Abstract
Neuroelectric oscillations reflect rhythmic shifting of neuronal ensembles between high and low excitability states. In natural settings, important stimuli often occur in rhythmic streams, and when oscillations entrain to an input rhythm their high excitability phases coincide with events in the stream, effectively amplifying neuronal input responses. When operating in a ‘rhythmic mode’, attention can use these differential excitability states as a mechanism of selection by simply enforcing oscillatory entrainment to a task-relevant input stream. When there is no low-frequency rhythm that oscillations can entrain to, attention operates in a ‘continuous mode’, characterized by extended increase in gamma synchrony. We review the evidence for early sensory selection by oscillatory phase-amplitude modulations, its mechanisms and its perceptual and behavioral consequences.
Introduction
Over 75 years ago, Bishop [1] raised the fundamental proposition that neuroelectric oscillations reflect cyclical variations in neuronal excitability. In the ensuing decades, increasingly specific linkages have been drawn between neuronal oscillations in defined frequency bands and a variety of cognitive functions. Linkages include (i) theta-band oscillations with phase-encoding of spatial information in hippocampus [2] and with formation of mnemonic neuronal representations [3], (ii) alpha-band oscillations with ‘internally-directed’ cognitive processes [4] and (iii) gamma-band oscillations with feature binding [5] and attention or sensory selection [6]. Thus, although the issue is not without controversy (e.g. Ref. [7]), there is gathering consensus that neuronal oscillations have an important role in brain operations to the extent that understanding of neuronal oscillation ‘rhythms’ now seems to be essential to our understanding of brain function [8,9].
We explore and advance the proposition that neuronal oscillations serve as crucial instruments of active input selection at the level of primary sensory cortex. Paradoxically, delta-band oscillations, long considered to index states of deep sleep and/or conditions of brain compromise [10], are at the heart of this phenomenon. In considering this proposition, we review findings about oscillations in four key areas: (i) their control of neuronal excitability, (ii) their mechanistic role in the amplification of sensory inputs, (iii) their control and utilization by attention and (iv) their variable modes of operation in response to task demands. We then describe how the conceptual framework generated by these findings converges with other theoretical positions and offers new explanation of prior behavioral and neurophysiological findings.
Four key issues
Oscillations control neuronal excitability
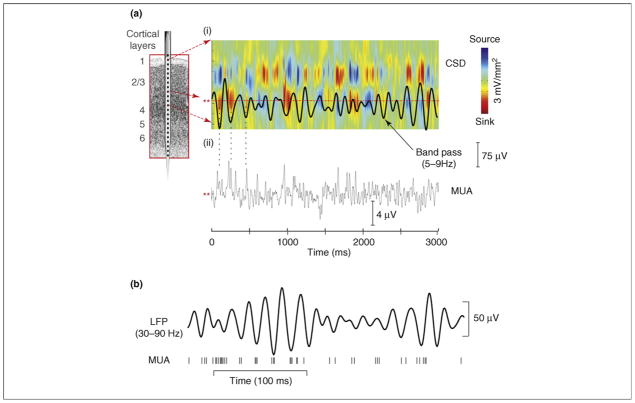
Local field potentials (LFPs) and their more macroscopic manifestations in the scalp electroencephalogram (EEG) are mainly generated by transmembrane currents occurring synchronously in ensembles of neurons [11,12]. Analysis of LFP distributions across cortical layers shows that the regular variations or ‘oscillations’ of voltage measured at any single point in the extracellular medium reflect the rhythmic (and synchronous) alternation of inward and outward transmembrane current flow in the local neuronal ensemble [13]. This point is illustrated in Figure 1a using a 7 Hz theta oscillation recorded from primary auditory cortex in an alert monkey superimposed on a color plot of its underlying current source density (CSD) profile. CSD analysis applied in this manner defines the laminar-time profile of net local transmembrane currents that generate an LFP. The point to note is that the succession of negative and positive voltage fluctuations comprising the oscillation reflects an underlying 7 Hz alternation of net inward and outward transmembrane current flow, producing extracellular current sinks (red, local negativity) and sources (blue, local positivity), respectively.
Figure 1.
(a) (i) Theta-band (5–9 Hz band pass) oscillatory activity from a lower supragranular site in primary auditory cortex (asterisks at left) superimposed on the underlying current source density (CSD) profile for the supregranular layers. Net outward transmembrane current flow generates net extracellular current sources (blue), whereas net inward current flow generates current sinks (red). The theta oscillation at this site represents the ‘underside’ of the superficial current dipole so that negative deflections correspond to current sinks and positive deflections reflect current sources, alternating at a theta rhythm. (ii) Multiunit activity (MUA) simultaneously recorded from the same site. Drop lines are provided to show the relationship between the initial three negative deflections and sinks at this site and MUA correlates. Note that current sinks and sources correspond to MUA peaks and troughs, indicating alternations in local neuronal excitability (adapted from Ref. [18]). (b) Relation between gamma-band (30–90 Hz) oscillatory phase and neuronal firing (MUA) from a recording in macaque visual area V4. Vertical lines at the bottom represent occurrence of action potentials (adapted from Ref. [14]).
