Abstract
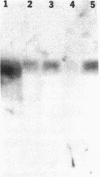
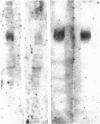
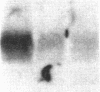
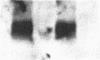
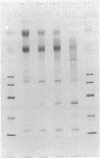
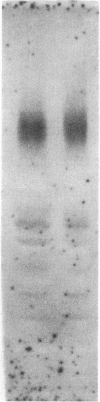
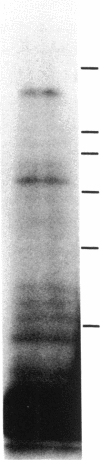
We report that a 120-kDa glycoprotein is the predominant laminin-binding protein detected within plasma membranes of rodent NG108-15 neural hybrid cells, embryonic chicken brain, and mouse 3T3 fibroblasts. This protein was detected when membrane extracts were separated by PAGE, transferred to nitrocellulose, and incubated with laminin at concentrations as low as 2.8 X 10(-11) M, under conditions of physiological ionic strength and pH and in the presence of calcium ions. It behaves as an integral membrane component, and its laminin-binding moiety is accessible to the external face of the cell surface. Moreover, it appears to bind to a site on laminin that is very sensitive to proteolysis. The properties of this protein, which we have termed "cranin," distinguish it from other known laminin receptors and make it a candidate to mediate some of the effects of laminin upon cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. J., Juliano R. L. Expression and function of a putative cell surface receptor for fibronectin in hamster and human cell lines. J Cell Biol. 1986 Oct;103(4):1595–1603. doi: 10.1083/jcb.103.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Shea E., Duggan K., Horwitz A. F. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J Cell Biol. 1986 Dec;103(6 Pt 1):2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary E. C., Saku T., Furthmayr H. Inhibition of laminin self-assembly and interaction with type IV collagen by antibodies to the terminal domain of the long arm. J Cell Biol. 1986 Nov;103(5):1689–1697. doi: 10.1083/jcb.103.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D., Timpl R., Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984 Jul;3(7):1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F., Buck C. A. A monoclonal antibody identifies a glycoprotein complex involved in cell-substratum adhesion. Exp Cell Res. 1985 Mar;157(1):218–226. doi: 10.1016/0014-4827(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B. Epithelial cell adhesion molecules. Exp Cell Res. 1986 Mar;163(1):1–21. doi: 10.1016/0014-4827(86)90554-9. [DOI] [PubMed] [Google Scholar]
- Paulsson M., Deutzmann R., Dziadek M., Nowack H., Timpl R., Weber S., Engel J. Purification and structural characterization of intact and fragmented nidogen obtained from a tumor basement membrane. Eur J Biochem. 1986 May 2;156(3):467–478. doi: 10.1111/j.1432-1033.1986.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Maxwell G. D., Shur B. D. Evidence for a novel enzymatic mechanism of neural crest cell migration on extracellular glycoconjugate matrices. J Cell Biol. 1986 Feb;102(2):432–441. doi: 10.1083/jcb.102.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M. Isolation of an adhesion-mediating protein from chick neural retina adherons. J Cell Biol. 1985 Sep;101(3):1071–1077. doi: 10.1083/jcb.101.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Smalheiser N. R., Crain S. M., Reid L. M. Laminin as a substrate for retinal axons in vitro. Brain Res. 1984 Jan;314(1):136–140. doi: 10.1016/0165-3806(84)90184-6. [DOI] [PubMed] [Google Scholar]
- Smalheiser N. R., Schwartz N. B. Kinetic analysis of 'rapid onset' neurite formation in NG108-15 cells reveals a dual role for substratum-bound laminin. Brain Res. 1987 Jul;431(1):111–121. doi: 10.1016/0165-3806(87)90200-8. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Johansson S., van Delden V., Oberbäumer I., Hök M. Characterization of protease-resistant fragments of laminin mediating attachment and spreading of rat hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8922–8927. [PubMed] [Google Scholar]
- Turner D. C., Gibralter D. Regulation of cell interactions during skeletal muscle development. Curr Top Cell Regul. 1985;26:115–126. doi: 10.1016/b978-0-12-152826-3.50016-4. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]