Abstract
Airway remodeling in asthma contributes to airway hyperreactivity, loss of lung function, and persistent symptoms. Current therapies do not adequately treat the structural airway changes associated with asthma. The statins are cholesterol-lowering drugs that inhibit the enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase, the rate-limiting step of cholesterol biosynthesis in the mevalonate pathway. These drugs have been associated with improved respiratory health and ongoing clinical trials are testing their therapeutic potential in asthma. We hypothesized that simvastatin treatment of ovalbumin-exposed mice would attenuate early features of airway remodeling, by a mevalonate-dependent mechanism. BALB/c mice were initially sensitized to ovalbumin, and then exposed to 1% ovalbumin aerosol for 2 weeks after sensitization for a total of six exposures. Simvastatin (40 mg/kg) or simvastatin plus mevalonate (20 mg/kg) were injected intraperitoneally before each ovalbumin exposure. Treatment with simvastatin attenuated goblet cell hyperplasia, arginase-1 protein expression, and total arginase enzyme activity, but did not alter airway hydroxyproline content or transforming growth factor-β1. Inhibition of goblet cell hyperplasia by simvastatin was mevalonate-dependent. No appreciable changes to airway smooth muscle cells were observed in any of the control or treatment groups. In conclusion, in an acute mouse model of allergic asthma, simvastatin inhibited early hallmarks of airway remodeling, indicators that can lead to airway thickening and fibrosis. Statins are potentially novel treatments for airway remodeling in asthma. Further studies utilizing sub-chronic or chronic allergen exposure models are needed to extend these initial findings.
Keywords: Statin, HMG-CoA reductase, mevalonate, asthma, goblet cell hyperplasia, arginase, hydroxyproline, TGFβ1, nitric oxide, remodeling
INTRODUCTION
Asthma is a chronic inflammatory disease that can progress to irreversible airflow obstruction. The constellation of structural airway changes that occur in persistent asthma, termed ‘airway remodeling,’ contribute to loss of lung function and occur in multiple different lung compartments, manifesting as goblet cell hyperplasia, smooth muscle cell proliferation and hypertrophy, subepithelial fibrosis/collagen deposition, loss of airway epithelial barrier, angiogenesis, and fibroblast-to-myofibroblast changes[1, 2]. Airway remodeling has not been eradicated or prevented despite wide-use of anti-inflammatory agents[3]. Asthmatic patients who have established structural changes to their airways are poorly responsive to corticosteroid therapy[4], and no other current asthma therapy is effective in treating this remodeling.
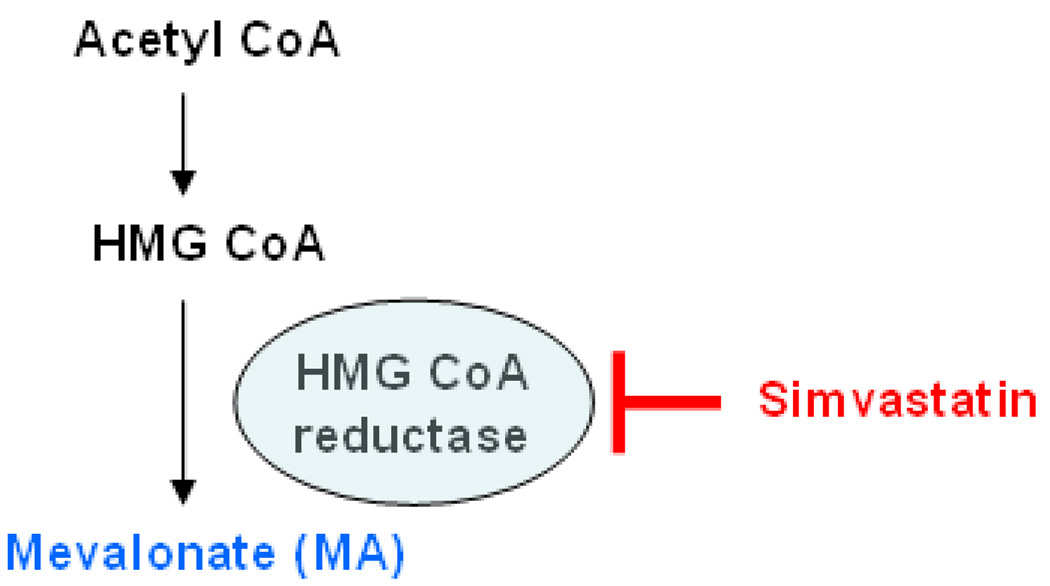
The statin drugs are cholesterol-lowering agents that inhibit the enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase), the rate-limiting step of cholesterol biosynthesis in the mevalonate (MA) pathway (Scheme 1). These drugs also have pleiotropic anti-inflammatory, anti-proliferative, and immunomodulatory effects beyond their lipid-lowering capacity[5, 6]. Statin use in patients with asthma and COPD has been associated with improved lung health in several observational clinical studies[7, 8]. Statins have also been proposed as potential new treatments for lung disease[9, 10]. Two short-term (4–8 weeks) clinical trials in asthma did not show a clinical benefit to statin treatment despite improvements in sputum markers of inflammation[11, 12]. However, the effects of statins on pre-established asthmatic airway remodeling may not be apparent after such brief treatment duration where lung function has already significantly declined in response to chronic inflammation.
Scheme 1. The mevalonate (MA) pathway and statin mechanism of action.
Simvastatin inhibits the rate-limiting enzyme hydroxymethylglutaryl (HMG)-CoA reductase to prevent the conversion of HMG-CoA to mevalonate (MA). Reversal of observed statin effects with MA co-treatment indicates that HMG-CoA reductase is the likely target enzyme of the statin.
In smoke-exposed rats, simvastatin treatment can attenuate small airway thickening[13] and inhibit the development of emphysema[14]. However, to date, the effects of statins on the development of asthmatic airway remodeling remain unknown in both animal models and humans. We previously showed that simvastatin inhibits airway hyperreactivity (AHR) in a mouse model of acute allergic inflammation[15]. This statin-mediated improvement in lung function was independent of inflammatory cell influx, where simvastatin likely had additional effects on airway resident cells. Because statins also have cellular anti-proliferative effects[5], this observation led us to investigate whether statins can attenuate the development of airway remodeling in the ovalbumin (OVA) mouse model.
One of earliest changes in allergen-induced asthma is goblet cell hyperplasia[16], an important component of long-term remodeling. A host of mechanisms may be responsible for “early remodeling”, such as goblet cell hyperplasia, but recent interest has focused on arginase and transforming growth factor-β1 (TGFβ1)[17–20]. Arginase enzymes convert the substrate L-arginine to ornithine which can be further metabolized to proline and hydroxyproline, important components of collagen. The Th2 cytokines IL-4 and IL-13 induce arginase expression in macrophages and lung tissue leading to subepithelial airway fibrosis[21, 22]. Similarly, TGFβ1 is a critical link between inflammation and fibrosis, a complex mediator that has both pro- and anti-inflammatory properties, as well as being pro-fibrogenic.
Given the clinical implications for asthma treatment, we examined whether systemic treatment with simvastatin, in an acute (2 week) OVA-exposure mouse model, attenuates airway remodeling such as goblet cell hyperplasia and pro-fibrogenic changes. We hypothesized that simvastatin treatment inhibits airway goblet cell hyperplasia, and lung arginase-1 (Arg1) and TGFβ1 protein expression by a MA-dependent mechanism. We used simvastatin to inhibit HMG-CoA reductase, and provided MA simultaneously to reverse the statin effect in order to test the hypothesis that simvastatin was acting specifically on the target enzyme HMG-CoA reductase (i.e. via the MA pathway (Scheme 1)).
We found that simvastatin attenuated airway goblet cell hyperplasia and inhibited lung Arg1 protein expression and enzyme activity. The simvastatin effect on goblet cell hyperplasia was reversed with MA co-treatment suggesting a MA-dependent mechanism for this early hallmark of remodeling. The inhibition of Arg1 by simvastatin is a novel finding in allergic asthma. The statin drugs may be an important modulator of airway remodeling with implications for longer-term asthma treatment.
