Summary
Objective
Magnetic resonance imaging (MRI) has greater sensitivity to detect osteoarthritis (OA) damage than radiographs but it is uncertain which MRI findings in early OA are clinically important. We examined MRI abnormalities detected in knees without radiographic OA and their association with incident knee symptoms.
Method
Participants from the Multicenter Osteoarthritis Study (MOST) without frequent knee symptoms (FKS) at baseline were eligible if they also lacked radiographic features of OA at baseline. At 15 months, knees that developed FKS were defined as cases while control knees were drawn from those that remained without FKS. Baseline MRIs were scored at each subregion for cartilage lesions (CARTs); osteophytes (OST); bone marrow lesions (BML) and cysts. We compared cases and controls using marginal logistic regression models, adjusting for age, gender, race, body mass index (BMI), previous injury and clinic site.
Results
36 case knees and 128 control knees were analyzed. MRI damage was common in both cases and controls. The presence of a severe CART (P = 0.03), BML (P = 0.02) or OST (P = 0.02) in the whole knee joint was more common in cases while subchondral cysts did not differ significantly between cases and controls (P > 0.1). Case status at 15 months was predicted by baseline damage at only two locations; a BML in the lateral patella (P = 0.047) and at the tibial subspinous subregions (P = 0.01).
Conclusion
In knees without significant symptoms or radiographic features of OA, MRI lesions of OA in only a few specific locations preceded onset of clinical symptoms and suggest that changes in bone play a role in the early development of knee pain. Confirmation of these findings in other prospective studies of knee OA is warranted.
Keywords: MRI, Knee osteoarthritis, BML, Epidemiology
Introduction
Osteoarthritis (OA) is the leading cause of physical disability in the US1 accounting for an estimated $7 billion in lost productive time per annum in the US. In longitudinal studies, the onset of knee pain is associated with a marked and persistent reduction in function2. However, there is only a modest correlation between clinical features and structural changes of knee OA as measured by radiographs3–5 and change in symptoms is poorly predicted by baseline radiographic features6.
Magnetic resonance imaging (MRI) has a greater sensitivity than radiographs to detect bone and soft tissue changes which are considered features of OA7,8. In early knee OA, before the appearance of radiographic features, MRI findings of structural abnormalities may provide clues to the subsequent course of disease. Identifying the structural characteristics that are prognostic would aid decision making for both the individual and treating physician as well as highlighting potential pathological mechanisms for the research community.
Bone marrow lesions (BMLs) are, in some cohorts, associated with clinical outcomes. Felson et al. have previously reported that in persons with knee OA or at risk of knee OA, an increase in size of BMLs seen on knee MRI is related to the contemporaneous onset of knee pain9. Our objective was to determine, in radiographically normal knees without significant knee pain, whether MRI findings are predictive of incident knee symptoms 15 months later.
Methods
STUDY DESIGN AND SUBJECTS
The Multicenter Osteoarthritis Study (MOST) is a prospective epidemiologic study of adults, aged 50–79 years, in which the goal is to identify risk factors for incident knee OA and progressive knee OA. Details of the study design and methods have been reported previously9. Study subjects either had knee OA or were at high risk of developing the disease. Factors considered to contribute to a high risk of knee OA included being overweight (defined as having a body weight higher than the median weight for each age- and sex-specific group based on the data from the Framingham OA Study10), a prior knee injury that made it difficult to walk for at least 1 week, previous knee surgery or having frequent knee symptoms (FKS) without radiographic OA. Those weighing more than 350 pounds were excluded as they would be unable fit in the extremity MRI scanner. Subjects in MOST were recruited from two communities in the US, Birmingham, Alabama and Iowa City, Iowa, through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. Each center also recruited ethnic minorities according to their representation in the recruitment population. Subjects were excluded if they screened positive for rheumatoid arthritis11, had ankylosing spondylitis, psoriatic arthritis, reactive arthritis, experienced problems with the kidneys that resulted in the need for hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), undergone bilateral knee replacement surgery, were unable to walk without the help of another person or walker, or were planning to move out of the area in the subsequent 3 years.
The study protocol was approved by the institutional review boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco, and Boston University Medical Center.