In agreement with Bishop’s fundamental proposition, analysis of concomitant local neuronal firing (Figure 1a(ii), multiunit activity [MUA]) indicates that this current-flow alternation reflects shifting between net depolarized and hyperpolarized states in the local neuronal ensemble [13]. That is, negative deflections and current sinks in lower infragranular layers are attended by increases in firing, whereas the opposite is true for positive deflections and current sources. Figure 1b illustrates a similar phase-excitability relationship for gamma-band oscillations and single-unit firing for macaque visual area V4 [14]. Systematic relationships between oscillatory phase and excitability have been substantiated for the very low-frequency (<1 Hz) oscillations [15–17], and the more recent work shows that the idea extends to neuronal oscillations throughout the traditional frequency bands (e.g. delta, theta and gamma [13]). Moreover, the fact that oscillations exhibit strong cross-frequency coupling (Box 1) means that excitability can be modulated in a coordinated manner on multiple time scales.
Box 1.
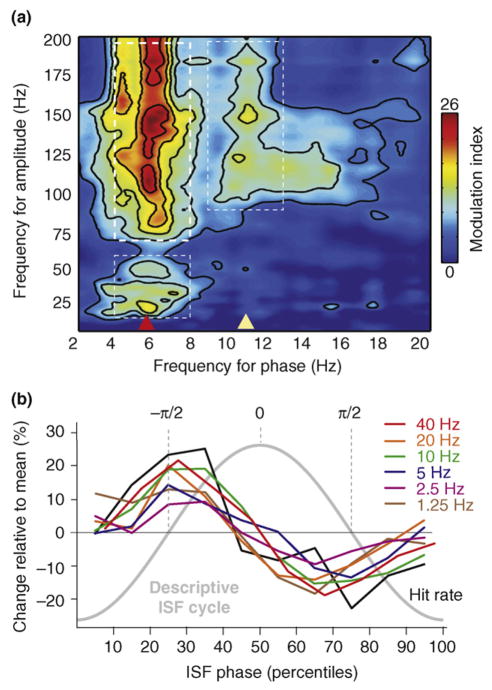
Building on early observations by Buzsaki and colleagues [68] in the rat hippocampus, more recent studies have explored the issue of oscillatory cross-frequency coupling in several neocortical regions monkeys [13,28] and humans [64,66,67]. This phenomenon entails a systematic relationship between the amplitude or power of one or more higher-frequency oscillation bands and the phase of a lower-frequency oscillation. As illustrated in Figure Ia, amplitude in the high gamma band (thick-dashed white box, upper left) is grouped by, or ‘nested within’, the phase of a theta-band oscillation (red arrowhead, bottom). Coupling between theta and gamma (thin-dashed white box, lower left) and between a ~10 Hz oscillation (yellow arrowhead, bottom) and high gamma (thin-dashed white box, right) is also apparent. Cross-frequency coupling has elements of hierarchical organization; that is, as gamma amplitude is coupled with theta phase, theta amplitude is coupled to delta phase [13]. Coupling extends throughout the >1 Hz frequency range typically studied [13] and extends down into the ‘slow’ (0.1–1 Hz) and ‘infraslow’ (0.01–0.1 Hz) range (Figure Ib); note that, as this area of investigation expands, methodological concerns (e.g. Ref. [56]) have emerged.
Oscillatory mechanisms of sensory enhancement
Because the oscillatory phase reflects excitability in a local ensemble (see earlier), the momentary phase at the time an input arrives in the cortex will determine whether it is attenuated or amplified, particularly if the input is near threshold [6,13,14,18]. In fact, neuronal oscillations seem to be part of the mechanism by which modulatory inputs control sensory selection. Figure 2 elaborates key aspects of this point.
Figure 2.
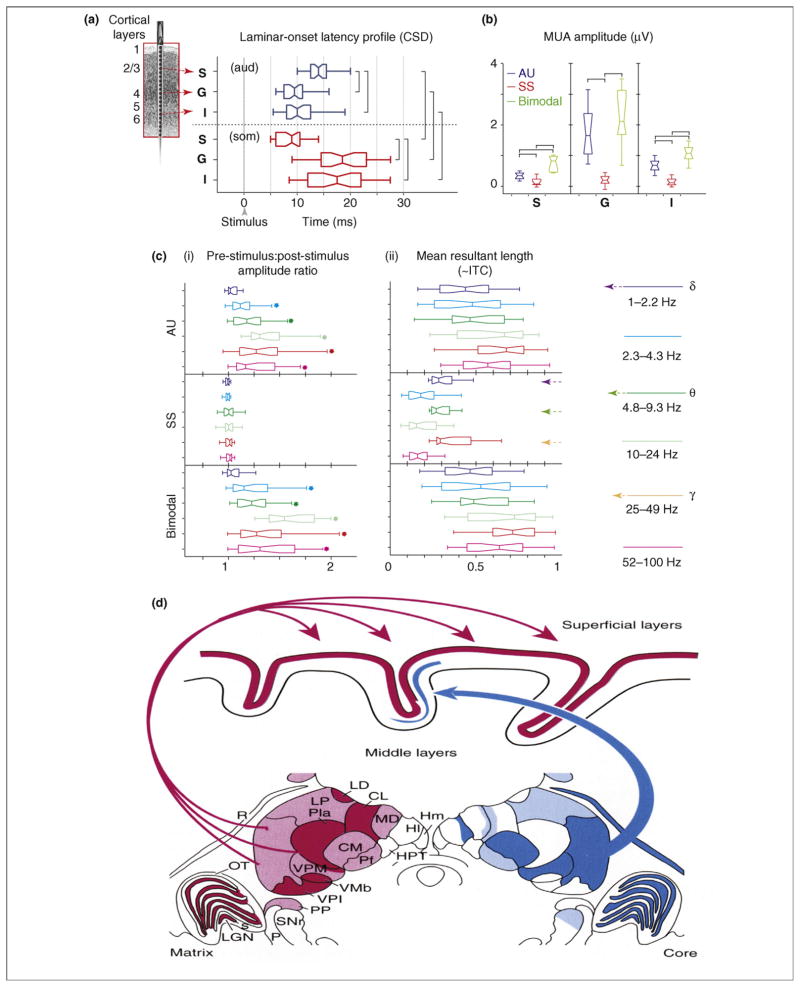
Mechanisms of driving and modulatory inputs. (a) Box plots show pooled onset latencies of the characteristic frequency-tone- (aud; blue) and somatosensory-stimulus (som; red)-related CSD response in supragranular (S), granular (G) and infragranular (I) layers across experiments. The boxes have lines at the lower quartile, median and upper quartile values and the notches in boxes graphically show the 95% confidence interval about the median of each distribution. Brackets indicate the significant post hoc comparisons calculated using Games-Howell tests (P < 0.01). (b) Box plots show pooled (n = 38) CSD and MUA amplitudes on the selected channels (S, G and I) averaged for the 15–60 ms time interval for the same conditions as (a), plus the bimodal condition. Brackets indicate the significant post hoc comparisons calculated using Games-Howell tests (P < 0.01). (c) (i) Pooled (n = 38) post-stimulus:pre-stimulus single-trial oscillatory amplitude ratio (0 to 250 ms: −500 to −250 ms) for different frequency intervals (different colors) of the