Abstract
Objective
To examine the safety and efficacy of liquid risperidone to reduce duration of rages in children with severe mood dysregulation (SMD) or possible bipolar disorder (BP).
Method
There were 151 admissions of 5–12 year old children to a psychiatric inpatient unit. Diagnostic information and history of prior rages were obtained at admission. In hospital, a first rage outburst was treated with seclusion. If a 2nd rage occurred, the child was offered liquid risperidone to help him/her regain control. Risperidone dose was increased by 0.02 mg/kg for any successive rages. Duration of unmedicated and last medicated rage were compared. Rage frequency in children with SMD and several definitions of BP were compared.
Results
Although 82 of 151 admissions were prompted by rages, they occurred during only 49 hospitalizations, and occurred more than once in only 24. In 16 multiply-medicated children duration of rages dropped from a baseline of 44.4± 20.2 minutes to 25.6 ± 12.5 minutes at the child’s last dose. Neither SMD nor any definition of BP influenced rage response in this small sample. The average liquid risperidone dose was 0.02 mg/kg. All but 2 children also took atypical antipsychotics daily. No adverse events were observed.
Conclusions
Liquid risperidone may be a safe and effective way to shorten the duration of rage episodes regardless of diagnosis. However, definitive conclusions cannot be drawn in the absence of a placebo control as children were also receiving other behavioral and psychopharmacologic treatments.
BACKGROUND
Children with severe and prolonged anger responses to events viewed by others as relatively minor have been variously conceptualized. Recently, there has been a debate about whether they have a particularly virulent subtype of bipolar disorder (1,2) or a less diagnostically specific syndrome called “severe mood dysregulation” (SMD, 3). In the former, irritable mood should co-occur with excessive energy, grandiosity, rapid speech, and reduced need for sleep. The latter is operationalized as chronic (>1 year), under age 12 onset of frequent (several times a week), severe outbursts (markedly increased reactivity to negative stimuli) in the context of a sad or angry mood, and accompanied by symptoms of hyperarousal.
Currently recommended evidence-based treatments are aimed at acute mania (in the case of bipolar disorder), and include lithium, divalproex, and/or atypical antipsychotics (4). Unless SMD is conceptualized as ADHD, with aggression, there have been few attempts to treat it (5,6). Treatment may be aimed at the ADHD with aggression additionally treated with atypical antipsychotics, lithium, divalproex or an alpha 2 agonist (7). Interestingly, however, with neither conceptualization is treatment specifically aimed at the aggressive outbursts that often culminate in psychiatric hospitalization because of their severity.
For many years, agitated outbursts (which we will call rage outbursts) were treated in hospitals with seclusion or restraint (8). There are a few published reports of medication alternatives in children. These include diphenydramine (9), droperidol (10), and ziprasidone (11,12) but these have been intramuscular injections.
The drug risperidone, which is now FDA-approved for the treatment of mania down to age 10, has also been used with success for short term (2–3 months) treatment for aggressive behavior in children with conduct disorder, mild mental retardation and autism (13, 14). The goal of these trials was an overall lowering of chronic behavior problems rather than the termination of an episode of agitation, but the studies provided rough dosing guidelines. In general, children received total daily risperidone doses of between 0.02 and 0.06 mg/kg or between 0.5–3.5 mg.
Finally, liquid risperidone (with oral lorazepam) has been used with success to treat agitated adults in the emergency room (15), thereby allowing them to be discharged more quickly with less time spent in restraints. There are no comparable data in children.
The goals of this pilot study were to determine acceptability (whether the child would take the liquid medication when angry), safety and efficacy of liquid risperidone in rage outbursts in general, and in children with severe mood dysregulation and/or possible bipolar disorder in particular, and to compare liquid risperidone to usual treatment (i.e. seclusion and restraint) in terms of time to behavioral control, and need for a 2nd intervention.