The participants from this study were a subset of those selected for a nested case–control study of the onset of FKS at the 15-month follow-up exam. Details of this study have been published elsewhere9,12 and are briefly described here. At baseline, all study subjects were asked about FKS as follows: “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?” This question was posed to subjects both by phone interview and during a clinic visit 1 month thereafter. If the subject answered no regarding the presence of pain, aching, or stiffness in the knee at both time points at baseline, that knee was considered eligible for the nested case–control study. At 15 months' follow-up, this same question regarding knee pain was posed to subjects, again both in a phone interview and at a clinic visit. This question was intended to identify pain, aching, or stiffness “on most days,” and because the two time points were, on average, 1 month apart, we characterized a positive response at both time points as consistent FKS. Knees with consistent frequent symptoms at follow-up were considered to be case knees. For the nested case–control study, knees from the same source population as cases but without consistent frequent symptoms at the 15-month follow-up were randomly selected as controls, in a ratio of two per case knee. Those knees that had frequent symptoms at one but not the both follow-up assessments were eligible to be controls. Both case and control knees were also required to have an acceptable MRI at baseline.
At the baseline clinic examination, subjects were weighed (without shoes) on a balance-beam scale to determine the body mass index (BMI) computed as weight (kg)/height squared (m2). Subjects completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)13, for each knee at both the baseline and 15-month visits.
Radiographic assessment
At baseline, all subjects underwent weight-bearing, posteroanterior (PA) fixed-flexion radiography, using the protocol by Peterfy14 and a Plexiglass positioning frame (SynaFlexer, TM) and weight-bearing lateral radiographs using the Framingham Study protocol15. A musculoskeletal radiologist (PA) and a rheumatologist (DF) experienced in reading study films and who were blinded to both the case/control status and clinical data, graded all of the PA radiographs for radiographic features of tibio-femoral OA and the lateral films for radiographic features of patello-femoral (PFJ) OA using the published osteoarthritis research society international (OARSI) atlas16. The lateral view was also assessed for tibio-femoral joint space narrowing (JSN) and anterior and posterior tibio-femoral osteophytes15.
MRI
MRIs were obtained with a 1.0 T dedicated MR system (OrthOne™; ONI Medical Systems, Wilmington, MA) with a circumferential extremity coil. All MRIs were performed using fat suppressed, fast spin-echo, proton density-weighted sequences in two planes, the sagittal plane (repetition time [TR] 4800 ms, time to echo [TE] 35 ms, slice thickness 3 mm, interslice gap 0 mm, field of view [FOV] 14 cm × 14 cm, matrix 288 × 192 pixels, number of excitations [NEX] 2) and the axial plane (TR 4700 ms, TE 13.2 ms, slice thickness 3 mm, interslice gap 0 mm, FOV 14 cm × 14 cm, matrix 288 × 192 pixels, NEX 2), and a STIR sequence in the coronal plane (TR 7820 ms, TE 14 ms, inversion time 100 ms, slice thickness 3 mm, interslice gap 0 mm, FOV 14 cm × 14 cm, matrix 256 × 256 pixels, NEX 2).
Two musculoskeletal radiologists (AG and FR), who were blinded to the case–control status and clinical data, semi-quantitatively assessed the MRIs for evidence of CARTs, osteophytes (OST), BMLs, subchondral cysts and cruciate ligament damage according to the Whole-Organ MRI Score (WORMS)17. Both, the tibio-femoral and the PFJ compartments were evaluated. Scores were applied in subregions defined by the WORMS method for the following features: CART (0 = no damage, 1 = signal change, 2 = focal partial thickness, 3 = multi-focal partial thickness with <75% region, 4 = multifocal partial thickness >75% region, 5 = full thickness <75% region, 6 = full thickness >75% region), OST (0 = none, 1 = equivocal, 2–3 = small, 4–7 = moderate/large), BMLs (0–3) and subchondral cysts (0–3) in each subregion of the knee and cruciate ligament damage (Fig. 1/Table I). For BMLs and subchondral cysts a score of 1 = <25% of region, 2 = 25–50% and 3 ≥ 50% of the region (Fig. 2). The weighted kappa coefficients of inter-reader reliability for the readings were 0.51 for OST (comparing 0–7 scores in each subregion); 0.78 (0.76–0.81) for cartilage (comparing 0–6 scores in each subregion); 0.62 (0.57–0.68) for BMLs (comparing 0–3 scores in each subregion) and 0.63 (0.57–0.69) for bone attrition. As a cartilage grade of 1 represents a signal abnormality and not a morphological change, we combined grades 0 and 1.