auditory (AU), somatosensory (SS) and bimodal supragranular responses. Stars denote where the amplitude ratio is significantly different across the pre- and post-stimulus periods (one-sample t tests, P < 0.01). (ii) Pooled intertrial coherence (ITC) expressed as a vector quantity (mean resultant length) measured at 15 ms post-stimulus (the time of the initial peak response). Note that in the case of somatosensory events an increase in phase concentration only occurs in the low-delta (1–2.2 Hz), theta (4.8–9.3 Hz) and gamma (25–49 Hz) bands, indicated by colored arrows on the right. (d) Relative distributions and concentrations of calbindin-positive matrix cells (bottom left) and parvalbumin-positive core cells (bottom right) in a frontal section through the middle of a macaque monkey thalamus. The projections of the matrix to superficial layers of cortex over a wide extent and unconstrained by areal borders is shown at the top. Core cells restricted to individual nuclei (e.g. the ventral posterior nucleus) project in a topographically ordered manner to the middle layers of single functional cortical fields. Abbreviations: CL, central lateral nucleus; CM, centre median nucleus; Hl, lateral habenular nuclei; Hm, medial habenular nuclei; LD, lateral dorsal nucleus; LGN, lateral geniculate nucleus; LP, lateral posterior nucleus; MD, mediodorsal nucleus; OT, optic tract; P, color-coded retinal ganglion cells; Pla, anterior pulvinar; PP, peripeduncular nucleus; R, reticular nucleus; s, s laminae; SNr, substantia nigra pars reticularis; VMb, basal ventral medial nucleus; VPi, ventral posterior inferior nucleus; VPM, ventral posterior medial nucleus (figure adapted, with permission, from Ref. [23]).
Laminar-onset latency profiles (Figure 2a) show that an auditory input produces a classic feedforward response that begins in layer 4, whereas a somatosensory input produces a clearly different type of response that begins in the supragranular layers [18]. Quantified MUA (Figure 2b) amplitude data for the same laminar groupings indicate that auditory input provokes a robust action-potential response, whereas somatosensory input has little or no impact on local action potentials. When the auditory and somatosensory inputs are combined (bimodal condition) there is significant enhancement of both CSD and MUA responses (only MUA is shown). Based on these characteristics, we suggest that the auditory and somatosensory responses reflect ‘driving’ and ‘modulatory’ inputs, respectively, similar to the proposition of Sherman and Guillery [19]. Of equal importance here is that the modulatory inputs seem to use ongoing neuronal oscillations as an instrument for enhancement of auditory processing. To make this point, Figure 2c(i) displays pre-stimulus:post-stimulus amplitude ratios for single-trial oscillatory activity in six frequency bands ranging from low delta to high gamma. Consistent with its modulatory status, the somatosensory input provokes no change in the power of ongoing oscillations; however, it produces a significant increase of phase coherence across trials (intertrial coherence or ITC) at the peak of the initial response (15 ms post-stimulus) in the low delta, theta and gamma bands [Figure 2c(ii)]. The somatosensory (modulatory) input contrasts with the auditory (driving) input, which provokes both an increase in oscillatory power and phase coherence (ITC) across the spectrum; this is typical of an ‘evoked’ type of response. These indices are important because they provide a means of distinguishing between the two chief mechanistic alternatives for generation of LFPs in response to synaptic input, ‘stimulus-evoked response’ and ‘phase-resetting’ of ongoing oscillatory activity [20,21]. In essence, stimulus-evoked responses require a pre- to post-stimulus increase in spectral power, whereas phase resetting does not; for different reasons [21], both entail a pre- to post-stimulus phase concentration (increase in phase-locking index).