METHODS
This study was approved by Stony Brook’s Committee on Research Involving Human Subjects. All parents/caregivers gave consent at admission to permit observations and ratings of a possible rage episode, as well as permission to use liquid risperidone with their child if needed. No one refused, but 3 children were not included in the medication aspect because they had had previous dystonic reactions taking risperidone. (Two ultimately never required risperidone).
A comprehensive, semistructured diagnostic interview was completed with parents/guardians of all admitted children based on their having completed the Child Symptom Inventory, a DSM IV-based rating scale, in advance (16, 17). Endorsed items from this rating scale were then reviewed with parents. A history of the presence/absence and frequency of rage outbursts at home was also obtained at admission. The diagnostic assessment has been described elsewhere (18). For study purposes, a best estimate diagnosis (19), was made by the first author (GAC) and the inpatient medical director (ZG), and included parent- provided history and child mental status, hospital course, and nurse and inpatient teacher observations. Since 141 (93.4%) admissions lasted more than one week, adequate observation of children after admission was possible in most cases. Diagnostic reliability was assessed in a random sub-sample of 25% of cases (between authors GAC and DM) using the procedure of Klein et al (20). Cohen’s kappas for the diagnostic categories relevant to this study were: for mania, learning and language disorders, externalizing disorder (either or both attention deficit hyperactivity disorder and oppositional defiant/conduct disorder) and psychosis, k=1.0; attention deficit disorder (ADHD), k=0.94; pervasive developmental disorder (PDD), k=0.91, anxiety k=0.85, depression k=0.83, oppositional defiant/conduct disorder k=0.74.
For this report, the diagnosis of bipolar disorder was examined from 5 viewpoints to address both broad and narrow diagnostic approaches to bipolar disorder: 1) bipolar diagnosis made by referring clinician; 2) DSM IV manic symptoms (elated/irritable mood + any of the “B” criteria) with or without episodes elicited from parent/guardian at admission 3) “narrow phenotype” mania/bipolar disorder (BP) requiring a clearly defined current episode with symptoms of mania described by parents and concurrent symptoms observed by the child psychiatrist, child psychologist or nursing staff as occurring most of the day and fulfilling unmodified DSM IV criteria (21); 4) The diagnosis of BP NOS/possible mania made if DSM IV symptoms of mania were transient, i.e, periods of behavior that lasted an hour or less. (The current operationalization of BPNOS (22) was not available when this study began); 5) Severe Mood Dysregulation (SMD), because it has been the subject of investigation as a possible “broad bipolar phenotype” (3). However, since this study was designed before SMD criteria were published, the condition was assigned retrospectively if the child had a history of chronic aggression lasting at least a year, frequent rages at home of sufficient severity and frequency that hospitalization resulted (i.e. markedly increased reactivity to negative emotional stimuli), and a history of irritability and 3 or more of the following: insomnia, agitation, distractibility, flight of ideas, pressured speech, intrusiveness as elicited in obtaining a history of manic symptoms, ADHD and/or oppositional defiant/conduct disorder.
In terms of safety, laboratory tests (CBC, electrolytes, BUN, creatinine, liver function tests, thyroid panel, cholesterol), and an EKG are done routinely on all admissions. No additional lab work was done with children who had rage outbursts. Any child who was given oral risperidone was evaluated for sedation and sleepiness as part of an Agitation Inventory developed for use in this project (19). The Abnormal Involuntary Movements Scale (AIMS, 24), Simpson-Angus scale for extrapyramidal symptoms (SAS, 25) and Barnes Akathisia Scale (BAS, 26) were completed within 2 hours of the end of each rage outburst for children who received liquid risperidone.
Procedure
A rage outburst was defined as sufficient agitation and loss of control such that the child was unable to “time out” (i.e. sit in a chair for 10 minutes on being told to do so) or was a danger to himself or others and a higher level of intervention was needed. In order to compare the efficacy of medication against usual treatment (i.e. seclusion/restraint), a first rage outburst in the hospital was treated non-medically; that is, the child was placed in an isolation room. The door remained open if the child was able to regain control and take the time out in the room; otherwise the door was closed. If there was a second episode, the child was told “You need some medicine to help you get back in control. Take this medicine or we may have to give you a shot”. If agreeable, the child was given 0.015 mg/kg of liquid risperidone.