Fig. 1.
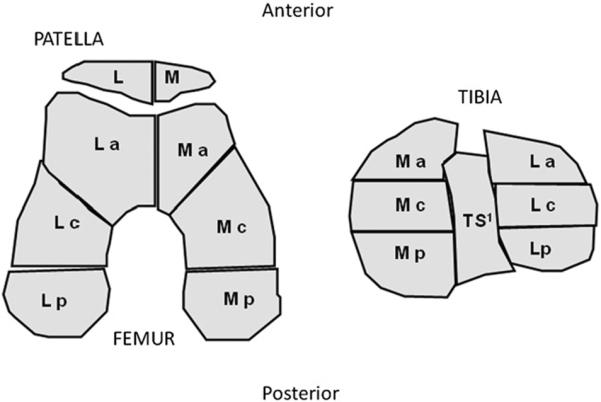
Schematic diagram to illustrate the subregions scored for BMLs and subchondral cysts using WORMS. Legend: M = medial; L = lateral; a = anterior; c = central; p = posterior. 1The TS region is only scored for BMLs and subchondral bone cysts and not OST nor CARTs.
Table I.
Definitions of ANY and severe feature scores from WORMS used to derive worsening (≥1 change) and severe changes at each subregion
| Pathologic features | Scale range | Sub regions | ANY | Severe |
|---|---|---|---|---|
| Cartilage lesion (CART) | 0–6 | T, F, Pt | >1 | ≥5 |
| Osteophytes (OST) | 0–7 | T, F, Pt | >0 | ≥4 |
| Bone marrow lesion (BML) | 0–3 | T, F, Pt, Ts | >0 | ≥2 |
| Bone cysts (CYST) | 0–3 | T, F, Pt, Ts | >0 | ≥2 |
T = tibia medial/lateral anterior/central and posterior. F = femur medial/lateral anterior/central and posterior. Pt = patella medial and lateral. Ts = tibial subspinous.
Fig. 2.
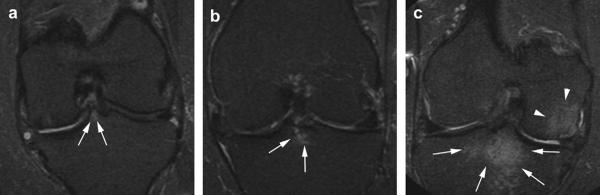
MOST OrthOne MRIs of the knee illustrating grades 1, 2 and 3 BMLs at the TS subregion. (a) Coronal STIR image. WORMS grade 1 bone marrow lesion in the medial spine of the intercondylar eminence in the TS region (arrows). (b) Coronal STIR image. WORMS grade 2 bone marrow lesion (arrows) in the TS region directly adjacent to the tibial insertion of the anterior cruciate ligament. (c) Coronal STIR image. Large grade 3 bone marrow lesion in the TS region. BML extends into the central subregions of the medial and lateral tibia (arrows). Note also large BML central medial femur (arrowheads).
As there is no consensus on the definition of a mild, moderate or severe MRI feature, using clinical judgment, each subregion were categorized as any and severe for cartilage (any >1, severe = 5–6); OST (any >0, severe = 4–7); BMLs and subchondral cysts (any >0, severe = 2–3) (Table I).
DATA ANALYSIS
For the present study, we analyzed a subset of knees from the nested case–control study of frequent symptom onset, selecting only those that had no baseline radiographic OA, defined as grade 0 using the OARSI atlas for all the radiographic features (OST, JSN, cysts and sclerosis) in the traditional PA projection for the tibio-femoral joint (TFJ) and in the lateral projection for the PFJ.