The significant pre- to post-stimulus phase concentration, in the absence of an accompanying increase in power, indicates that in A1 somatosensory input impacts mainly by resetting the phase of ongoing oscillations. Thus, somatosensory input ‘modulates’ the dynamics of activity in A1 but does not cause auditory neurons to respond. In more general terms, the effect of a modulatory somatosensory input to A1 is most obvious when it coincides with a driving auditory input [18]. Kayser et al. [22] found similar effects of visual inputs on auditory processing in primary and secondary auditory cortical areas, thus it seems that this is a general mechanism whereby non-preferred stimuli can affect specific stimulus processing at early stages. Moreover, both the extremely short latency of the effect and its preferential supragranular impact implicate extralemniscal thalamic afferents as a neuronal substrate. Although it is beyond the scope of this paper to cover this topic in detail, it is noteworthy that so-called ‘non-specific’ thalamic afferents are widespread and project both within [23] (Figure 2d) and across modalities [24].
Oscillations are used in attentional selection
Given the fundamental role of neuronal oscillations in control of neuronal excitability and sensory processing, one would expect them to play a part in attentional selection, and there is strong support for this expectation [25–28]. Gamma-band (30–70 Hz) oscillations are associated with active, attentive aspects of visual processing [14,29–31] and, interestingly, it seems that different portions of the gamma band might be used for different aspects of discriminative visual processing [31]. Delta-band (1–4 Hz) oscillations, by contrast, more often seem to be linked to deep-sleep states and compromise of neuronal function [10]. The latter findings contribute to the belief that low-frequency oscillations might actually interfere with active processing. Consistent with this view, Fries and colleagues showed that, along with attentional enhancement of gamma synchrony, there is apparent suppression of low-frequency (delta- and theta-band) synchrony. Others have reported, however, that low-frequency oscillatory synchrony can be enhanced by attention [25–28]. The contrast between these findings and between their underlying task structures has important implications that will be developed more fully in the next section.
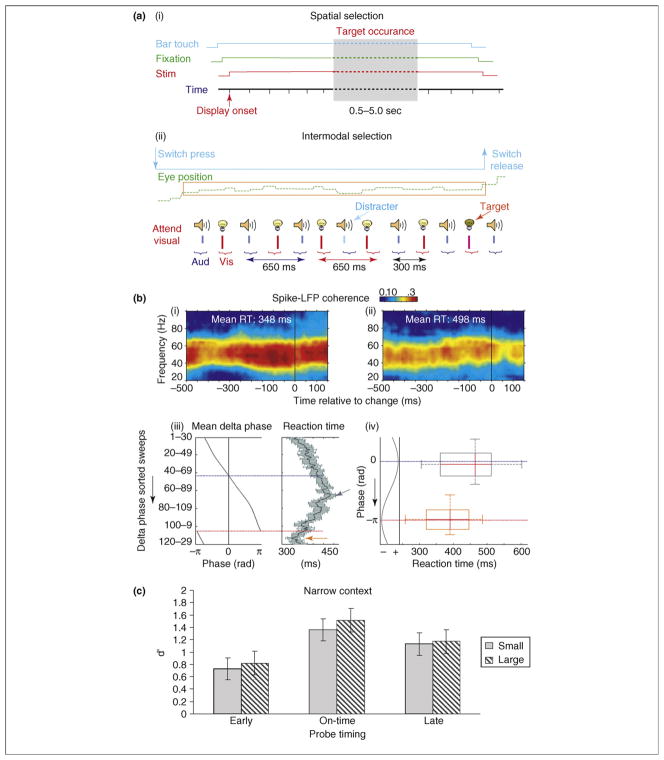
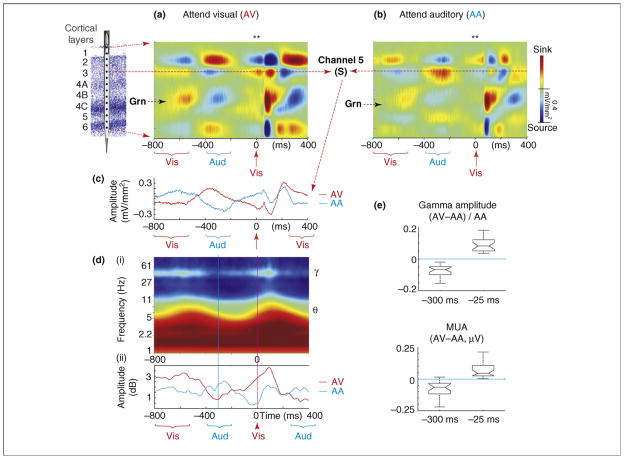
In any case, recent findings show that delta-band oscillations can function as an instrument of attentional selection [28]. Monkeys performed an intermodal selection task (Figure 3) in which auditory and visual stimuli (beeps and flashes) were delivered in rhythmic, interdigitated streams. In alternate trial blocks, the monkey had to attend to either the visual or the auditory stimulus stream and make a manual response to an infrequently presented ‘oddball’ stimulus. This paradigm combines the rhythmic structure and variability characteristic of many natural event patterns. A striking cascade of attention effects on V1 activity is evident in this context (Figure 4). Displayed are averaged laminar CSD profiles in the attend visual condition (Figure 4a) and the attend auditory condition (Figure 4b), shown for a long time frame, including the response to the visual and auditory stimuli (brackets below time axis) preceding the one used as the trigger (red arrow). The key observation is that, whereas activity in the thalamic-input (granular, Grn) layer entrains reliably to visual input, activity in the extra-granular layers entrains to the attended input stream, whether visual or auditory. The difference is most apparent in the supragranular (S) laminae. Throughout the entire duration of the epoch, delta oscillations in the supragranular layers are in opposite phase in the two attention conditions (Figure 4c). This effect contrasts with that in the Grn layer, which shows amplitude but not phase modulation by attention (i.e. sources and sinks seem to be more intense but occur in the same progression); note that the amplitude effect in layer 4 contrasts with the earlier conclusions based on analysis of averaged responses that attention does not affect the initial feedforward response in V1 [32,33]. Time-frequency dissection of the S-channel signal in the attend condition [Figure 4d(i)] reveals predictable coupling of theta-band and gamma-band amplitude to the entrained delta phase (Box 1), and comparison of gamma-band amplitude variations in this signal across attention conditions [Figure 4d(ii)] shows that this phase-amplitude coupling is maintained throughout (i.e. gamma amplitude follows delta phase).