Four highly experienced day and evening nursing “shift leaders” were trained to a reliability of K>0.8 to use the Children’s Agitation Inventory (23), an observational list developed to code the presence or absence of specific tantrum behaviors at 5, 15, 30, 60, 90 and 120 minutes or termination of the rage. Behaviors rated included physical aggression (e.g. hitting, kicking, pushing, throwing things), verbal aggression (e.g. cursing, screaming, threatening), other behaviors like throwing self on the floor and stamping feet, and mood and psychiatric symptoms (e.g. crying, pacing, being withdrawn and unresponsive, reporting or appearing to be having hallucinations). Also noted was whether the child was sedated or asleep. Reliability ranged from k=0.66 for pacing/psychomotor agitation to k=1.0 for physical and verbal aggression.
If the patient showed no evidence of improvement by 30 minutes following drug administration, the dose for a next rage was increased to 0.02 mg/kg. Since children were also often taking other scheduled atypical antipsychotics, the IRB required a ceiling dose on total amount of risperidone (or equivalent) that could be administered in one day. The ceiling dose was based on the child’s weight and ranged from 1.4 mg in a child weighing 20–24 kg to 4 mg in a child of 60–64 kg. We examined improvement using time to behavioral control or if there was the need for a 2nd intervention (locked door seclusion or, if the child was in danger of injuring himself, intramuscular dyphenhydramine which had been the rescue medication used prior to starting this study).
Adverse events such as sedation (as noted on the Agitation Inventory), extrapyramidal symptoms and akathisia were measured at the end of a rage outburst in which medication was administered using the coding inventory described above (24–26).
Data Analysis
Sample characteristics, and rates of SMD, parent-described manic symptoms and referring clinician-diagnosed BP rates prior to each admission, best estimate BP diagnoses and classes of discharge medication were compared using the Chi square statistic between admissions with no rages, one to two, and three or more rages. For BP, current mania and BP NOS were counted separately from lifetime mania since the former were the object of treatment. The paired t-test was used to calculate significance between non-medicated and maximally-medicated rage durations. The duration of rages in children with two or more liquid risperidone administrations were compared to those from the non-medicated state to account for any effect of the novelty of intervention.
RESULTS
SAMPLE
There were 151 admissions between January 2003 and June 2004. There were no rage outbursts in 102 hospitalizations and in 25 hospitalizations there was only one rage. Eight children had 2 rages, 7 had 3 rages, 3 had 4 rages, 2 had 5, and another 2 had 7. The highest number of rages (n=9) occurred in one child in each of 2 hospitalizations. Groupings of interest compare hospitalizations with no rages, 1–2 rages (received no risperidone or received only one dose) and 3 or more rages (received 2 or more doses of liquid risperidone) to highlight differences between these groups. Demographic and diagnostic information are described in Table 1. We analyzed rage duration in addition to rage number. As we have reported elsewhere (18), children with multiple rages were significantly younger than children with few or no rages and were more likely to have an externalizing disorder diagnosis.
Table 1.