METHODS
Animals
All procedures were performed as per an IACUC-approved protocol, following institutional standards and regulations for animal care and use. All mice were maintained in a HEPA-filtered laminar flow cage rack with a 12-hour light/dark cycle and allowed free access to food and water. Animals were housed and cared for by the veterinary staff of Animal Resource Services at the University of California, Davis in AALAC- accredited facilities, in plastic cages over autoclaved bedding in HEPA-filtered cage racks. Animals were routinely screened for health status by serology and histology by our veterinary animal resources facility. Eight week old BALB/c mice were purchased from Charles River and allowed to acclimatize for 1 week prior to initiation of OVA sensitization protocol. All experiments conform to the relevant ethical guidelines for animal research.
Exposure of Mice to OVA Aerosol
All mice were sensitized by intraperitoneal (i.p.) injection of chicken egg albumin (Ovalbumin (OVA), grade V, ≥98% pure, Sigma, St. Louis, MO, 2 × 10 µg/0.1 mL, 2 weeks apart) with alum as an adjuvant[23] starting on day 0. After a 4-week period of sensitization, mice were then exposed to aerosolized OVA (for 30 minutes) three times per week for 2 weeks (for six total exposures or ‘6OVA’). Mice were divided into six treatment groups: 6OVA+EtOH (where ethanol (EtOH) solution is the drug vehicle), 6OVA+Simvastatin (Sim), 6OVA+Sim+Mevalonate (MA), FA+EtOH, FA+Sim, and FA+PBS. Simvastatin was purchased from Sigma-Aldrich and prepared as described in Zeki et al. 2009[15]. OVA-exposed groups received intraperitoneal injections of 0.3 mL of 10% ethanol (simvastatin drug vehicle), 0.3 mL of Sim (40 mg/kg), or 0.3 mL of Sim plus 0.1 mL of MA (20 mg/kg). Filtered air (FA)-exposed mice also received their corresponding intraperitoneal injections of 0.3 mL of PBS (1x), 0.3 mL of 10% ethanol, or 0.3 mL of simvastatin (Sim, 40 mg/kg). Drug injections were administered 30 minutes before exposure to FA or 1% OVA aerosol in PBS (10 mL of a 10-mg/mL OVA solution).
To determine whether HMG-CoA reductase inhibition by simvastatin alters measured endpoints, we exposed mice to inhaled OVA or FA for 2 weeks, and treated them with simvastatin (40 mg/kg, with and without MA (20 mg/kg) co-treatment) or drug vehicle (EtOH) 30 minutes before all exposures. We previously reported no statistically significant differences in inflammatory cell influx between the 6OVA+EtOH and 6OVA+MA groups or their respective air controls[15]. To decrease the unnecessary use of mice, the experiments in the present study do not include these MA control groups (6OVA+MA and FA+MA).
Exposures to OVA aerosols were performed with chambers and generators as previously described[24]. Aerosol delivery was conducted via side stream nebulizer (Invacare Corp., Elyria, OH) and air compressor (Invacare Corp., Sanford, FL). Mass concentration and aerodynamic size distributions were determined with a Mercer-type cascade impactor[24]. Histological evaluations of OVA-exposed and FA-exposed control mice lungs demonstrated that we were able to induce airway inflammation, epithelial cell sloughing, and goblet cell hyperplasia in the OVA-exposed mice.
After completion of the final exposure, all mice underwent lung physiology studies with a full-body plethysmograph for restrained animals. Immediately thereafter, mice were killed with intraperitoneally administered Beuthanasia-D (0.3 mL at 1:6 dilution), followed by collection of tissue for analysis (blood draw, lung lavage, and lung isolation).
Airway Inflammation
Lungs were lavaged with two, 1-mL aliquots of phosphate-buffered saline (PBS), at pH 7.4. Each aliquot was passaged twice through the lungs. Lavage fluid was centrifuged (at 2,500 rpm for 10 minutes) to pellet the cells, and the supernatant was removed for inflammatory cytokine profiling. The remaining cell pellet was suspended in AKC lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.3) to lyse red blood cells, and finally resuspended in 0.5 mL PBS.
Total lavage live cell numbers were determined using Trypan blue exclusion and total live cells per mL lavage was calculated using a hemacytometer. Aliquots of 100 µL of the remaining cell suspension were processed onto slides using a cytocentrifuge at 1650 rpm for 15 minutes. Slides were air dried and stained with a Hema3 stain set as described in the manufacturer’s instructions (Fisher Scientific, Kalamazoo, MI) and sealed using Cytoseal (Richard Allen Scientific, Kalamazoo, MI). Cell percent differentials for alveolar macrophages were determined by counting 10 fields under a 40x objective based upon morphological characteristics and staining profiles.
Histological Preparation
Half of the animals had their lungs fixed for histological evaluation at 30 cm pressure using 1% paraformaldehyde in PBS (pH 7.5). After 24 hours of fixation, the left lung lobes were placed in 70% ethanol and embedded in paraffin. Lung sections of 5 µm thickness were made with special attention to cutting through the larger lobar bronchi in parallel then slides were dried at 37°C overnight. Lung sections were deparaffinized and processed for Arg1 or TGFβ1-specific immunohistochemistry or Alcian Blue-Periodic acid-Schiff (PAS) staining to quantify the number of mucus-containing goblet cells.
Whole Lung Lavage Cytokine Assays
The concentrations of helper T-cell type 2 (Th2) cytokines (IL-4, IL-13) and TGFβ1 in lung lavage supernatant were measured with commercially available multiplex assays (Millipore, St. Charles, MO). The assay for TGFβ1 measured the active dimerized form of the protein. For cytokine measurements that were below the lower detection limit of the assay, results were assigned a value equal to the minimal detection limit for the specific assay to facilitate statistical analysis.
Lung Compliance and Resistance Measurements
Mice were anesthetized and sedated with medetomidine, 0.5 mg/kg (Dormitor, Orion Pharma, Finland), and tiletamine/zolpidem, 50 mg/kg (Telazol, Fort Dodge Laboratories, Fort Dodge, IA) and ventilated at 7–8 cm3/kg with a mouse ventilator (MiniVent, Harvard Apparatus, Cambridge, MA) at a frequency of 150 breaths per min for the duration of the procedure. Dynamic lung compliance (Cdyn, as mL/cm of H2O) and respiratory system resistance (Rrs, as cm of H2O*s/mL) measurements were made at baseline and following serial 3-minute nebulizations of saline and methacholine (MCh) at doses 0, 0.5, 1.0 and 2.0 mg/mL. Dynamic compliance and respiratory system resistance were measured using a plethysmograph for restrained animals (Buxco Inc., Troy, NY) as described previously[25]. The percent change (% Decrease in Cdyn or % Increase in Rrs) is calculated as the difference between the physiologic parameter (Cdyn or Rrs) measured at baseline and after the third MCh bronchial challenge, divided by the baseline value (for % Decrease in Cdyn) or by the value measured after the third MCh bronchial challenge (for % Increase in Rrs).
Exhaled Nitric Oxide Measurements
Prior to the methacholine dose-response challenge, a 5-minute sample of exhaled gases was collected from the cannulated mice (immediately after insertion of a mouse into the plethysmograph), via the ventilator exhalation port during the PBS exposure and recovery periods (i.e. saline time-point). Samples were collected in specially constructed Mylar bags and the fraction of exhaled nitric oxide (FeNO) was measured in parts per billion (ppb) by a chemiluminescence assay using the Sievers Nitric Oxide analyzer (Sievers Inst., Boulder, CO). Placement of the Mylar bag did not affect pressure measurements[26].