Figure 3.
Effects of task demands on oscillatory dynamics and behavior. (a) Depiction of a vigilance paradigm (i) (adapted, with permission, from Refs [14,29,30]), and a rhythmic stream paradigm (ii) (adapted, with permission, from Ref. [28]). In both cases the subjects make manual responses to target stimuli. The key difference is that stimuli occur randomly in the first case but are arranged in a rhythmic stream in the second. The former suppresses low-frequency oscillatory entrainment, whereas the latter facilitates it. (b) Behavioral correlates of oscillatory modulation by attention in the same two studies. Reaction time (RT) is predicted by gamma-band amplitude in the former (i and ii) and by delta-band phase in the latter (iii and iv). (c) Variations in stimulus discriminability (d′) in a tone discrimination, depending on whether targets occurred in (middle) or out of phase (left and right) with an attended rhythm (adapted, with permission, from Ref. [36]).
Figure 4.
Attentional modulation of delta phase and its related cascade of effects. (a,b) Color maps show CSD profiles related to standard visual (Vis) stimuli in the attend visual (AV) and attend auditory (AA) conditions for the −800 to +400 ms time frame from a representative experiment. Red arrow indicates the visual event used as a trigger (0 ms). Blue and red brackets indicate the time frame where adjoining auditory (Aud) and visual events occur; because stimuli are jittered the responses to prior stimuli are somewhat ‘smeared’ over time. (c) Overlay of CSD waveforms from supragranular site (S) in the AV and AA conditions. (d) (i) Time–frequency plot of the average oscillatory amplitude of the wavelet transformed single trials from the supragranular site in (A); note variations in theta (~6 Hz) and gamma (~40 Hz) amplitudes are coupled with stimulus-entrained delta phase. (ii) An overlay of the variations in time course of averaged (37–57 Hz) gamma amplitude in the AV and AA conditions. (e) Pooled (n = 24) normalized gamma amplitude and MUA differences between AV and AA conditions ([AV−AA]/AA) for the −325 to −275 and −50 to 0 ms time frames. Notches in the boxes depict a 95% confidence interval about the median of each distribution.
Despite the complexities in this cascade of effects, at its base is the relationship between delta-oscillation phase and neuronal excitability. Comparison of gamma-band and MUA amplitudes in the S-channel signal during the immediate pre-stimulus period with another point at which there is large delta-phase opposition (~300 ms pre-stimulus) reflects this relationship, in addition to that between gamma amplitude and excitability [29]. In broad terms, the laminar configuration of sinks and sources at stimulus onset in the attend visual condition reflects a high-excitability state permissive to transmission of inputs from granular to extragranular laminae (and onward), whereas in the ignore visual (attend auditory) condition the laminar CSD configuration at this time point reflects a relative depression of excitability in this circuit. These different facilitative and suppressive peristimulus states seem to be responsible for the amplitude differences between visual responses in attend visual and attend auditory (ignore visual) conditions.
Oscillatory mode of the system reflects task demands and predicts behavioral performance
It is at first paradoxical that selective attention seems to both suppress delta oscillations [29] and use them as an instrument of response amplification (see earlier). Consideration of task dynamics, however, indicates an interesting and plausible explanation. That is, the suppression of low-frequency oscillations might be due to the use of a ‘vigilance’ paradigm in some of the earlier studies [14,29]. This paradigm entails a task in which the time at which target stimuli might occur is completely unpredictable [Figure 3a(i)]. It models an important circumstance that occurs in natural behavior, for example, when a cat watches a mouse hole waiting for the mouse to appear. However, a great deal of natural stimulation (e.g. biological motion and vocal communication) has an explicitly rhythmic and predictable pattern and, under these circumstances, neuronal oscillations can entrain (phase-lock) to the structure of the stimulus stream [34]. The ‘intermodal’ paradigm described earlier [Figure 3a(ii)] is of this type.
We propose that the brain is biased toward either a ‘rhythmic’ or a ‘continuous’ mode of operation, depending on the dynamics of task demands. When there is task-relevant temporal structure that sensory systems can entrain to, lower-frequency oscillations can become instrumental in sensory processing. Rhythmic-mode operation entails: (i) sensory cortical entrainment (phase-locking) to the temporal structure of an attended stream, (ii) alignment of ‘high-excitability’ oscillation phases with events in the attended stream and (iii) systematic enhancement of responses to attended events and suppression of responses to events that occur out of phase with attended events. These effects typify the findings reviewed in the last section in addition to psychophysical results from normal human adults [35]. Using a rhythmic-tone discrimination paradigm that is like that in Figure 3a(ii), Jones and colleagues (Figure 3c) show that stimulus discriminability (d′) is affected by whether task-relevant stimuli fit or conflict with an anticipated low-frequency rhythm [36]. Recent findings show that these effects are paralleled increases in reaction time [37].