Characteristics of Rages Sample N (%)
| variable | No Rages In hospital N=102 | 1–2 Rages n=33 | 3 or more Rages n=16 | Total N=151 | stat | significance |
|---|---|---|---|---|---|---|
| Mean age (mean ± SD) | 9.79 ± 2.1 | 9.76 ±2.2 | 8.19 ±2.0 | 9.64 ± 2.09 | F=4.55 | .012 |
| N (%) | N (%) | N(%) | N(%) | Chi sq | ||
| male | 79 (77.5) | 29 (87.9) | 12 (75.0) | 120 (79.5) | 1.88 | .390 |
| living with a parent | 79 (77.5) | 24 (72.7) | 11 (68.8) | 114 (74.5) | .741 | .690 |
| History of domestic violence | 37 (36.3) | 13 (39.4) | 6 (37.5) | 56 (37.9) | .105 | .949 |
| ≥ 1 severe academic delay | 39 (58.2) | 17 (68.0) | 7 (70.0) | 62 (61.4) | 1.06 | .589 |
| ≥1 psychiatric hospitalization | 35 (34.3) | 13 (39.4) | 6 (37.5) | 54 (37.9) | .304 | .859 |
| Best Estimate externalizing disorder diagnosis | 66 (67.5) | 29 (87.9) | 16 (100) | 111 (73.5) | 13.33 | .001 |
| History of: | ||||||
| Rages prior to admission | 40 (39.2) | 28 (84.8) | 14 (87.5) | 82 (54.3) | 28.87 df2 | .000 |
| Severe mood dysregulation | 26 (25.5) | 20 (60.6) | 12 (75.0) | 58 (38.4) | 23.12 df2 | .000 |
| Parent manic symptoms | 23 (22.5) | 10 (30.3) | 8 (50.0) | 41 (27.2) | 5.48 df2 | .065 |
| Community clinician bipolar diagnosis | 16 (15.7) | 13 (39.4) | 4 (25.0) | 33 (21.9) | 8.31 df2 | .016 |
| Best estimate bipolar diagnosis | ||||||
| Current mania | 3 (2.9) | 4 (12.1) | 1 (6.2) | 8 (5.3) | 6.151df6 | .407 |
| BP NOS | 2 (2.0) | 1 (3.0) | 1 (6.2) | 4 (2.6) | ||
| Lifetime mania | 2 (2.0) | 0 | 0 | 2 (1.3) | ||
| Total Current mania/BPNOS | 5 (4.9) | 5 (15.2) | 2 (12.5) | 12 (7.9) | 4.09 df2 | .130 |
The number of rages significantly correlated with length of stay (r=0.317, p<0.001). Eighty-two (54.3 %) admissions were precipitated by frequent rage episodes at home or school. However, 40 of these 82 (48.8%) children who had had a history of rages at home did not have them in hospital. As shown in table 1, a total of 58 (38.4%) children were considered to have symptoms of SMD by our definition (27.5%), and 75% continued to have multiple rages in hospital. Community clinicians were significantly more likely to diagnose BP in children who subsequently had rages in hospital (p=0.016). A trend showed that parents who had endorsed manic symptoms (not necessarily occurring during discrete episodes) prior to 41 of 151 admissions (27.2%), had children with multiple hospital rages (p=.065). Of note, these groups were not mutually exclusive. For instance, adding columns 2 and 3 of table 2, of 49 hospitalizations with rages, 42 (85.7%) had been preceded by rages at home, 18 (36.7%) parents had described manic symptoms, and 17 (34.6%) had been given a diagnosis of bipolar disorder by the referring clinician. In 10 hospitalizations (20.4%) there were both parent and clinician-described manic symptoms/bipolar diagnoses.
Table 2.
Discharge medications in children with and without multiple rages
| variable | No Rages In hospital N=102 | 1–2 Rages n=33 | 3 or more Rages n=16 | Total N=151 | Statistic | significance |
|---|---|---|---|---|---|---|
| Number of discharge medications (mean ± SD) | 1.8 ± 97 | 1.9 ± 84 | 2.3 ± .58 | F=1.60 Df 2 | .205 | |
| N (%) | N (%) | N (%) | N (%) | Chi sq | ||
| Any atypical antipsychotic | 65 (63.7) | 27 (81.8) | 16 (100) | 108 (71.5) | 11.13 | .004 |
| Any medication for ADHD | 38 (37.3) | 14 (42.4) | 9 (56.2) | 61(40.4) | 2.14 | .342 |
| Any Antidepressant | 45 (44.1) | 8 (24.2) | 4 (25.0) | 57 (37.7) | 5.43 | .066 |
| Any mood stabilizer | 19 (18.6) | 8 (24.2) | 6 (37.5) | 33 (21.9) | 3.03 | .220 |
Formally diagnosed mania/bipolar NOS observed in hospital appeared to be independent of rage numbers.
