Abstract
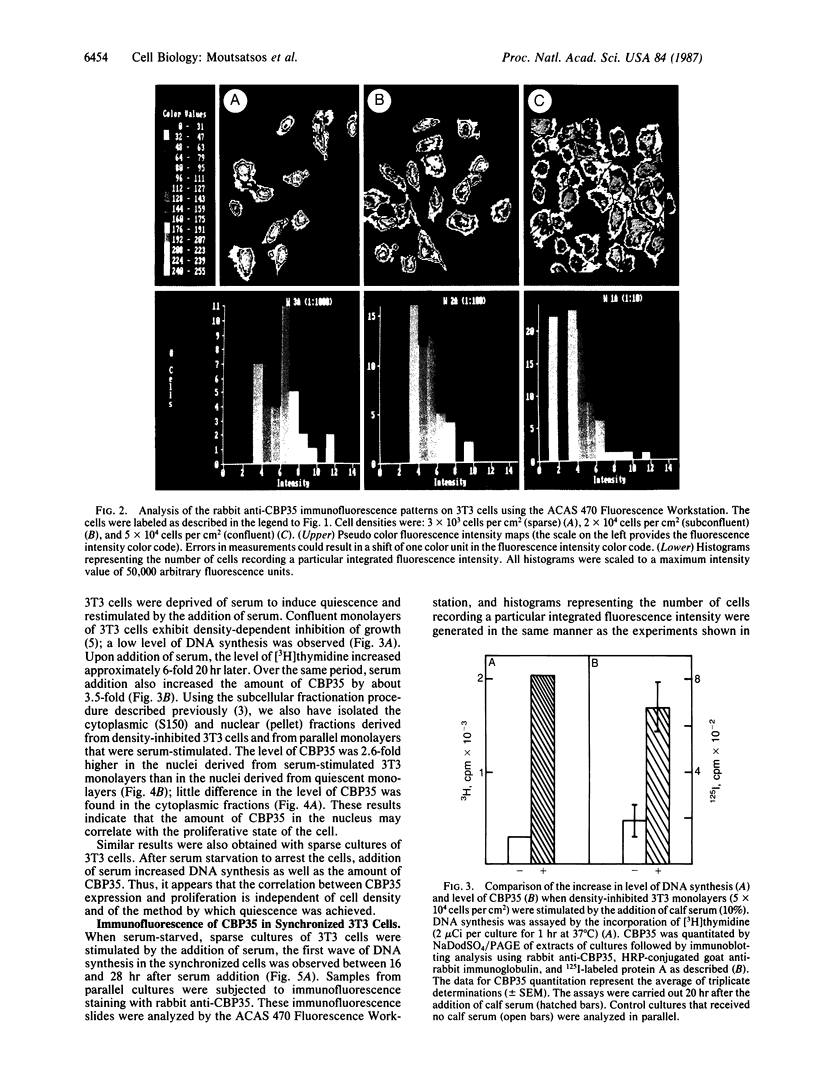
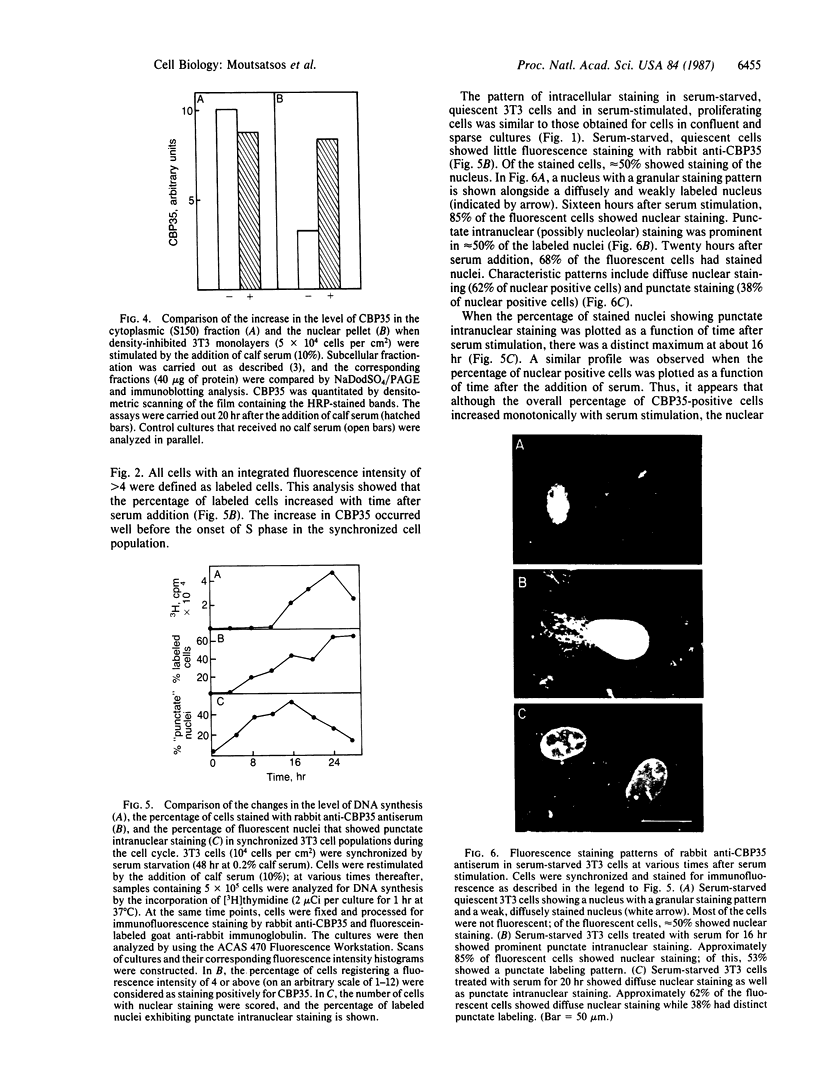
Proliferating 3T3 mouse fibroblasts contain higher levels of the lectin carbohydrate-binding protein 35 (CBP35) than do quiescent cultures of the same cells. An immunofluorescence study was carried out with a rabbit antiserum directed against CBP35 to map the cellular fluorescence distribution in a large population of cells under different growth conditions. This cytometric analysis showed that the lectin is predominantly localized in the nucleus of the proliferating cells. In quiescent 3T3 cultures, the majority of the cells lost their nuclear staining and underwent a general decrease in the overall fluorescence intensity. Stimulation of serum-starved quiescent 3T3 cells by the addition of serum resulted in an increase in the level of CBP35. The percentage of cells showing distinct punctate intranuclear staining reached a maximum at about the same time as the onset of the first S-phase of the cell cycle. All of these results suggest that CBP35 may be a protein whose presence in the nucleus, in discrete punctate distribution, is coordinated with the proliferation state of the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C., Barondes S. H. Chicken tissue binding sites for a purified chicken lectin. J Supramol Struct. 1980;13(2):219–227. doi: 10.1002/jss.400130210. [DOI] [PubMed] [Google Scholar]
- Carding S. R., Thorpe S. J., Thorpe R., Feizi T. Transformation and growth related changes in levels of nuclear and cytoplasmic proteins antigenically related to mammalian beta-galactoside-binding lectin. Biochem Biophys Res Commun. 1985 Mar 15;127(2):680–686. doi: 10.1016/s0006-291x(85)80215-1. [DOI] [PubMed] [Google Scholar]