By contrast, when there is no task-relevant rhythm that the system can entrain to, low-frequency oscillations are actually detrimental to processing because, by definition, they entail long periods of low excitability during which detection of a subtle random stimulus would be less likely. We propose that under these conditions a continuous (vigilance) mode of operation is implemented, low-frequency oscillations are suppressed and the system is pushed as much as possible into a continuous state of high excitability. Several behavioral observations are consistent with the differential operation of these two processing modes. In continuous or vigilance mode [Figure 3a(i)], where the oscillatory correlates of attention are enhanced by gamma amplitude and lower-frequency suppression [29], variations in gamma synchrony are predictive of reaction-time variations [14] [Figure 3b(i)]. In rhythmic mode [Figure 3a(ii)], where attentional modulation harnesses low-frequency rhythmic entrainment [28] (Figure 4), variations of low-frequency (delta) phase predict reaction-time variations [Figure 3b(ii)].
In this framework, the rhythmic mode would be the preferred state of the system, in part because of its efficiency and because inputs that are out of phase with the attended stream are automatically suppressed. Additionally, gamma-band activity seems to be more metabolically demanding than low-frequency oscillations [38,39] and, because of hierarchical coupling, gamma activity is ‘rationed’ or selectively enhanced at critical time points when a high-excitability state is most useful. This thinking is in line with the common subjective experience that the continuous or vigilance mode is difficult to maintain for extended periods, which makes the further prediction that periodic breaks in attention will be associated with lapses into rhythmic mode accompanied by a phasic increase in delta-band amplitude. This prediction is yet to be tested, but even in an established continuous mode of operation there are small variations of gamma synchrony indicative of low-frequency modulation. In Figure 3b, for example, gamma synchrony seems to vary on a scale of ~500 ms, which is the period of a 2 Hz delta oscillation.
Rhythmic processing: converging theory and retrospection on earlier findings
A proposition most akin to rhythmic-mode operation and pre-dating it by several years is the dynamic attending theory. As developed by Jones, Large and colleagues [35,40,41] and in a more ‘motor perspective’ by Praamstra and colleagues [37], the idea is that attending itself can be an oscillatory process that entrains to environmental rhythms, thus improving discriminative performance (Figure 3c). Nobre and colleagues [42–44] suggest that ‘attention to time’ is one of several attentional varieties and that it is linked to a specific network of brain structures with a central node in the parietal cortex. Ghose and Maunsell [45] suggest that in a structured task monkeys can form an internal representation of task timing that can guide the temporal allocation of attentional resources to maximize behavioral performance. In all of these cases, entrained neuronal oscillations operating in a steady-state mode and/or re-setting on a trial-by-trial basis [22,26,28,25,46] provide likely physiological substrates for the effects of attention. The fact that oscillations can be re-set to low and high excitability states on multiple time scales [34] increases the flexibility and range of the mechanism.
It is unlikely that rhythmic-mode operation reflects simply a variety, such as spatial or feature attention, because diverse varieties such as object attention [47] and intermodal attention [28] can operate in a rhythmic mode with excitability and gamma bursting coupled with the rhythm of the task. Additionally, singular varieties such as spatial attention can operate in either a continuous mode (with the effect of a tonic increase in excitability [14,29]) or a rhythmic mode (with periodic variations in excitability coupled with the temporal structure of the task) [45].
The idea that these two basic processing modes impact the operation of attention might help to resolve some of the apparent discrepancies between attention effects reported by different laboratories (e.g. Refs [48,49] versus Refs [50,51]). Phase resetting of low-frequency oscillations might help to explain cueing effects on attentional performance and event-related potentials (ERPs), whose precise neuronal substrates have proven elusive for decades. For example, the frontal contingent negative variation [52] probably reflects a phase reset of frontal low-frequency oscillations by a warning cue. Similarly, attentional blink [53] and inhibition of return (IOR) [54] would result when stimuli are delivered during the low-excitability phase of a low-frequency oscillation that has been reset by the appearance of either a salient target or a cue to attend.
Questions and caveats for future research
Generality of rhythmic-mode processing?
The foregoing framework makes numerous empirical predictions. Sensory selection in a typical ERP spatial attention paradigm, for example, could be accomplished by entraining low-frequency oscillations in the neuronal representations of the relevant locations to the basic rhythm of stimulus presentation. The representations of all other locations could be left to wander in random phase, thus passively and stochastically degrading the processing of an irrelevant event stream, or could be pushed into counterphase, producing more active and stronger degradation of processing; the latter would be useful for dealing with the representations of distractor events. A similar logic would apply to feature- and object-based selection. In testing these it is crucial to keep in mind that, whereas primary cortical activity seems to be driven exclusively by one input modality, it can be modulated by heteromodal inputs [18,22,28]. Thus, realistic stimulus representations in the brain are much broader and more complex than indicated by a ‘unisensory’ perspective [55]. When performing a demanding task, most of the brain becomes dynamically engaged and regional patterns of phase coherence, lack thereof or phase opposition are dictated by task demands.
Importance of, and pitfalls in, cross-frequency coupling?
A good case can be made for the use of multiple-nested frequencies for representing complex events such as vocal communication [34]; however, numerous other possibilities such as use in sensory representation of external biological motion or generation of complex motor sequences remain to be explored. Serious concerns have been raised about artifacts in these investigations, but they clearly can be mitigated by careful data inspection of unfiltered signals with emphasis on the prominence and bi-coherence spectra of higher-frequency harmonics and, possibly, by use of analytic strategies involving ‘causality’ indices [56]. For direct neuronal recordings, rhythmic variations in local firing patterns can help to confirm the separation of some components [13].