RISPERIDONE ACCEPTABILITY AND SAFETY
Over multiple episodes, only one child occasionally refused a dose of oral medication. The rest were willing to take medication and did not spit it back.
The average dose of liquid risperidone given for rage outbursts was 0.02 mg/kg, or between 0.5 and 1.2 mg per dose. However, 21 of the 23 children who received PRN risperidone were also taking atypical antipsychotics on a daily basis as well. Total daily doses of risperidone ranged from 1.4 to 3.1 mg. None of the children experienced extrapyramidal symptoms or akathisia as measured by the AIMS, SAS and BAS (24–26). Sedation or sleepiness was noted in 7/67 (10.44%) of medicated episodes and 8/46 (17.4%) of non-medicated episodes (NS).
RISPERIDONE EFFICACY
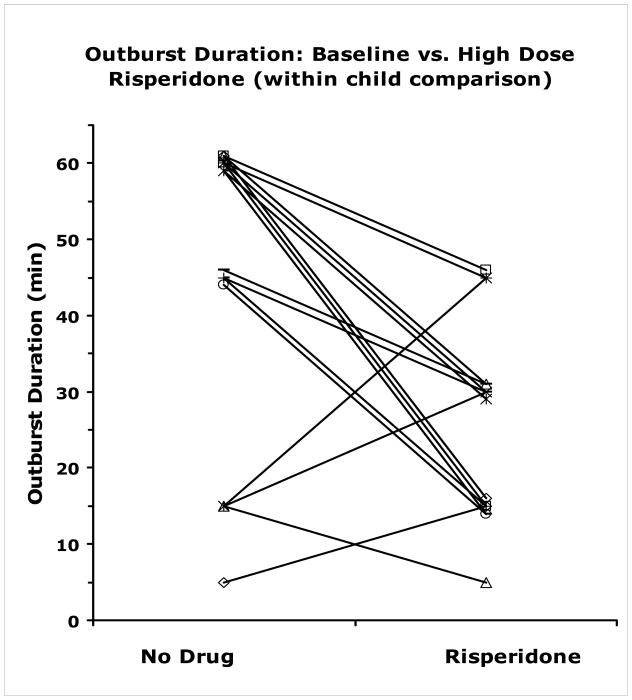
The average duration of the initial rage outburst for all children with rages was 50.8 ± 22.9 minutes. Of the 16 children who had more than 3 outbursts (and thus received liquid risperidone on more than one occasion) comparison of outburst duration between the unmedicated state and highest dose state revealed a significant drop in duration from 44.36 ± 20.15 minutes to 25.62 ± 12.5 minutes. The related measures t-test is t(15)=3.43, p<0.004. See figure 1.
Figure 1.
For each child with a least one acutely unmedicated and more than one acutely risperidone treated outburst, each line connects outburst duration at baseline (no drug) to outburst duration on highest dose. Lines which would otherwise overlap have been offset by ± 1 minute for display purposes
Response to liquid risperidone did not vary by any diagnostic variable measured, though only 16 children had multiple rages making data analysis difficult to interpret because of small sample size. On the other hand, there was no obvious pattern of medication combination that occurred during hospitalization that accounted for why some children had few and some had many outbursts or why some children responded sooner than others to treatment.
Reasoning that the type of medications prescribed at discharge was the best gauge of what was helpful during the hospitalization, we examined number and classes of medication used. As seen in table 2, atypical antipsychotics were used most frequently, usually in combination with an ADHD treatment, an antidepressant or a mood stabilizer. There were no significant differences in number of discharge medications based on whether or not a child received liquid risperidone (no risperidone, 1.8 ±0.97 discharge medications, 1 dose, 1.9 ± 0.84 medications, more than one dose, 2.3 ± 0.58 medications, F=1.602, df2, p=0.205).
