differences in anti-type II collagen IgG1 or IgG2a were observed between CD200R1−/− and wild-type mice (data not shown). A modest increase in proliferation was observed in collagen-stimulated lymph node cells (LNC) from CD200R1−/− mice (Fig. 3). However, no differences in the production of IL-2, IL-4, IL-5, IL-10, IL-17 or IFN-γ were observed in LNC culture supernatants from CD200R1−/− and wild-type mice (Fig. 4). Similarly, no differences in T cell cytokine production were observed in anti-CD3-stimulated spleen cell cultures from naive CD200R1−/− mice (Fig. 4). It was concluded that the absence of CD200R1 had minimal effects on the cellular or humoral response in either naive or immunized mice.
Fig. 3.
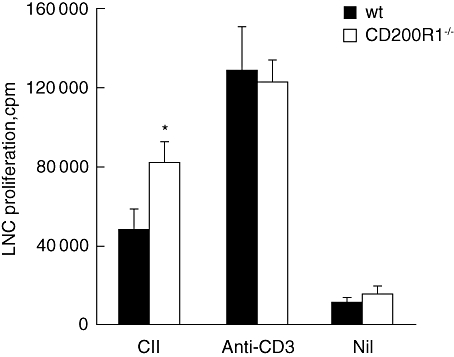
Modestly increased T cell proliferation in CD200R1−/− mice. Lymph node single cell suspensions were prepared 14 days after type II collagen immunization and cells were stimulated in the presence of type II collagen or anti-CD3 monoclonal antibody. T cell proliferation was assessed by incorporation of tritiated thymidine. Data are expressed as mean ± standard error of the mean (n = 12) and are representative of two independent experiments. *P < 0·05.
Fig. 4.
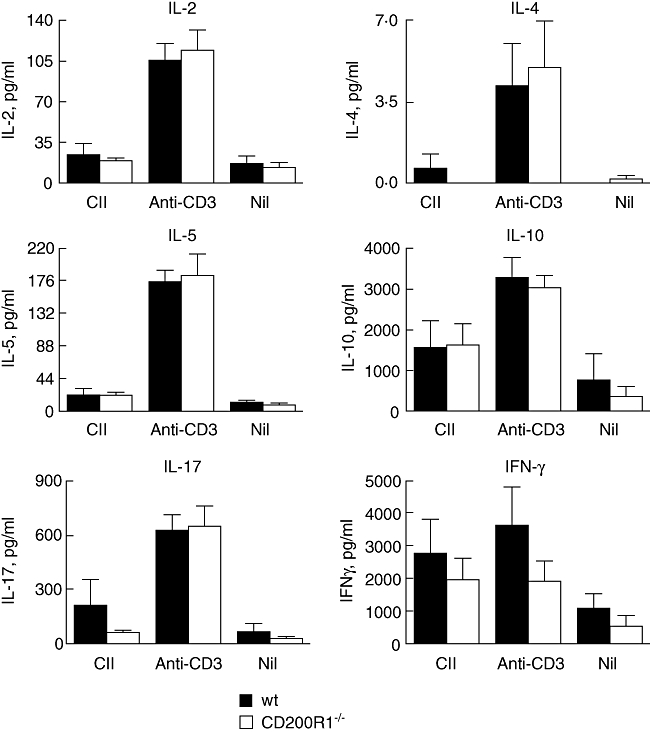
Naive CD200R1−/− mice have a normal T cell cytokine profile. Spleen cells were isolated from naive CD200−/− mice and age- and sex-matched control wild-type mice. Cells were stimulated with soluble anti-CD3 or a combination of anti-CD3 and anti-CD28. After 48 h, concentrations of cytokines were measured in cell culture supernatants by bead-based assay. Data are expressed as mean ± standard error of the mean (n = 3) and are representative of two independent experiments.
Discussion
The first finding to emerge from this study is that mRNA for both CD200R1 and CD200 is expressed in arthritic joints from DBA/1 mice and follows similar kinetics of expression. Thus, expression of both the ligand and the receptor are up-regulated after immunization and reach peak levels at the height of the inflammatory response, probably reflecting changes in the type and numbers of infiltrating cells. CD200R1 is reported to be expressed mainly on cells of myeloid lineage, including macrophages, dendritic cells, neutrophils and mast cells [6,13–15]. Hence, it is likely that myeloid cells are the principle cell type expressing CD200R1 in the joint, although we did not address this question in this study. Similarly, the identity of the CD200 expressing cells is unknown but is likely to include myeloid cells themselves, as well as B cells, T cells and endothelial cells. The finding that CD200 and CD200R1 are expressed in arthritic joints suggests strongly that this pathway is active at the site of disease activity in arthritis and probably represents a regulatory mechanism to down-regulate immune-mediated inflammation, as proposed by [16,17].