We compared the presence of feature damage in each compartment using clustered logistic models. We then compared frequent symptom onset case and control knees for (1) the presence of a baseline MRI finding of each type, and (2) the presence of a severe baseline MRI finding of each type (see Table I for definitions). These comparisons were made for MRI findings of articular damage in the whole knee and in each compartment, medial tibio-femoral (MTFJ), lateral tibio-femoral (LTFJ) and PFJ. We also examined whether MRI findings were more or less common in specific subregions of cases compared to controls. For example, we determined whether the presence of CART in the medial subregion of the PFJ differed between cases and controls. Thirteen participants contributed both knees to the analysis so we used generalized estimating equations (GEE) to take into account the clustering by person. Differences in the presence and subregion location of MRI findings between cases and controls were evaluated using marginal logistic models. We compared the total number of subregions in the whole knee with MRI findings of each an ordinal outcome for cartilage, subchondral cysts and BMLs and as a continuous outcome for OST.
For non-parametric distributions medians and inter-quartile ranges (IQR) are given. Models were adjusted for age, gender, race, BMI, reported previous injury, baseline WOMAC pain score and clinic site. All statistical tests were two-tailed and were calculated using Stata for Windows, version 10.0 (Statacorp, TX, USA).
As the number of cases was modest, we tested the stability of the multivariate regression model estimates and standard errors using a bootstrap analysis with 1000 replications as part of a sensitivity analysis. In addition, to account for the original sampling method we repeated the models using adjusted sampling weights.
Results
164 knees (155 participants) were included that at baseline had neither FKS nor radiographic features of OA. 36 of these knees went on to develop consistent FKS at 15 months (cases) and 128 remained without consistent FKS (controls). The baseline characteristics of the participants are shown in Table II. At baseline 53% of cases and 63% of control knees had WOMAC pain scores of zero (P = 0.25). As anticipated, case knees had substantially greater increases in WOMAC pain scores from baseline to 15 months. There was no difference in reported injury in cases vs controls (P = 0.6).
Table II.
Baseline characteristics of cases and controls from 155 participants
| Incident FKS |
|||
|---|---|---|---|
| Cases‡ (n = 33) | Controls (n = 122) | P value | |
| Age (SD) | 59.2 (7.6) | 59.3 (7.9) | 0.93 |
| Female (%) | 22 (67%) | 67 (55%) | 0.23 |
| White (%) | 30 (91%) | 103 (84%) | 0.34 |
| BMI kg/m2 (SD) | |||
| Women | 26.9 (4.7) | 28.4 (4.7) | 0.20 |
| Men | 28.7 (3.4) | 29.0 (3.5) | 0.43 |
| Knees |
|||
|---|---|---|---|
| n = 36 | n = 128 | ||
| Reported previous injury | 5 (14%) | 22 (17%) | 0.63 |
| Baseline total WOMAC >0 | 29 (81%) | 98 (77%) | 0.62 |
| Baseline WOMAC (pain) >0 | 17 (47%) | 47 (37%) | 0.25 |
| Baseline WOMAC (stiffness) >0 | 17 (47%) | 50 (39%) | 0.38 |
| Change in total WOMAC† | 18.4 (14.5) | 1.8 (9.9) | <0.001 |
| Change in pain WOMAC† | |||
Mean (SD) shown.
From baseline to 15 months.
Case defined by incident FKS OA at 15 months.
PRE-RADIOGRAPHIC MRI FEATURES AND PREDICTORS OF THE ONSET OF FKS AT 15-MONTH FOLLOW-UP
Although these knees had no radiographic features of knee OA, MRI evidence of abnormalities or damage to articular tissues was common in the whole knee and in each compartment (Table III). In case and control knees combined (data not shown in tables), CARTs were more common in the PFJ (P < 0.001) than in other compartments, OST more common in the MTFJ (P < 0.001) than any other compartments and BMLs were more common in the tibial subspinous (TS) and PFJ (P < 0.001).
Table III.