Discussion
At least one rage outburst occurred in one third of admissions (49/151) occurring over 18 months. In 24 of those instances, multiple rages occurred and liquid risperidone was administered to 23 children with consent. Over 16 hospitalizations, multiple medication administrations occurred because 3 or more rage outbursts occurred (i.e. the first outburst was unmedicated, and two medication administrations were necessary to control for novelty of intervention). Within that small sample, risperidone appeared to shorten the duration of the last rage episode, though the episodes still continued for almost 30 minutes. Whether a higher dose of medication might have had a greater impact is not clear. It is also possible that order effects and other interventions are responsible for the decreased episode duration. The absence of a placebo control is an obvious limitation. Finally, the inability of an oral medication to further shorten an episode of agitation may simply be due to its rate of absorption. Onset of action begins roughly 30 minutes after oral administration (package insert).
The study by Currier and Simpson (15) also reported significant improvement in agitation scores in adults presenting to a psychiatric emergency room over the same time frame though those patients also received concurrent lorazepam.
Given the relatively low doses of risperidone used in the current study (0.02 mg/kg/dose), adverse events were negligible even superimposed on daily doses of atypical antipsychotic medication.
The procedure was acceptable to all of the children almost all of the time (only one child refused the oral medication occasionally). In other words, even when quite angry, children were willing to take something that was deemed possibly helpful, or to avoid a “shot”. For some children, this may have been therapeutic in and of itself.
We have reported elsewhere on the absence of relationship between observed acute mania and rage frequency in hospital (18). Although there was a relationship between number of hospital rages and both SMD “diagnosis” and community clinician antecedent diagnosis of BP (that is, children with severe mood dysregulation or clinician diagnosed bipolar disorder had more rages both prior to admission and while hospitalized), decrease in rage duration was independent of those and other diagnoses. While the absence of a formal structured interview may have been a limitation, there is currently no reason to think that such a medication response would be diagnosis-specific (27).
In conclusion, liquid risperidone may be a safe and effective way to shorten the duration of rage episodes regardless of diagnosis. However, it is impossible to draw definitive conclusions in the absence of a placebo control as children were getting other behavioral and psychopharmacologic treatments. Nevertheless, given the relatively low doses that were used, and the pharmacokinetic delays in the action of a drug that was administered at outburst onset, the resulting reduction in duration is a surprisingly robust effect.
Acknowledgments
This study was funded by a grant to Dr. Carlson from Janssen Pharmaceutica. The study was registered at clinicaltrials.gov.
We are especially grateful for the staff of the children’s inpatient unit without whose efforts this study would not have been possible.
Contributor Information
Gabrielle A. Carlson, Email: Gabrielle.Carlson@StonyBrook.edu, Professor of Psychiatry and Pediatrics, Director, Child and Adolescent Psychiatry, Stony Brook University School of Medicine, Putnam Hall-SUNY Stony Brook, Stony Brook, NY 11794-8790, Phone 631-632-8840, Fax 631-632-8953.
Michael Potegal, University of Minnesota, School of Medicine.
David Margulies, Stony Brook University School of Medicine.
Joann Basile, Stony Brook University School of Medicine.
Zinoviy Gutkovich, St. Lukes Hospital, New York.