Measurement of Nitrate/Nitrite in Whole Lung Homogenate
Nitrate and nitrite in isolated whole lung tissue homogenate were measured after reduction with acidified vanadium III using the Sievers NO Analyzer (Sievers, Boulder, CO) as an indicator of NO production. For specimens, 20 µg of total protein was deproteinized using 10% TCA. Samples were centrifuged at 14,000 rpm on a tabletop centrifuge and the supernatant was used to measure nitrate and nitrite content.
Alcian Blue-Periodic Acid-Schiff (PAS) Staining
Left lung sections prepared as described above were immersed in a 1% alcian blue, 3% glacial acetic acid solution (pH 2.5) for 30 minutes and then immediately rinsed in tap water. Slides were them immersed in a 1% periodic acid solution for 7 minutes. Slides were rinsed 3 times in distilled water and immersed in Schiff Reagent Solution consisting in 0.45% basic fuchsin, 10% HCl, 0.45% sodium bisulfite for 15 minutes. Slides were repeatedly immersed in running tap water until clear then counterstained by flash immersion in Harris’ Hematoxylin[27] and immediately rinsed repeatedly in running tap water. Slides were then dehydrated and mounted in cytoseal.
Each animal was represented by a single section of lung: 5 randomly selected regions were evaluated (two segments of the primary conducting airway, two segments from separate secondary conducting airways, and one segment from a tertiary conducting airway). A minimum of 100 sequential airway epithelial cells were counted from each region and the total number of PAS-positive cells per total epithelial cells was determined for each region. These regional values were then averaged to give a final PAS score per animal termed ‘mean goblet cell index’.
Western Blot Analysis of Tissue
Western blot methods are described in Bratt et al. 2009 with changes in methods described below[28]. Antibodies were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) unless otherwise stated. Right lung lobes were sonicated in a homogenization buffer and centrifuged on ice at 10,000 rpm for 20 minutes. The supernatant was stored at −80° C until further use. Total protein concentration was measured for each sample using the Micro BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Samples (75 µg total protein) were electroporated under reducing conditions and transferred to a polyvinylidene difluoride membrane. Membranes were probed using 0.8 µg/mL rabbit, anti-mouse NOS2; 0.6 µg/mL rabbit, anti-mouse NOS3; 0.4µg/mL of goat, anti-mouse Arg1; 0.4 µg/mL rabbit, anti-mouse TGFβ1 or 0.4 µg/mL rabbit, anti-mouse α-Actinin IgG in 5% dry milk in PBS and 40 ng/mL horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Pierce Biotechnology, Rockford, IL) or 40 ng/mL HRP-conjugated donkey anti-goat IgG (Pierce Biotechnology, IL) secondary antibodies in 5% milk in PBS. Bands were visualized using Western Lightning Plus-ECL substrate kit (PerkinElmer, Shelton, CT) and band intensity measured using the Fujifilm Image Reader LAS-3000 V2.1 (Fuji Photo Film Co, Cypress, CA).
Measurement of Total Arginase Activity
Arginase activity assay of tissue homogenates was adapted from Xu et al [29]. In brief, right lung lobe homogenates from OVA-exposed mice were aliquoted into volumes containing 10 µg total protein and FA-exposed animals were aliquoted to volumes containing 30 µg total protein. Tissue homogenate volumes were brought to a volume of 50 µL using homogenization buffer as described in the section on Western blot analysis.
Homogenate mixture was incubated with 100 µL 0.1% Triton X-100 for 30 minutes at room temperature on a shaker. After incubation, 100 µL of 25 mM Tris-HCl (pH 7.5) and 20 µL of 10 mM MnCl2 was added to each sample and incubated at 56° C for 10 minutes. Samples were then incubated with 100 µL 0.5 M L-arginine at 37° C for 1 hr and the reaction stopped with 900 µL of a solution containing 8.7% H2SO4, 23.2% H3PO4, 68% H2O (1:3:7 of 96% H2SO4: 85% H3PO4: water). Sample was mixed with 40 µL 9% ISPF dissolved in ethanol and heated to 95°C for 30 minutes. Relative urea concentration was calculated using a spectrophotometer at 540 nm, using a linear standard curve of arginase activity derived from a liver homogenate dilution series. The unit of measure for total arginase activity was represented by absorbance at 540 nm.
Arginase-1 and TGFβ1 Immunohistochemistry
Left lung sections were prepared as described. Slides were incubated in 1 mM ETDA, pH 7.5 at 100°C for 20 minutes to decloak the antigen. After rinsing in deionized water, sections were processed using the R&D Systems Cell and tissue staining kit HRP-DAB System (Minneapolis, MN) for rabbit antibodies. Sections were incubated overnight at 4°C in 1 µg/mL goat anti-mouse arginase-1 (Arg1) IgG diluted in 5% goat serum in 1% BSA, 1.33 µg/mL rabbit anti-mouse TGFβ1 IgG, 1 µg/mL goat IgG (isotype control), or serum+BSA only (negative control). All other reagents were diluted as per manufacturer’s instructions. Incubation times were as follows; 1 hr in biotinylated goat anti-rabbit or biotinylated donkey anti-goat secondary antibody, 30 minutes in HSS-conjugated HRP, and 15 minutes in diaminobenzidine.
Results were independently scored by two blinded observers for TGFβ1 immunohistochemical staining, with the lung tissue divided into six compartments; proximal airway epithelium, distal airway epithelium, airway and vascular smooth muscle cells, parenchyma, macrophages, and basement membrane. For the Arg1 assessment, the lung tissue compartments were divided into airway epithelial and subepithelial compartments for the qualitative assessment of immunohistochemical staining. Arg1 staining was defined as present or absent, and if present further defined as weak, moderate, or strong. For TGFβ1, a grading system of 0–5 was established prior to the grading based upon pre-stained slide standards. All slides were scored under x200 power using a linear intensity grading scale: (0 – no stain as compared to a primary antibody negative control to 5 – dramatically increased staining).
Hydroxyproline Measurement in Isolated Airways
The colorimetric assay method was based upon methods derived from Woessner[30] with the modifications described below. In brief, fresh right lung lobes were transferred to ethanol and microdissected, producing a preparation of airways separate from the associated parenchyma. These preparations consisted of the right mainstem bronchus to the terminal bronchioles of the four right lobes. Airways were then homogenized in 500 µL milliQH2O with a 50 µL aliquot removed for BCA assay of total protein content and the remaining sample centrifuged at 3000 rpm for 10 minutes. The supernatant was removed and the pellet resuspended in 100 µL of 6 M HCl. The suspension was transferred to a glass tube, sealed, and incubated overnight at 110°C. Tubes were then cooled to room temp and opened. A volume of 30 µL 0.1% phenol red was added to each sample. Samples were neutralized with 80 µL 6 M NaOH and 100 µL of solution, then transferred to glass test tubes for analysis. Samples were normalized to total protein content and treatment groups compared using statistical tests as described in Results.
Statistical Analysis
Results are presented as mean values ± SEM. Means were compared by unpaired Student's t-test or ANOVA (1-way or 2-way), with Tukey's or Bonferroni correction for multiple comparisons applied where appropriate, using the Prism 5 software package (Graphpad, Inc., San Diego, CA). Non-parametric analyses were done using Kruskal-Wallis test with Dunn’s post-test correction, or Mann Whitney test. Standard linear regression analysis was done for all two-dimensional correlations. Linear regression analysis of data was derived from individual animal measurements to determine correlations. The D’Agostino and Pearson omnibus test was used to evaluate data for normality (an α<0.05 was used to indicate normality). A p-value of 0.05 or less was taken to indicate statistical significance. Values that differed by more than two standard deviations from the mean were excluded from the statistical analysis where appropriate.