Frequency of any or severe articular damage on MRI at baseline in incident frequent knee pain cases (n = 36) and controls (n = 122), in the whole knee, knee compartments and TS region
| Knees | CART |
OST |
BML |
CYST |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Case |
Control |
Case |
Control |
Case |
Control |
|||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Whole knee * | ||||||||||||||||
| Any† | 29 | 80.6% | 86 | 67.2% | 36 | 100.0% | 127 | 99.2% | 27§ | 75.0% | 70 | 54.7% | 12 | 33.3% | 31 | 24.2% |
| Severe‡ | 8§ | 22.2% | 11 | 8.6% | 5§ | 13.9% | 5 | 3.9% | 11§ | 30.6% | 20 | 15.6% | 1 | 2.8% | 2 | 1.6% |
| Compartments | ||||||||||||||||
| Medial T–F | ||||||||||||||||
| Any† | 18 | 50.0% | 53 | 41.4% | 36 | 100.0% | 121 | 94.5% | 8 | 22.2% | 33 | 25.8% | 3 | 8.3% | 14 | 10.9% |
| Severe‡ | 1 | 2.8% | 1 | 0.8% | 2 | 5.6% | 4 | 3.1% | 2 | 5.6% | 7 | 5.5% | 1 | 2.8% | 1 | 0.8% |
| Lateral T–F | ||||||||||||||||
| Any† | 11 | 30.6% | 35 | 27.3% | 29 | 80.6% | 89 | 69.5% | 10§ | 27.8% | 18 | 14.1% | 3 | 8.3% | 4 | 3.1% |
| Severe‡ | 3§ | 8.3% | 1 | 0.8% | 2 | 5.6% | 0 | 0.0% | 3 | 8.3% | 4 | 3.1% | 0 | 0.0% | 0 | 0.0% |
| Patelofemoral | ||||||||||||||||
| Any† | 21 | 58.3% | 51 | 39.8% | 32 | 88.9% | 113 | 88.3% | 16∥ | 44.4% | 27 | 21.1% | 4 | 11.1% | 3 | 2.3% |
| Severe‡ | 5 | 13.9% | 9 | 7.0% | 1 | 2.8% | 2 | 1.6% | 5 | 13.9% | 7 | 5.5% | 1 | 2.8% | 0 | 0.0% |
| Other location | ||||||||||||||||
| TS | ||||||||||||||||
| Any† | 10§ | 27.8% | 17 | 13.3% | 6 | 16.7% | 15 | 11.7% | ||||||||
| Severe‡ | 4§ | 11.1% | 4 | 3.1% | 0 | 0.0% | 1 | 0.8% | ||||||||
Whole knee joint.
Knees or compartments with any feature damage.
Knee or compartments with severe feature damage.
From marginal logistic model adjusted for age, gender, race, BMI and clinic site comparing cases vs control knees: P < 0.05.
P < 0.01.
When we compared the presence of MRI lesions in the whole knee between cases and controls, the presence of only a few features differed between cases and controls: a severe CART OR 3.46 (1.2–10.3), a severe OST [OR 4.7 (1.3–18)] and the presence of any grade of BML [OR 2.8 (1.2, 6.5)] (Table III). Subchondral cysts did not differ significantly between cases and controls (P > 0.1) in any of the compartments. The number of subregions in the whole knee with any or severe damage for each feature, an indicator of the extent of abnormality, is shown in Table IV. The number of subregions affected with CARTs, BMLs and any OST size was significantly higher in cases than controls.
Table IV.
Number of subregions having any or severe damage in cases (n = 36) and controls (n = 122)
| Type of MRI damage | CART |
OST |
BML |
CYST |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
Case |
Control |
Case |
Control |
Case |
Control |
|||||||||
| Any | Sev* | Any | Sev* | Any | Sev* | Any | Sev* | Any | Sev* | Any | Sev* | Any | Sev* | Any | Sev* | |
| No. of affected subregions | ||||||||||||||||
| 0 | 19% | 78% | 33% | 92% | – | 86% | 1% | 96% | 25% | 69% | 44\5% | 84% | 67% | 97% | 76% | 98% |
| 1 | 25% | 14% | 20% | 9% | – | 14% | 4% | 3% | 36% | 22% | 34% | 13% | 22% | – | 20% | 2% |
| 2 | 22% | 8% | 21% | – | 6% | – | 9% | 1% | 25% | 6% | 16% | 3% | 6% | 3% | 3% | – |
| 3 | 19% | – | 14% | – | 17% | – | 18% | – | 11% | 3% | 3% | – | 6% | – | – | – |
| 4 | 3% | – | 8% | – | 8% | – | 16% | – | 3% | – | 2% | – | – | – | 1% | – |
| 5 | 6% | – | 4% | – | 6% | – | 8% | – | – | – | – | – | – | – | – | – |
| 6 | 3% | – | – | – | 11% | – | 9% | – | – | – | – | – | – | – | – | – |
| 7 | – | – | – | – | 8% | – | 13% | – | – | – | – | – | – | – | – | – |
| 8 | – | – | – | – | 28% | – | 7% | – | – | – | – | – | – | – | – | – |
| 9–12 | 3% | – | – | – | 17% | – | 15% | – | – | – | – | – | – | – | – | – |
| P value† | 0.05 | 0.01 | 0.02 | 0.06 | 0.01 | 0.03 | 0.09 | 0.24 | ||||||||
Severe feature damage (cartilage > 4, OST > 3, BML > 1 and subchondral cyst > 1).