References
- 1.Mick E, Spencer T, Wozniak J, Biederman J. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;1:58(7):576–82. doi: 10.1016/j.biopsych.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Wozniak J, Biederman J, Kwon A, Mick E, Faraone S, Orlovsky K, Schnare L, Cargol C, van Grondelle A. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biol Psychiatry. 2005 Oct 1;58(7):583–8. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Leibenluft E, Charney DS, Towbin KE, et al. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160(3):430–7. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 4.McClellan J, Kowatch R, Findling RL Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–25. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 5.Waxmonsky JG, Pelham WE, et al. The Impact of Manic-Like Symptoms on the Multimodal Treatment of Pediatric Attention Deficit Hyperactivity Disorder. J Child Adolesc Psychopharmacol. In press. [Google Scholar]
- 6.Dickstein DP, Towbin KE, et al. Randomized double-blind placebo-controlled trial of lithium in youth with severe mood dysregulation. J Child Adolesc Psychopharmacol. doi: 10.1089/cap.2008.044. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pliszka S AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 8.Masters KJ, Bellonci C, et al. American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002 Feb;41(2 Suppl):4S–2. doi: 10.1097/00004583-200202001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Vitiello B, Hill JL, et al. P.r.n. medications in child psychiatric patients: a pilot placebo-controlled study. J Clin Psychiatry. 1991 Dec;52(12):499–501. [PubMed] [Google Scholar]
- 10.Joshi PT, Hamel L, Joshi AR, Capozzoli JA. Use of droperidol in hospitalized children. J Am Acad Child Adolesc Psychiatry. 1998 Feb;37(2):228–30. doi: 10.1097/00004583-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Khan SS, Mican LM. A naturalistic evaluation of intramuscular ziprasidone versus intramuscular olanzapine for the management of acute agitation and aggression in children and adolescents. J Child Adolesc Psychopharmacol. 2006 Dec;16(6):671–7. doi: 10.1089/cap.2006.16.671. [DOI] [PubMed] [Google Scholar]
- 12.Barzman DH, DelBello MP, et al. A retrospective chart review of intramuscular ziprasidone for agitation in children and adolescents on psychiatric units: prospective studies are needed. J Child Adolesc Psychopharmacol. 2007 Aug;17(4):503–9. doi: 10.1089/cap.2007.5124. [DOI] [PubMed] [Google Scholar]
- 13.McCracken JT, McGough J, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002 Aug 1;347(5):314–21. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 14.Aman MG, De Smedt G, et al. Risperidone Disruptive Behavior Study Group Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002 Aug;159(8):1337–46. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 15.Currier GW, Simpson GM. Risperidone liquid concentrate and oral lorazepam versus intramuscular haloperidol and intramuscular lorazepam for treatment of psychotic agitation. J Clin Psychiatry. 2001;62:153–7. doi: 10.4088/jcp.v62n0303. [DOI] [PubMed] [Google Scholar]
- 16.Grayson P, Carlson GA. The utility of a DSM-III-R-based checklist in screening child psychiatric patients. J Am Acad Child Adolesc Psychiatry. 1991 Jul;30(4):669–73. doi: 10.1097/00004583-199107000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Sprafkin J, Gadow KD, et al. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Clin Child Adolesc Psychol. 2002 Dec;31(4):513–24. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GA, Potegal M, et al. Rages: What are they? Who has them? J Child Adolesc Psychopharmacol. 2009 doi: 10.1089/cap.2008.0108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 20.Klein DN, Ouimette PC, Kelly HS, Ferro T, Riso LP. Test-retest reliability of team consensus best-estimate diagnoses of axis I and II disorders in a family study. Am J Psychiatry. 1994;151:1043–1047. doi: 10.1176/ajp.151.7.1043. [DOI] [PubMed] [Google Scholar]
- 21.Carlson GA. Treating the bipolar controversy: A Tale of 2 Children. Am J Psychiatry. 2009;166:18–24. doi: 10.1176/appi.ajp.2008.08091362. [DOI] [PubMed] [Google Scholar]
- 22.Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potegal M, Carlson GA, et al. The behavioral organization, temporal characteristics, and diagnostic concomitants of “rage” outbursts in child psychiatry in-patients. Child Psychiatry and Human Development. doi: 10.1007/s11920-009-0020-2. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Rockville, MD: WU Department of Health, Education and Welfare; 1976. pp. 534–7. [Google Scholar]
- 25.Simpson GM, Angus JWS. A rating scale for extrapyrimidal side effects. Acta Psychiatr Scand. 1970;212:S11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Bames TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 27.Jensen PS, Youngstrom EA, et al. Consensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studies. J Am Acad Child Adolesc Psychiatry. 2007;46(3):309–22. doi: 10.1097/chi.0b013e31802f1454. [DOI] [PubMed] [Google Scholar]