RESULTS
Fraction of Exhaled Nitric Oxide (FeNO)
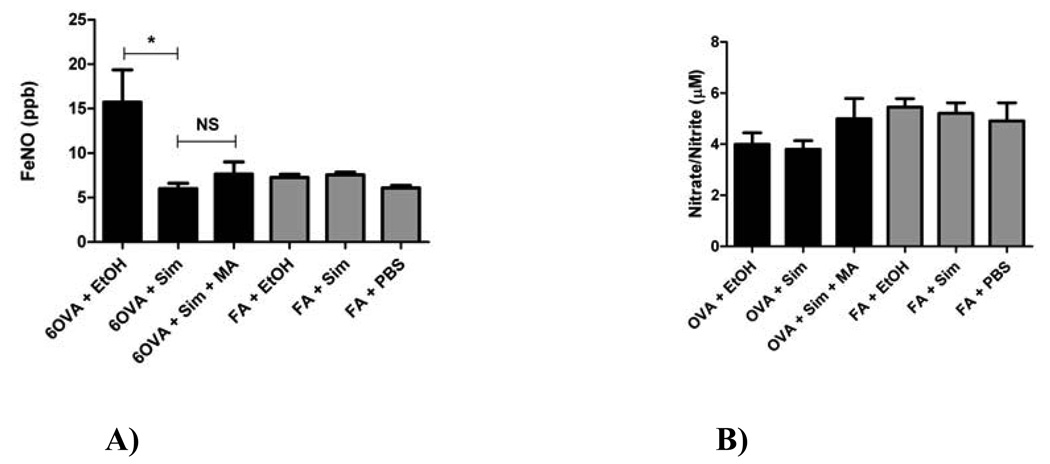
Simvastatin reduced in vivo FeNO by 61.7% (p=0.0019) to air control levels, suggesting that NO production is altered by simvastatin treatment (Figure 1A). Although there was a trend of increased FeNO production after MA co-treatment, this small reversal of simvastatin’s effect on FeNO did not reach statistical significance (p=NS), indicating a possible MA-independent mechanism of action.
Figure 1. A) Fraction of Exhaled Nitric Oxide (FeNO), B) Whole Lung Homogenate Nitrate/Nitrite Levels.
A). The effect of simvastatin on in vivo FeNO measurements as described in Methods. Simvastatin (40 mg/kg) treatment decreased FeNO by 61.7% (*p<0.0019 by Kruskal-Wallis test) down to air control levels. Simvastatin and MA (20 mg/kg) co-treatment did not reverse the inhibitory statin effect (p=NS, Kruskal-Wallis test). Each treatment group had between 5–8 mice.
B) Treatment with simvastatin did not alter whole lung nitrate/nitrite content, an indicator of NO production (p=NS by 1-way ANOVA). Each treatment group had between 6–9 mice.
Nitrate/Nitrite in Whole Lung Homogenate
Treatment with simvastatin did not alter whole lung nitrate/nitrite content, an indicator of NO production (p=NS) (Figure 1B). There was a trend towards higher nitrate/nitrite levels in the air control groups compared to the OVA groups. This observation was confirmed when the groups were analyzed in aggregate, i.e. all OVA animals compared to all FA animals, where the FA animals on average had 22.2% higher nitrate/nitrite levels than the OVA animals (p=0.0164 by Mann Whitney test, data not shown).
Lung NOS2 and NOS3 Protein Expression
Statins can alter the expression of both NOS2 and NOS3 in various tissues including lungs, airway epithelial cells, and endothelium[31–35]. In our experimental model, simvastatin treatment did not alter NOS2 or NOS3 protein expression (data not shown) in whole lung homogenate as measured by Western blot (p=NS by 1-way ANOVA). There was a trend towards higher NOS2 expression in the OVA groups compared to their corresponding FA controls, but this was not statistically significant. However, when all combined OVA groups are compared to all FA groups, the OVA mice had 38% higher NOS2 protein expression than FA mice (p=0.031 by t-test, data not shown). NOS1 was not assessed in this experiment.
Airway Goblet Cell Hyperplasia
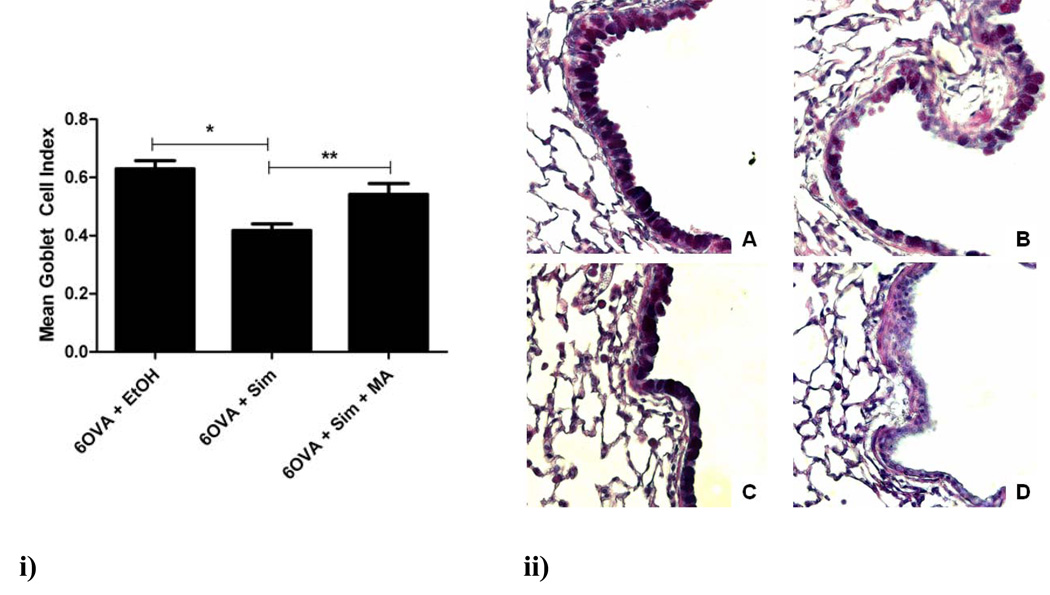
Airway goblet cell hyperplasia is one of the earliest hallmarks of airway remodeling in the OVA mouse model. In OVA-exposed mice, simvastatin treatment reduced goblet cell hyperplasia (measured as mean goblet cell index) by 33% (p<0.005), with MA co-treatment reversing this inhibitory statin effect (p<0.05) (Figure 2i). Alcian Blue-PAS staining of representative lung sections showed marked reductions in mucus staining with simvastatin treatment and reversal of this statin effect with MA co-treatment (Figure 2ii). As expected, the air controls showed no evidence of mucus-producing goblet cells staining.
Figure 2. Airway goblet cell hyperplasia.
i) Treatment with simvastatin (40 mg/kg) reduced the mean goblet cell index (see Methods) by 33% (*p<0.005 by 1-way ANOVA). Co-treatment with MA (20 mg/kg) reversed this inhibitory statin effect (**p<0.05 by 1-way ANOVA). The air controls are not shown since there were virtually no goblet cells visualized (see Fig 2ii image D). Each treatment group had between 4–6 mice.
ii) The four different treatment groups seen at an original magnification of x400 were stained with Alcian Blue-PAS to show goblet cells in the airway epithelium: A) 6OVA+EtOH, B) 6OVA+Sim, C) 6OVA+Sim+MA, and D) FA+PBS. The OVA group treated with simvastatin (6OVA+Sim) has significantly less goblet cell staining corresponding to the 33% decrease in mean goblet cell index as described above in i).