Significance from marginal ordinal model with case–control as outcome adjusted for age, gender, race, BMI and clinic site for any damage.
By location, findings in two specific subregions were more common in cases than controls: in the TS subregion [any BML (P = 0.04) and a severe BML (P = 0.02)] (Table III) and a severe BML in the lateral patella (cases 18.9%, controls 6.6%, P = 0.040). The multivariate logistic model adjusted for age, ethnicity, BMI, gender and baseline WOMAC pain score is shown in Table V. As the subspinous region is the insertion site for the anterior cruciate ligament (ACL), we compared the prevalence of ACL damage as measured by WORMS in cases and controls. Only six knees (three case knees) had detectable ACL damage using WORMS and after adding baseline ACL status to the model, baseline severe BML still predicted 15-month case status [OR 4.9 (1.0–24.5)]. The OR estimates for a severe BML at the TS region remained similar but were no longer statistically significant [OR 5.6 (0.75–41.92), P = 0.053] when we repeated the models using weights to account for the original sampling method and bootstrapping.
Table V.
Presence of a BML at the TS subregion predicts incident FKS at 15 months
| Variable (units) | OR | 95% CI |
|---|---|---|
| Severe BML lesion | 6.7 | (1.3–34.0) |
| present at TS region* | ||
| Age (years) | 1.0 | (1.0–1.0) |
| Race (white vs non-white) | 0.4 | (0.1–1.7) |
| BMI (kg/m2) | 0.9 | (0.8–1.0) |
| Gender (female vs male) | 0.7 | (0.3–1.5) |
| Baseline WOMAC pain score | 1.0 | (1.0–1.0) |
| N | 164 |
OR and 95% confidence interval (CI) shown for multivariate marginal logistic with bootstrap estimates for SE. Also adjusted for clinic site.
Severe (grades 2, 3) vs non-severe (grade 0, 1) BML.
Discussion
In this study of knees at risk for knee OA with little or no pain and without radiographic OST or JSN at baseline, while MRI features of OA such as OST, cartilage damage and BMLs were common, we found few MRI features which predicted the development of frequent knee pain 15 months later. Knees with baseline MRI findings of BMLs, and in particular BMLs in the PFJ and the TS region, were more likely to develop frequent symptoms, as were knees with patellar OST.
Our findings contrast with those of Karachalios et al. who obtained knee MRIs in a consecutive sample of patients with significant knee pain and doubtful or minimal radiographic findings at the tibio-femoral and PFJ18. They found a low prevalence of OST and CARTs and no BMLs. The different results between the two studies may be due to differences patient inclusion as MOST participants had risk factors for OA. Ding et al. examined a sample of 372 healthy community controls and offspring of patients who had undergone total knee arthroplasty for OA, and found that 74% of knees with CARTs did not have radiographic changes, defined using the Altman atlas16 in the tibio-femoral compartments19. However, the PFJ was not imaged by radiographs in this study.
In many epidemiological studies of knee OA, subjects have been classified for the presence or absence of disease using radiographic criteria. In this study, the vast majority of participants with risk factors for knee OA, but without any radiographic structural damage as measured using standard views, had MRI detected CARTs, OST, BMLs and/or subchondral cysts. A few of these MRI findings were associated with the onset of frequent symptoms within 15 months, suggesting that these abnormalities represent structural features of early knee OA and that studies based on radiographic criteria alone may entail substantial misclassification of disease status. Definitions of early knee OA based on pathology detected on MRI plus clinical characteristics remain to be developed and validated. This should be a priority for future investigation20.