- Crittenden S. L., Roff C. F., Wang J. L. Carbohydrate-binding protein 35: identification of the galactose-specific lectin in various tissues of mice. Mol Cell Biol. 1984 Jul;4(7):1252–1259. doi: 10.1128/mcb.4.7.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moutsatsos I. K., Davis J. M., Wang J. L. Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. J Cell Biol. 1986 Feb;102(2):477–483. doi: 10.1083/jcb.102.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Meromsky L., Carmi P., Karakash R., Lotan D., Lotan R. Monoclonal antibodies to endogenous galactose-specific tumor cell lectins. EMBO J. 1984 Dec 1;3(12):2979–2983. doi: 10.1002/j.1460-2075.1984.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Rosevear P. R., Wang J. L., Barker R. Identification of carbohydrate-binding proteins from mouse and human fibroblasts. Biochem J. 1983 Jun 1;211(3):625–629. doi: 10.1042/bj2110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Wang J. L. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J Biol Chem. 1983 Sep 10;258(17):10657–10663. [PubMed] [Google Scholar]
- Seve A. P., Hubert J., Bouvier D., Bouteille M., Maintier C., Monsigny M. Detection of sugar-binding proteins in membrane-depleted nuclei. Exp Cell Res. 1985 Apr;157(2):533–538. doi: 10.1016/0014-4827(85)90138-7. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck P. A., Voss P. G., Wang J. L. Growth control in cultured 3T3 fibroblasts. Assays of cell proliferation and demonstration of a growth inhibitory activity. J Cell Biol. 1979 Dec;83(3):562–575. doi: 10.1083/jcb.83.3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sève A. P., Hubert J., Bouvier D., Bourgeois C., Midoux P., Roche A. C., Monsigny M. Analysis of sugar-binding sites in mammalian cell nuclei by quantitative flow microfluorometry. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5997–6001. doi: 10.1073/pnas.83.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]