Oscillatory-phase re-setting effects in behavior and ERPs?
Sensory-evoked and phase-reset processes usually co-exist at all cortical processing stages and, despite clear criteria for distinguishing these processes, they are usually difficult to separate [20,21]. Nonetheless, as suggested earlier, low-frequency phase re-setting is likely to contribute to generation of cognitive ERP components and, in general, the weight of this contribution probably increases with the latency of the component.
Eye movements: baby or bathwater?
An important recent paper raised the concern that extraocular muscle electromyographic signals associated with mini-saccades can contaminate the so-called ‘induced-gamma’ effects noted to occur between 200 and 400 ms latency in scalp ERP recordings [57]. Although the problem is not believed to extend to intracranial recordings, it is of widespread concern. There are numerous potential solutions including use of first and second derivative approximations to reduce contamination, modeling and removal of artifacts using independent component analysis, and identification of suspect trials using high-resolution eye tracking. Each of these will probably prove useful with two caveats. First, saccades including mini-and micro-saccades might have systematic effects on ongoing oscillatory activity across the spectrum. Second, these effects might be useful in normal vision [58]. Thus, procedures that eliminate saccade trials from analysis might ‘throw out the baby with the bathwater’.
Concluding comments
Natural stimulation acquired through our own motor behavior or produced by that of another animal is usually rhythmic, in part because motor behavior is itself patterned by oscillatory mechanisms such as the 10 Hz mu rhythm [59,60]. In these and other common circumstances, when there is a relevant stimulus rhythm(s) that intrinsic brain oscillations can entrain to, attention operates in a rhythmic mode putting the range of ambient neuronal oscillations to work in amplifying relevant inputs and suppressing irrelevant ones. With random stimuli, the extended period of insensitivity during the low-excitability phase becomes a large cost. Thus, when relevant stimuli lack rhythm, attention operates in a continuous mode, maximizing the sensitivity of the system by suppressing lower-frequency oscillations and exploiting the advantages of extended continuous gamma-band oscillations [61].
Although there are numerous open questions about rhythmic-mode processing, several tentative conclusions are gathering weight. The fact that certain frequencies dominate the spectra of spontaneous oscillations in sensory cortices [13], plus the remarkable match between these oscillatory bands and the temporal structure of biologically relevant sensory inputs [34,62], is consistent with the idea of ‘special frequencies’ in perception. At the same time, cross-frequency oscillatory coupling provides obvious potential for improvement in the matching of brain oscillations with complex natural input patterns covering different time scales [34] in addition to more subtle computational benefits [63]. The modulation of low-frequency phase and of cross-frequency coupling by attention [28] yokes these dynamics to the current goals of an observer.
Figure I.
(a) Cross-frequency phase-amplitude coupling plot showing that high-gamma-band amplitude (thick-dashed white box) is grouped according to theta-band oscillatory phase (red arrow). More subtle phase-amplitude-coupling effects (thin-dashed white boxes) are also apparent between theta-phase and gamma amplitude, and between ~10 Hz (alpha and mu) band phase (yellow arrow) and high gamma amplitude (adapted, with permission, from Ref. [64]). (b) Based on EEG recorded from a scalp electrode (vertex to mastoid reference), 1–40 Hz oscillation amplitudes are coupled with infraslow frequency (ISF) phase. Amplitude values are represented as a percentage change from the mean for each frequency as a function of ISF phase. ISF phase ranges from −π to +π in 10%-ile bins. Note that the phase difference (mean +/− standard error of the mean) between the amplitude envelope of faster oscillations and ISF is consistently at ~ −π/2. Note also that behavioral responding accuracy (hit rate, black line) is coupled with ISF phase in the same way as the >1 Hz oscillations (adapted, with permission, from Ref. [65]).
Acknowledgments
We are grateful to our colleagues in the Columbia University Oscillation Journal Club for helpful commentary. We thank J.M. Palva, P. Fries, T. Womelsdorf, R. Desimone, E.G. Jones, E.W. Large and M.R. Jones for advice and help in preparing illustrations. This work is supported the National Institute of Mental Health (MH 060358 and MH 061989; www.nimh.nih.gov).