Previous literature has focused on the presence of BMLs and their association with clinical outcomes9,21. However there are few data examining the location of the BML; in this study, a BML grade >0 in the TS region was associated with the onset of knee pain. The subspinous region is a non weight-bearing site that is not considered part of the MTFJ or LTFJ. The subspinous region is instead exposed to stresses from the anterior and posterior cruciate ligaments. As BMLs have been proposed to correlate with bone loading22, a BML in the subspinous region is likely to reflect abnormal cruciate ligament strain23. A second less common type of subspinous BML is the result of extension of BMLs from the medial or lateral compartments in the subspinous subregion. This latter type of BML has been associated with subsequent medial compartment cartilage loss23. The findings at the TS region suggest a hypothesis that abnormal loading of the cruciate ligaments, with tensional forces on the subchondral bone, identifies those at risk of developing significant knee symptoms.
In cross-sectional surveys of knee pain, radiographic OA of the PFJ is common and, independent of tibio-femoral disease, and is associated with disability24,25. The presence of PFJ BMLs was one of the few MRI features associated with prevalent knee pain26. Our study supports the hypothesis that ascertainment of patello-femoral disease is substantially underestimated by radiographic techniques. Furthermore, the association between damage to the patella with the subsequent onset of knee symptoms suggests that MRI detected damage in the PFJ is of clinical importance.
Our study has several limitations. By design, MOST assessed the development of FKS, a patient-centred outcome encompassing pain, aching and stiffness27,28. Despite this focus of MOST, a major limitation of this analysis is the small number of radiographically normal knees without frequent symptoms at baseline that developed frequent symptoms at follow-up. In addition, this was an exploratory analysis evaluating associations with a large number of structural features of OA and P-values were not corrected for multiple analyses: these results require replication in future studies29. Knee OA has a generally slow course in its early stages, with symptoms that vary considerably over time before becoming more persistent and severe30. Longer follow-up is needed to fully evaluate the importance of these early OA findings on MRI for long-term clinical outcomes, such as the development of persistent pain. We used the PA projection radiograph to replicate current clinical practice for the TFJ and the lateral projection for the PFJ to identify knees without any radiographic features of OA. The lateral projection was also graded for TFJ JSN and OST formation, but as this is not a standard assessment we did not use these findings to define the presence of radiographic OA. However, neither femoral OST nor TFJ JSN were present on the lateral view for any of the knees we included in this analysis. Anterior tibial OST were found in one control knee and posterior tibial OST were found in one control and three case knees from the lateral view. As a sensitivity analysis, excluding these knees did not alter the findings of the study. As only complete ACL tears are measured using WORMS, we were unable to exclude an effect of partial ACL tears on incident symptoms. We did not exclude referred pain from the ipsilateral hip or lumbar spine. However, this would bias the estimates for the association between knee pain and knee damage towards the null. Some of the knees we studied already had mild or infrequent symptoms at baseline. As with most measures of disease onset, onset of knee symptoms requires defining a threshold that must be crossed to reach the endpoint. In this study, we considered consistent FKS (on most days of a month at two time points about a month apart) to be the threshold for clinically important symptoms. Knees with incident symptoms had substantially greater increases in WOMAC symptom scores than controls knees, indicating a high degree of consistency between our pain endpoint and changes on this validated pain instrument. In addition, we adjusted for baseline WOMAC knee pain scores and this did not affect our results.
In this cohort at high risk for knee OA, we found a high frequency of tissue damage, as detected by MRI, in knees with minimal or no symptoms and without any radiographic features of OA. Several specific MRI lesions predicted onset of clinically significant symptoms at 15 months, giving potential insights into the early structural determinants of pain in knee OA. Given the small numbers of knees studied, these findings require replication in larger studies.
Acknowledgements
We would like to thank the journal reviewers for their helpful comments. This work was supported by grants from the NIH/NIA (Felson – AG18820; Torner – AG18832; Lewis – AG18947; Nevitt – AG19069) and the NIHR Musculoskeletal Biomedical Research Unit (BRU), UK.