Lung Arginase Expression, Enzyme Activity, and Immunohistochemistry
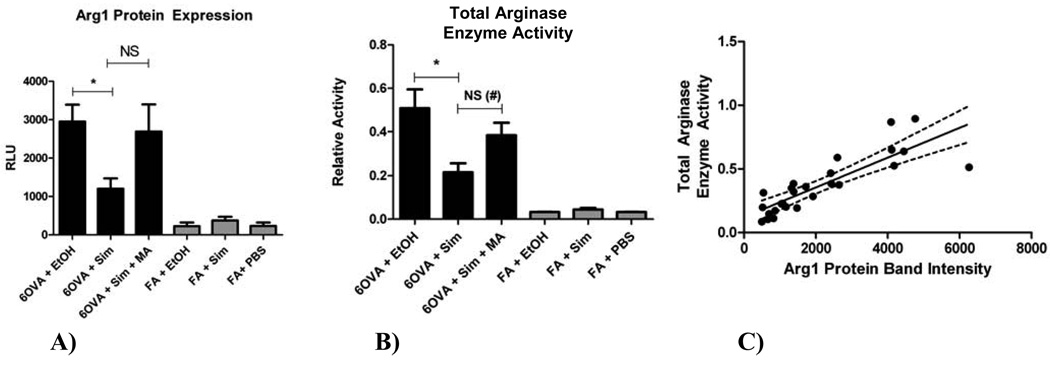
To determine whether simvastatin treatment affects lung arginase, we evaluated Arg1 protein expression, arginase enzyme activity, and Arg1 tissue localization. Arg1 protein expression and total enzyme activity were evaluated using whole lung homogenate. Simvastatin treatment reduced Arg1 protein expression, as measured by Western blot, by 59.4% (p<0.05) (Figure 3A), with MA co-treatment partially reversing this inhibitory statin effect, however, this was not significant (p=NS). Total arginase enzyme activity (Arg1 and Arg2) followed the same pattern as Arg1 protein expression, with simvastatin reducing arginase activity by 57% (p<0.005) in OVA-exposed mice (Figure 3B), with MA co-treatment reversing the statin effect (p=0.0275 by t-test). Arg1 protein expression was barely detectable in the air control (FA) groups (Figure 3A) with concordant very low total arginase enzymatic activity (Figure 3B). Total arginase enzyme activity correlated positively with Arg1 protein expression (r2=0.70, p<0.0001) (Figure 3C).
Figure 3. A) Arginase-1 (Arg1) protein expression in whole lung homogenate by Western blot, B) Total arginase enzyme activity in whole lung homogenate, and C) Correlation between total arginase enzyme activity and Arg1 protein expression.
A) Simvastatin (40 mg/kg) reduced Arg1 protein expression by 59.4% (*p<0.05 by 1-way ANOVA). Co-treatment with MA (20 mg/kg) reversed the inhibitory statin effect on Arg1 expression, but this was not statistically significant (p=NS by 1-way ANOVA, p=0.057 by t-test). Each treatment group had between 8–12 mice.
B) Simvastatin (40 mg/kg) reduced total arginase enzyme activity by 57% in the OVA group (*p<0.005 by 1-way ANOVA). Co-treatment with MA (20 mg/kg) reversed the statin effect (not significant by 1-way ANOVA, but significant by t-test (#p=0.0275)). Each treatment group had between 6–9 mice.
C) Arg1 protein expression is represented by protein band intensity (measured by Western blots of whole lung homogenates). Total arginase enzyme activity correlated positively with Arg1 protein expression (r2=0.70, p<0.0001). The dotted lines above and below the best fit line represent the 95% confidence interval. Each dot represents one mouse.
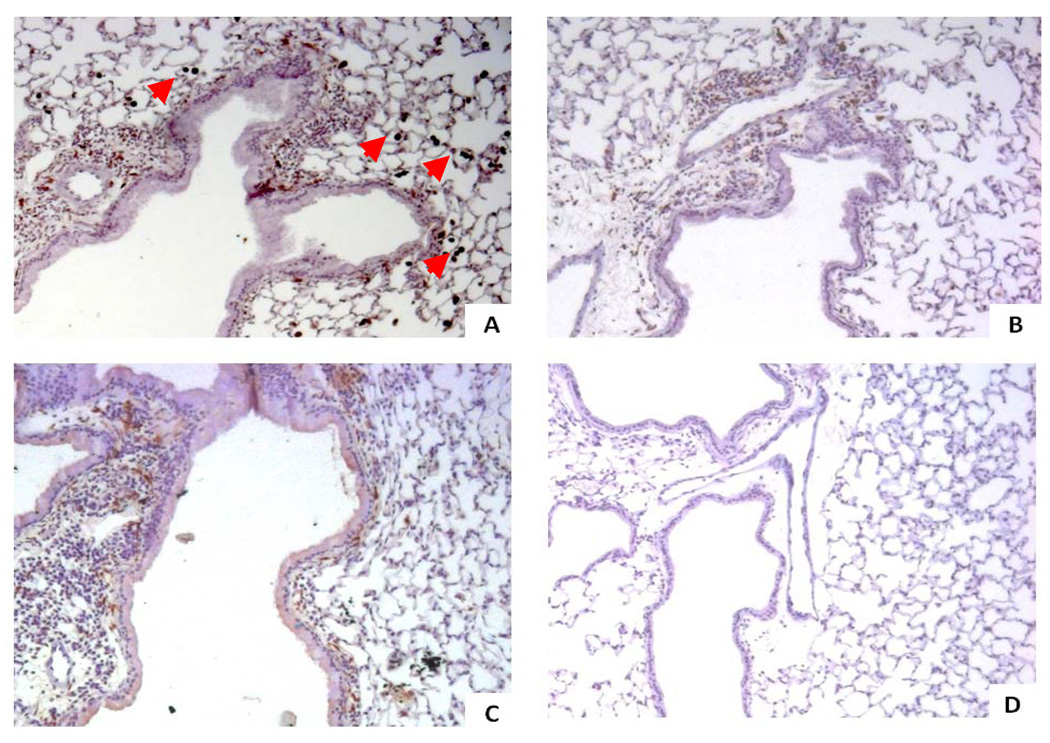
Immunohistochemical staining of lung sections for localization of Arg1 protein expression showed no Arg1 staining in the FA control groups (Figure 4D), however, there was intense staining of inflammatory alveolar macrophages and components of the airway subepithelial compartment in the OVA groups (Figure 4A–C). There was an apparent trend with simvastatin reducing Arg1 subepithelial staining in the OVA group (Figure 4B), with MA co-treatment only partially abrogating this simvastatin effect (Figure 4C), however, there was no difference in the intensity of Arg1 staining in peribronchiolar and alveolar macrophages. Changes observed in Arg1 staining of the subepithelial compartment were likely due to the simvastatin-dependent reduction in peribronchiolar inflammation, including a 48.3% reduction in lung lavage macrophages (p<0.005) that is MA-dependent (Figure 7B). Most eosinophils did not stain positively for Arg1 in the OVA groups. All treatment groups showed no evidence of Arg1 staining in airway smooth muscle cells. Nearly all OVA-exposed mice showed no appreciable Arg1 staining in the airway epithelium (Figure 4A–C); however, we did not distinguish between columnar ciliated cells and goblet cells. Negative control experiments with isotype control antibody showed no brown color staining (data not shown).
Figure 4. Immunohistochemical staining of Arginase-1 (Arg1).
The four different treatment groups are seen at an original magnification of x100, where Arg1 stains brown. We evaluated the epithelial and subepithelial compartments, of which the latter contains the airway smooth muscle cells, inflammatory cells, and extracellular space. There was no Arg1 staining of airway smooth muscle cells in any of the treatment groups.
A) 6OVA+EtOH. Arg1 stains dark brown and localizes to the subepithelial space and in macrophages (red arrow heads). There is little-to-no Arg1 staining visualized in airway epithelial cells.
B) 6OVA+Sim. Simvastatin treatment (40 mg/kg) of OVA-exposed mice attenuated Arg1 staining in the subepithelial space. No differences were seen compared to 6OVA+EtOH in macrophage Arg1 staining by this qualitative assessment.
C) 6OVA+Sim+MA. Co-treatment with MA (20 mg/kg) partially abrogated the simvastatin effect on Arg1 staining in the subepithelial compartment. No significant differences were seen with respect to macrophage Arg1 staining.
D) FA+PBS. There is no Arg1 staining noted in this air control group.