References
- 1.Bishop G. Cyclical changes in excitability of the optic pathway of the rabbit. Am J Physiol. 1933;103:213–224. [Google Scholar]
- 2.Maurer AP, McNaughten BL. Network and intrinsic cellular mechanisms underlying theta phase precession of hippocampal neurons. Trends Neurosci. 2007;30:325–333. doi: 10.1016/j.tins.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Jensen O, et al. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Palva S, Palva JM. New vistas for α frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 6.Fries P, et al. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–77. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- 8.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G. The structure of consciousness. Nature. 2007;446:267. doi: 10.1038/446267a. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Mitzdorf U. Current source-density method and application in cat cerebral cortex: Investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder CE, et al. Localization of ERP generators and identification of underlying neural processes. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:55–75. [PubMed] [Google Scholar]
- 13.Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 14.Womelsdorf T, et al. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- 15.Steriade M, et al. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras D, et al. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 18.Lakatos P, et al. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makeig S, et al. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Shah AS, et al. Neural dynamics and fundamental mechanisms of event-related potentials. Cereb Cortex. 2004;14:476–485. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- 22.Kayser C, et al. Visual modulation of neurons in auditory cortex. Cereb Cortex. 2008;18:1560–1574. doi: 10.1093/cercor/bhm187. [DOI] [PubMed] [Google Scholar]
- 23.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 24.Hackett TA, et al. Multisensory convergence in auditory cortex. II. Thalamocortical connections of the caudal superior temporal plane. J Comp Neurol. 2007;502:924–952. doi: 10.1002/cne.21326. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, et al. Attentional modulation of SSVEP power depends on the network tagged by the flicker frequency. Cereb Cortex. 2006;16:1016–1029. doi: 10.1093/cercor/bhj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YJ, et al. Attention induces synchronization-based response gain in steady state visual evoked potentials. Nat Neurosci. 2007;10:117–125. doi: 10.1038/nn1821. [DOI] [PubMed] [Google Scholar]
- 27.Morgan ST, et al. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc Natl Acad Sci U S A. 1996;93:4770–4774. doi: 10.1073/pnas.93.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakatos P, et al. Oscillatory entrainment as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 29.Fries P, et al. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 30.Womelsdorf T, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 31.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 32.Mehta AD, et al. Intermodal selective attention in monkeys II: Physiologic mechanisms of modulation. Cereb Cortex. 2000;10:359–370. doi: 10.1093/cercor/10.4.359. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder CE, et al. Determinants and mechanisms of attentional modulation of neural processing. Front Biosci. 2001;6:D672–D684. doi: 10.2741/schroed. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder CE, et al. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci. 2008;12:106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones MR, et al. Effects of auditory pattern structure on anticipatory and reactive attending. Cognit Psychol. 2006;53:59–96. doi: 10.1016/j.cogpsych.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Jones MR, et al. Temporal aspects of stimulus-driven attending in dynamic arrays. Psychol Sci. 2002;13:313–319. doi: 10.1111/1467-9280.00458. [DOI] [PubMed] [Google Scholar]
- 37.Praamstra P, et al. Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci. 2006;26:5448–5455. doi: 10.1523/JNEUROSCI.0440-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 39.Niessing J, et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 40.Jones MR, Boltz M. Dynamic attending and responses to time. Psychol Rev. 1989;96:459–491. doi: 10.1037/0033-295x.96.3.459. [DOI] [PubMed] [Google Scholar]
- 41.Large EW, Jones MR. The dynamics of attending: how we track time-varying events. Psychol Rev. 1999;106:119–159. [Google Scholar]
- 42.Correa A, Nobre AC. Neural modulation by regularity and passage of time. J Neurophysiol. 2008;100:1649–1655. doi: 10.1152/jn.90656.2008. [DOI] [PubMed] [Google Scholar]
- 43.Correa A, Nobre AC. Spatial and temporal acuity of visual perception can be enhanced selectively by attentional set. Exp Brain Res. 2008;189:339–344. doi: 10.1007/s00221-008-1429-2. [DOI] [PubMed] [Google Scholar]
- 44.Nobre A, et al. The hazards of time. Curr Opin Neurobiol. 2007;17:465–470. doi: 10.1016/j.conb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Ghose GM, Maunsell JH. Attentional modulation in visual cortex depends on task timing. Nature. 2002;419:616–620. doi: 10.1038/nature01057. [DOI] [PubMed] [Google Scholar]
- 46.Morgan ST, et al. Selective attention to stimulus location modulates the steady state visual evoked potential. Proc Natl Acad Sci U S A. 1996;93:4770–4774. doi: 10.1073/pnas.93.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor K, et al. Coherent oscillatory activity in monkey area V4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- 48.Luck SJ, et al. Neural mechanisms of spatial selective attention in areas V1, V2 and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 49.Moran J, Desimone R. Selective attention gates visual information processing in extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 50.McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci. 1999;19:7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter WG, et al. Contingent negative variation: an electrical sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- 53.Raymond JE, et al. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- 54.Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- 55.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 56.Kramer MA, et al. Sharp edge artifacts and spurious coupling in EEG frequency comodulation measures. J Neurosci Methods. 2008;170:352–357. doi: 10.1016/j.jneumeth.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Yuval-Greenberg S, et al. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Rajkai C, et al. Transient cortical excitation at the onset of visual fixation. Cereb Cortex. 2007;18:200–209. doi: 10.1093/cercor/bhm046. [DOI] [PubMed] [Google Scholar]
- 59.Pfurtscheller G, et al. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin Neurophysiol. 2000;111:1873–1879. doi: 10.1016/s1388-2457(00)00428-4. [DOI] [PubMed] [Google Scholar]
- 60.Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Brain Res Rev. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Borgers C, Kopell NJ. Gamma oscillations and stimulus selection. Neural Comput. 2008;20:383–414. doi: 10.1162/neco.2007.07-06-289. [DOI] [PubMed] [Google Scholar]
- 62.Luo H, Poeppel D. Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron. 2007;54:1001–1010. doi: 10.1016/j.neuron.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krupa M, et al. Mixed-mode oscillations in a three time-scale model for the dopaminergic neuron. Chaos. 2008;18:015106. doi: 10.1063/1.2779859. [DOI] [PubMed] [Google Scholar]
- 64.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1629. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monto S, et al. Very slow EEG fluctuations predice the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008;28:8262–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freeman WJ, et al. Spatial spectra of scalp EEG and EMG from awake humans. Clin Neurophysiol. 2003;114:1053–1068. doi: 10.1016/s1388-2457(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 67.Vanhatalo S, et al. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A. 2004;101:5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Csicsvari J, et al. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]