Footnotes
Conflict of interest Ali Guermazi is President of Boston Imaging Core Lab, LLC (BICL), Boston, MA, a company providing radiological image assessment services. He is shareholder of Synarc, Inc.
Frank Roemer is shareholder of BICL. The other authors have no conflicts of interest to declare.
References
- 1.Theis KA, Helmick CG, Hootman JM. Arthritis burden and impact are greater among U.S. women than men: intervention opportunities. J Womens Health (Larchmt) 2007;16(4):441–53. doi: 10.1089/jwh.2007.371. [DOI] [PubMed] [Google Scholar]
- 2.Jinks C, Jordan K, Croft P. Osteoarthritis as a public health problem: the impact of developing knee pain on physical function in adults living in the community: (KNEST 3) Rheumatology (Oxford) 2007;46(5):877–81. doi: 10.1093/rheumatology/kem013. [DOI] [PubMed] [Google Scholar]
- 3.Dieppe PA, Cushnaghan J, Shepstone L. The Bristol `OA500' study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5(2):87–97. doi: 10.1016/s1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 4.Szebenyi B, Hollander AP, Dieppe P, Quilty B, Duddy J, Clarke S, et al. Associations between pain, function, and radiographic features in osteoarthritis of the knee. Arthritis Rheum. 2006;54(1):230–5. doi: 10.1002/art.21534. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R, Peat G, Thomas E, Hay E, McCall I, Croft P. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66(1):86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paradowski PT, Englund M, Lohmander LS, Roos EM. The effect of patient characteristics on variability in pain and function over two years in early knee osteoarthritis. Health Qual Life Outcomes. 2005;3:59. doi: 10.1186/1477-7525-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19(7):822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 8.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl A):A87–94. doi: 10.1016/j.joca.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 11.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56(12):4048–54. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 14.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 15.LaValley MP, McLaughlin S, Goggins J, Gale D, Nevitt MC, Felson DT. The lateral view radiograph for assessment of the tibiofemoral joint space in knee osteoarthritis: its reliability, sensitivity to change, and longitudinal validity. Arthritis Rheum. 2005;52(11):3542–7. doi: 10.1002/art.21374. [DOI] [PubMed] [Google Scholar]
- 16.Altman RD, Hochberg M, Murphy WA, Jr., Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 17.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Karachalios T, Zibis A, Papanagiotou P, Karantanas AH, Malizos KN, Roidis N. MR imaging findings in early osteoarthritis of the knee. Eur J Radiol. 2004;50(3):225–30. doi: 10.1016/j.ejrad.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Ding C, Garnero P, Cicuttini F, Scott F, Cooley H, Jones G. Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage. 2005;13(3):198–205. doi: 10.1016/j.joca.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Guermazi A, Hunter D, Roemer FW, Niu J, Neogi T, McLennan CE, et al. MRI prevalence of different features of knee osteoarthritis in persons with normal knee X-rays. Arthritis Rheum. 2007;56(9):S128. [Google Scholar]
- 21.Berry PA, Davies-Tuck ML, Wluka AE, Hanna FS, Bell RJ, Davis SR, et al. The natural history of bone marrow lesions in community-based middle-aged women without clinical knee osteoarthritis. Semin Arthritis Rheum. 2008 doi: 10.1016/j.semarthrit.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52(9):2814–21. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Molina G, Guermazi A, Niu J, Gale D, Goggins J, Amin S, et al. Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum. 2007;58(1):130–6. doi: 10.1002/art.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844–9. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund M, Lohmander LS. Patellofemoral osteoarthritis coexistent with tibiofemoral osteoarthritis in a meniscectomy population. Ann Rheum Dis. 2005;64(12):1721–6. doi: 10.1136/ard.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 27.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 28.Thomas E, Peat G, Mallen C, Wood L, Lacey R, Duncan R, et al. Predicting the course of functional limitation among older adults with knee pain: do local signs, symptoms and radiographs add anything to general indicators? Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.080945. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 30.Peat G, Thomas E, Duncan R, Wood L, Hay E, Croft P. Clinical classification criteria for knee osteoarthritis: performance in the general population and primary care. Ann Rheum Dis. 2006;65(10):1363–7. doi: 10.1136/ard.2006.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]