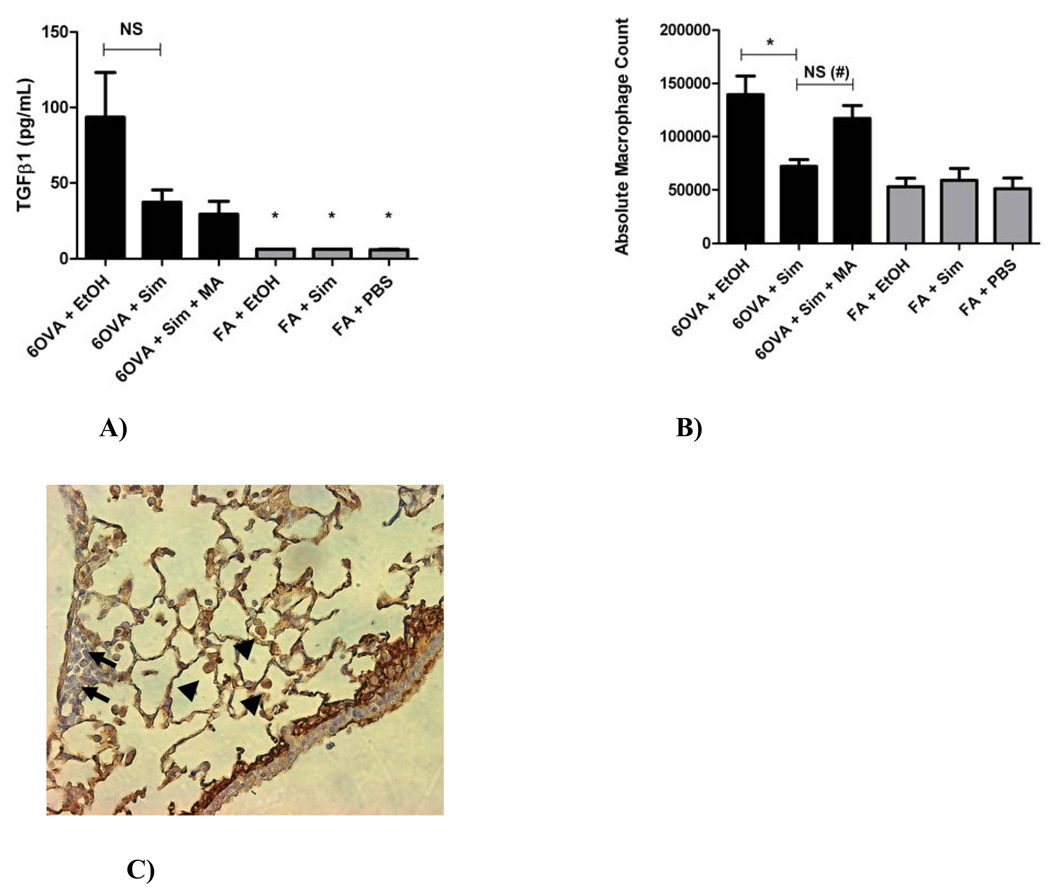
Figure 7. A) Concentration of TGFβ1 in whole lung lavage, B) Whole lung lavage Absolute Macrophage Counts, and C) TGFβ1 immunohistochemical staining of lung and inflammatory cells.
A) The group 6OVA+EtOH had significantly higher TGFβ1 concentration than any of the FA controls (*p<0.005 by 1-way ANOVA). Simvastatin (40 mg/kg) reduced TGFβ1 concentration in lung lavage by 60.4% in the OVA group (p=NS by 1-way ANOVA or t-test). Co-treatment with MA (20 mg/kg) did not reverse this inhibitory statin effect on TGFβ1. Each treatment group had between 5–6 mice.
B) Simvastatin (40 mg/kg) treatment decreased lung lavage absolute macrophage count by 48.3% (*p<0.005 by 1-way ANOVA) in OVA exposed animals. Co-treatment with MA (20 mg/kg) reversed the simvastatin inhibitory effect (p=NS by 1-way ANOVA, but #p=0.0029 by t-test). Each treatment group had between 10–17 mice.
C) By qualitative immunohistochemical assessment, macrophages appear to be the main inflammatory cells responsible for TGFβ1 production under OVA-induced allergic inflammation (image shows the 6OVA+EtOH treatment group: representative slide where TGFβ1 stains brown seen at x400 magnification). Arrows represent inflammatory cells with little-to-no staining for TGFβ1 (likely eosinophils or lymphocytes). Arrow heads represent macrophages that stain densely brown for TGFβ1.
Correlations of Arginase to Inflammation and Lung Function
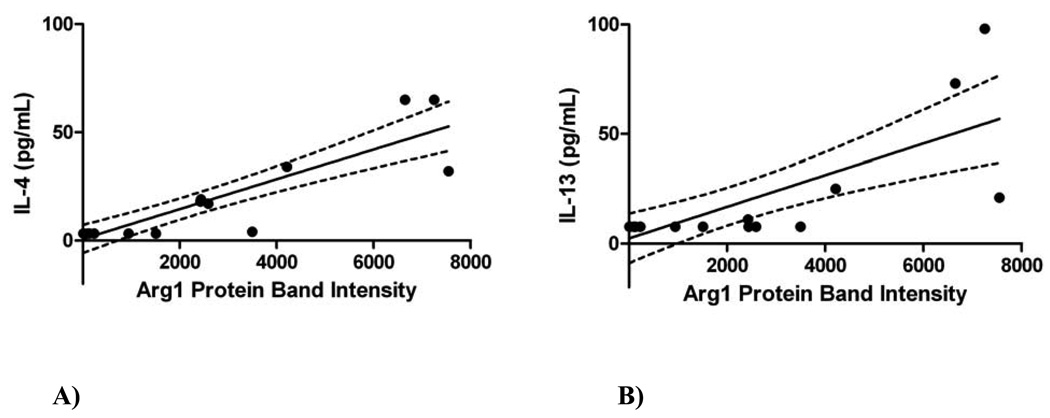
Since IL-13 and IL-4 are known to increase Arg1 expression, we evaluated whether Arg1 protein expression correlated with inflammation or lung lavage IL-4 and IL-13 concentrations. We previously published IL-4/IL-13 measurements[15]; however, these current comparisons with Arg1 are new and not previously reported. Whole lung Arg1 positively correlated with lung lavage total cell count (r2=0.56, p<0.0001), eosinophil count (r2=0.56, p<0.0001), macrophage count (r2=0.42, p<0.0001), and lymphocyte count (r2=0.46, p<0.0001) (data not shown). Both IL-4 and IL-13 also positively correlated with Arg1 protein expression (r2=0.78, p<0.0001 for IL-4; and r2=0.56, p=0.0003 for IL-13) (Figure 5).
Figure 5. Correlations between Th2 Cytokines and Arginase-1 (Arg1) protein expression.
Arg1 protein expression is represented by protein band intensity (equivalent to RLU) as measured by Western blots of whole lung homogenates. The dotted lines above and below the best fit line represent the 95% confidence interval. Each dot represents one mouse.
A) The whole lung lavage IL-4 levels correlated positively with increased Arg1 protein expression in whole lung (r2=0.78, p<0.0001).
B) The whole lung lavage IL-13 levels correlated positively with increased Arg1 protein expression in whole lung (r2=0.56, p=0.0003).