Secondly, we showed that C57BL/6 mice lacking CD200R1 show increased development of arthritis following immunization with chicken type II collagen. This shows clearly that CD200R1 plays a disease-limiting role in this model of arthritis and is consistent with previous studies demonstrating that CD200-Fc is therapeutically effective in CIA. The findings are also consistent with the observations that CD200−/− mice manifest increased severity of EAE, CIA and delayed resolution of lung inflammation following infection with influenza [5].
The expression of CD200R1 by myeloid cells, including dendritic cells, raises the question of whether it plays a role in modulating antigen presenting cell (APC) function. Hence, we set out to address the question of whether CD200R1 plays a significant role in shaping the immune response to type II collagen in CIA. First, we compared serum levels of anti-type II collagen antibody in CD200R1−/− and wild-type mice from the above study, but found no significant differences.
Previously we showed that treatment with CD200-Fc, an agonist of CD200R1, caused a significant reduction in the expression of TNF and IL-1β in the joints of mice with CIA without having any obvious effect on the immune response [2]. To address this question more fully, we analysed LNC responses in CD200R1−/− and wild-type mice immunized 14 days previously with type II collagen in CFA. This time was chosen because it represents the time of maximal T cell activity in draining (inguinal) lymph nodes. A small increase in T cell proliferation was observed in CD200R1−/− mice, without any differences in T cell cytokine production. Similarly, no differences in cytokine production by anti-CD3-stimulated spleen cells were observed between CD200−/− and wild-type mice and we concluded that the CD200R1/CD200 pathway does not play a major role in modulating the adaptive immune response, at least in our experimental system. This is consistent with our previous study, which showed that treatment of arthritic DBA/1 mice with CD200-Fc did not affect anti-collagen antibody levels or T cell cytokine production [2]. These results suggest that the CD200R1/CD200 pathway inhibits primarily the innate, rather than the adaptive immune response.
In conclusion, we have shown that CD200R1 plays a physiological role in suppressing joint inflammation in CIA and acts locally at the site of disease activity, without affecting systemic immune responses. These findings are likely to increase interest in therapeutic opportunities in targeting the CD200R1/CD200 pathway.
Acknowledgments
This work was funded by the Arthritis Research Campaign of Great Britain. I.B. received an international Travel Fellowship from the Royal Society.
Disclosure
There are no competing interests.
References
- 1.Gorczynski RM, Chen Z, Yu K, Hu J. CD200 immunoadhesin suppresses collagen-induced arthritis in mice. Clin Immunol. 2001;101:328–34. doi: 10.1006/clim.2001.5117. [DOI] [PubMed] [Google Scholar]
- 2.Simelyte E, Criado G, Essex D, Uger RA, Feldmann M, Williams RO. CD200-Fc, a novel antiarthritic biologic agent that targets proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis. Arthritis Rheum. 2008;58:1038–43. doi: 10.1002/art.23378. [DOI] [PubMed] [Google Scholar]
- 3.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–71. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 4.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–77. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snelgrove RJ, Goulding J, Didierlaurent AM, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–83. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 6.Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–46. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 7.Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172:7744–9. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- 8.Hatherley D, Cherwinski HM, Moshref M, Barclay AN. Recombinant CD200 protein does not bind activating proteins closely related to CD200 receptor. J Immunol. 2005;175:2469–74. doi: 10.4049/jimmunol.175.4.2469. [DOI] [PubMed] [Google Scholar]
- 9.Boudakov I, Liu J, Fan N, Gulay P, Wong K, Gorczynski RM. Mice lacking CD200R1 show absence of suppression of lipopolysaccharide-induced tumor necrosis factor-alpha and mixed leukocyte culture responses by CD200. Transplantation. 2007;84:251–7. doi: 10.1097/01.tp.0000269795.04592.cc. [DOI] [PubMed] [Google Scholar]
- 10.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–8. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simelyte E, Rosengren S, Boyle DL, Corr M, Green DR, Firestein GS. Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum. 2005;52:1876–84. doi: 10.1002/art.21099. [DOI] [PubMed] [Google Scholar]
- 12.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–8. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 13.Cherwinski HM, Murphy CA, Joyce BL, et al. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J Immunol. 2005;174:1348–56. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–93. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- 15.Wright GJ, Puklavec MJ, Willis AC, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–42. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 16.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–90. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 17.Gorczynski RM. CD200 and its receptors as targets for immunoregulation. Curr Opin Investig Drugs. 2005;6:483–8. [PubMed] [Google Scholar]