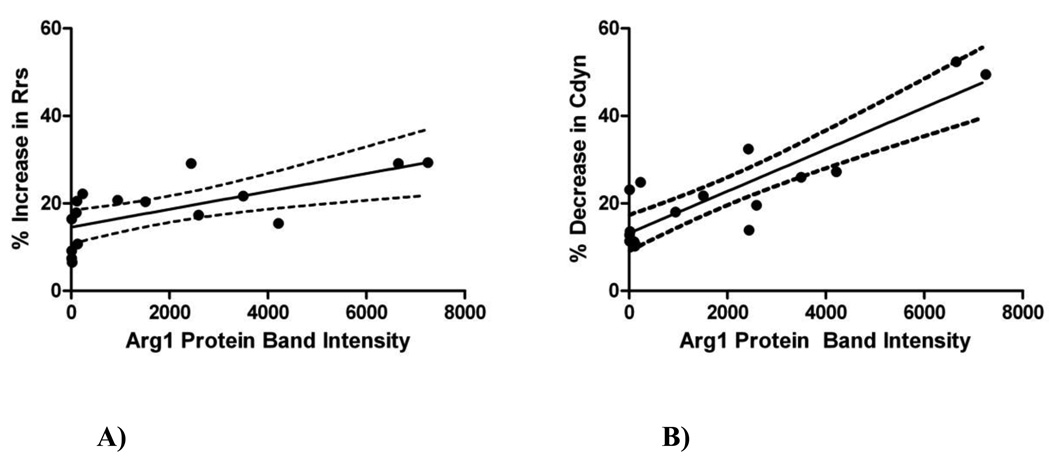
Arg1 protein expression was also positively correlated with AHR as calculated by percent increase in Rrs (r2=0.45, p=0.0044) (Figure 6A) and percent decrease in Cdyn (r2=0.78, p<0.0001) (Figure 6B), in response to MCh bronchial challenge. We previously published aspects of this lung physiology data (Cdyn and Rrs), however, the data presented as “percent increase” or “percent decrease” and the comparisons to Arg1 are new and not previously reported.
Figure 6. Correlations between lung physiology and Arginase-1 (Arg1) protein expression.
Arg1 protein expression is represented by protein band intensity (equivalent to RLU) as measured by Western blots of whole lung homogenates. The dotted lines above and below the best fit line represent the 95% confidence interval. Each dot represents one mouse.
A) The percent increase in respiratory system resistance (Rrs) correlated positively with increased Arg1 protein expression in whole lung (r2=0.45, p=0.0044).
B) The percent decrease in dynamic lung compliance (Cdyn) correlated positively with increased Arg1 protein expression in whole lung (r2=0.78, p<0.0001).
Airway Hydroxyproline Content
Measurement of total hydroxyproline content from microdissected airways indicated no significant differences between all six treatment groups at the time-point examined (p=NS by 1-way ANOVA). In general, the OVA groups did not demonstrate higher hydroxyproline content compared to air controls. There was also no significant difference between the combined OVA versus combined FA groups (p=0.128 by t-test, data not shown). Simvastatin did not alter hydroxyproline content in the OVA-exposed mice, nor was it different from the other OVA groups or air controls (p=NS, by 1-way ANOVA) (data not shown).
Lung TGFβ1: Lung Lavage Concentration, Protein Expression, and Immunohistochemistry
TGFβ1 protein is expressed in several different forms in tissues, where we focused on the precursor and active, dimerized forms of this protein. We evaluated the levels and locations of TGFβ1 expression in mouse lung and in different compartments after OVA and FA exposures, with simvastatin and MA co-treatment.
In the lung lavage fluid, the 6OVA+EtOH group had significantly higher TGFβ1 concentration than any of the FA controls (p<0.005), but simvastatin treatment or MA co-treatment did not significantly affect lavage TGFβ1 concentration (Figure 7A).
There were no statistically significant differences in total TGFβ1 (precursor and dimer) protein expression between the OVA and air (FA) control groups (p=NS by Kruskal-Wallis test with Dunn’s post-test correction, data not shown). ‘Total’ TGFβ1 protein was calculated as the sum of precursor and dimer protein levels as measured by Western blot simultaneously (i.e. relative light units (RLU) or band intensity). The combined OVA groups versus FA groups showed a trend of higher total TGFβ1 expression in the OVA animals, but this did not reach statistical significance (p=0.061 by Mann Whitney test, data not shown). Separate evaluations of TGFβ1 precursor and dimer protein expression also did not yield any statistically significant differences amongst the groups (p=NS by 1-way ANOVA, data not shown). Simvastatin (40 mg/kg) treatment and MA co-treatment both did not alter total TGFβ1 protein expression in the OVA groups (by 1-way ANOVA, data not shown). In the OVA treatment groups there were 16–18 mice per group, and in the air controls there were 6 mice per group.
Simvastatin treatment decreased lung lavage absolute macrophage count by 48.3% (p<0.005) in OVA exposed animals, with MA co-treatment reversing the simvastatin inhibitory effect (p=0.0029 by t-test) (Figure 7B). By qualitative immunohistochemical assessment, macrophages appear to be the main inflammatory cells that stain intensely for TGFβ1, as compared to eosinophils or lymphocytes (Figure 7C).
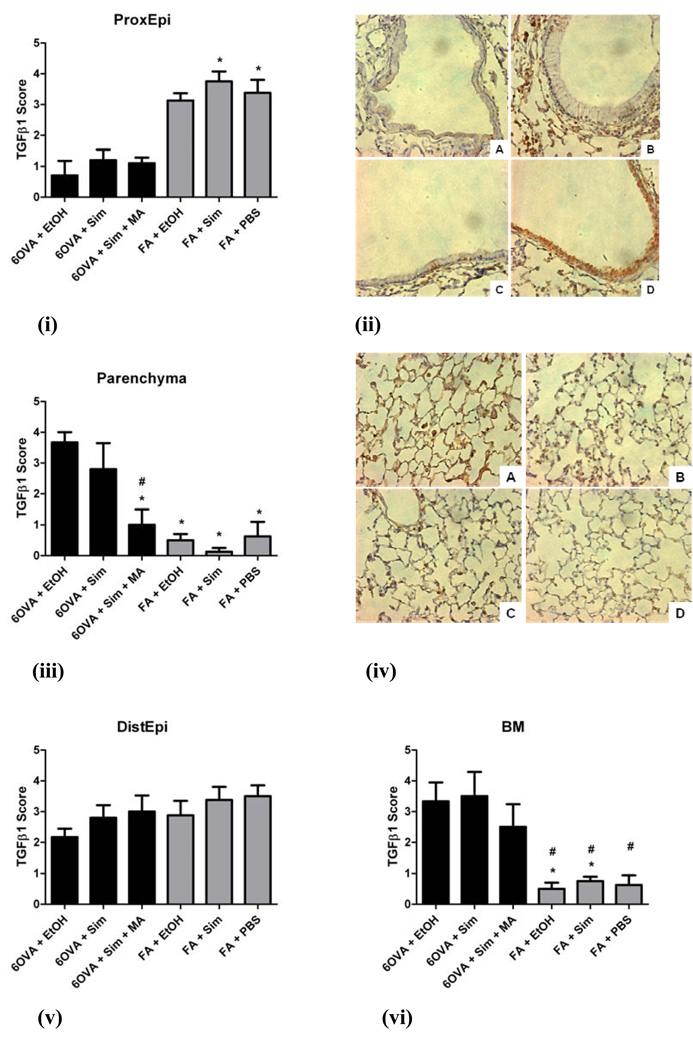
Semiquantitative assessment of TGFβ1 immunohistochemical staining in six different lung compartments was completed as described in Methods. Simvastatin treatment did not significantly alter TGFβ1 staining in any of the six different lung compartments evaluated (p=NS) (Figure 8). In the proximal airway epithelium (ProxEpi), the air controls (FA+Sim, FA+PBS) had greater TGFβ1 content compared to the OVA groups, especially 6OVA+EtOH (p<0.05) (Figures 8i and 8ii). In contrast, the lung parenchyma of all three air control groups had much lower TGFβ1 content compared to the 6OVA+EtOH group (p<0.005), but with the 6OVA+Sim+MA group showing reduced TGFβ1 content compared to the 6OVA+EtOH group (p<0.005) (Figures 8iii and 8iv).
Figure 8. TGFβ1 immunohistochemical staining and scoring in different lung compartments.
The groups illustrated in the histology images are seen at an original magnification of x400, where TGFβ1 stains brown: A) 6OVA+EtOH, B) 6OVA+Sim, C) 6OVA+Sim+MA, and D) FA+PBS. Simvastatin (40 mg/kg) had no statistically significant effect on total TGFβ1 content in the different lung compartments evaluated. Each treatment group had between 4–6 mice. All analyses were done by 1-way ANOVA.
i) In the proximal airway epithelium (ProxEpi), the air controls (FA+Sim, FA+PBS) had greater TGFβ1 content compared to the OVA groups, specifically 6OVA+EtOH (*p<0.05).
ii) Histology corresponding to i) where TGFβ1 stains brown most intensely in the epithelium of the FA+PBS group.
iii) In the lung parenchyma, all three air control groups had much lower TGFβ1 content compared to the 6OVA+EtOH group (*p<0.005). The treatment group 6OVA+Sim+MA showed less TGFβ1 content than the 6OVA+EtOH group (#p<0.005).
iv) Histology corresponding to iii) shows the highest intensity TGFβ1 staining in group A) 6OVA+EtOH, greater than the other OVA groups, and significantly greater than all of the air controls.
v) In the distal airway epithelium (DistEpi), there were no statistically significant differences amongst all six groups (p=NS).
vi) In the basement membrane (BM), the air controls had significantly less TGFβ1 content than the OVA groups, specifically 6OVA+EtOH (*p<0.05) and 6OVA+Sim (#p<0.05).
In the basement membrane (BM), the air controls had significantly less TGFβ1 content than the OVA groups, specifically 6OVA+EtOH (p<0.05) and 6OVA+Sim (p<0.05) (Figure 8vi). Distal airway epithelium (DistEpi) (Figure 8v), and airway and vascular smooth muscle cells (SMC) showed no differences between the six treatment groups (p=NS, by 1-way ANOVA) (data not shown).
Macrophages had a 2-fold higher TGFβ1 content in the combined OVA groups compared to air controls (p=0.0003 by t-test, data not shown), but this did not demonstrate individual significance across the different treatment groups (p=NS, by 1-way ANOVA) (data not shown). These lung macrophages appear to be the predominant inflammatory cells responsible for TGFβ1 production during OVA-induced allergic inflammation, with TGFβ1 immunohistochemically staining strongest in lung macrophages, and little or no staining in eosinophils or lymphocytes. Negative control experiments with isotype control antibody showed no brown color staining (data not shown).
DISCUSSION
In our previous study, we established that treatment with simvastatin decreases total inflammatory cell load and eosinophilic airway inflammation via a MA-dependent mechanism, and that the MA-independent inhibition of AHR by simvastatin was likely due to additional effects on airway resident cells[15]. Using this same acute mouse model of allergic airway inflammation, we have now studied the effects of simvastatin on the development of early hallmarks of airway remodeling. We investigated whether simvastatin reduces the expression of remodeling markers such as goblet cell hyperplasia, and pro-fibrogenic mediators in the lung such as Arg1, TGFβ1, and hydroxyproline. Given the “pleiotropic effects” of statins, we also performed experiments with simultaneous simvastatin and MA co-treatment, to test whether simvastatin’s mechanism of action was via inhibition of the target enzyme HGM-CoA reductase (Scheme 1).
The key findings from this study are that systemically administered simvastatin attenuated goblet cell hyperplasia, and inhibited lung Arg1 protein expression and total arginase enzyme activity. Arginase-1 protein expression correlated positively with measurements of AHR (increased lung resistance and decreased lung compliance), and also with increased lung lavage IL-4 and IL-13 concentration. Despite a reduction of exhaled NO by simvastatin, we did not see changes in NOS2 or NOS3 protein content in the lung, or lung tissue intracellular nitrite/nitrate content. Histologic examination of lung sections did not reveal significant airway smooth muscle cell hypertrophy or hyperplasia in any of the 2-week OVA-exposed mice.
For all measured endpoints the simvastatin effect was MA-independent except for goblet cell hyperplasia where the inhibitory statin effect was MA-dependent, indicating statin inhibition of HMG-CoA reductase (Figure 2, Scheme 1). The MA pathway leads to cholesterol and other crucial lipid intermediates (the isoprenoids FPP and GGPP) which are necessary for small GTPase (e.g. Ras and RhoA, respectively) cell membrane-anchoring and subsequent intracellular signal transduction. Cellular inflammation, transmigration, and proliferation are dependent on the availability of these isoprenoids downstream of MA. This suggests that FPP and/or GGPP may be playing a role with respect to goblet cell hyperplasia.
Kim and co-authors also found a reduction in the percentage of goblet cells (approximately 69% reduction) in BALB/c mice treated with simvastatin; however, the authors did not suggest a potential mechanism for this inhibition other than a decrease in IL-5[36]. The reduction in goblet cell hyperplasia found by Kim and co-authors is more than twice that in our study (69% versus 33%), perhaps due to methodological differences. In a rat model of LPS-induced airway mucus hypersecretion, Ou and co-authors suggest that the inhibition of goblet cell hyperplasia by simvastatin is dose-dependent and mediated via inhibition of Muc5AC expression and RhoA/p38 pathways[37]. Alternatively, the statin effect on goblet cells may be driven by potent IL-13 inhibition[15], a key cytokine that drives Muc5AC expression, mucin synthesis, and ciliated cell to goblet cell phenotypic change[38]. More work is needed to further elucidate the underlying mechanism(s) of this observation, where goblet cell hyperplasia and mucus production are key elements of airway remodeling and fatal asthma[39].
Arginase plays an important role in asthma and has been correlated with allergic lung inflammation, AHR, and airway remodeling in both humans and animal models[40, 41]. Treatment with simvastatin significantly reduced lung Arg1 protein expression and overall arginase enzyme activity, a novel finding in asthma (Figures 3A and 3B), and may potentially be mediated by the MA pathway. The arginase activity assay measures total arginase (which includes both isoforms Arg1 and Arg2), however, linear regression analysis of arginase activity versus Arg1 protein expression shows a strong correlation between these two parameters (Figure 3C), indicating that the Arg1 isoform contributes to a significant portion of total arginase enzyme activity in the inflamed lung.
Immunohistochemical staining showed Arg1 throughout the lung, where positive, strong staining was seen in the subepithelial peribronchiolar space, particularly among infiltrating inflammatory cells (predominantly macrophages) (Figure 4A), consistent with previous reports in mouse models[18–20]. Although no changes were seen in the intensity of Arg1 staining in macrophages, simvastatin reduced Arg1 staining in the subepithelial peribronchiolar space (Figure 4B) by the reduction in total macrophages as depicted in lung lavage macrophage counts (Figure 7B).
Most OVA-exposed mice showed no appreciable Arg1 staining in the airway epithelium (Figures 4A–C). Evaluating specimens obtained from human asthmatics and mouse models of allergic asthma, North and co-authors found significant Arg1 staining in the airway epithelium of both acute and chronic OVA-exposed mice, perhaps more intensely in the chronically (12-week) exposed mice[19]. Their acute exposure was for 3 weeks while ours was for 2 weeks, but it is not clear if the time-course alone can explain the difference between our results and that of North et al. Evidence to support this possibility comes from Takemoto and co-authors, where Df-exposed (Dermatophagoides farinae (Df) extract) mice at day 12 also showed no evidence of Arg1 staining in airway epithelial cells[20]. Similarly, in an OVA exposure model for 12 days, no Arg1 staining in the airway epithelium was noted[18]. Zimmermann and co-authors noted mostly subepithelial Arg1 mRNA signal (by in situ hybridization) in OVA and Aspergillus fumigatus extract mouse models of asthma, however, patchy areas of Arg1 mRNA positive airway epithelial cells were noted in an endobronchial specimen from a human asthmatic[42]. One potential explanation for the lack of Arg1 expression in airway epithelia in our model is the high density of goblet cells seen at 2 weeks of OVA exposure, which we previously showed to be as high as 74.3% of all epithelial cells at this time-point[16]. Goblet cell percentage decreases to 9.2% after 8 weeks of OVA exposure, thus a chronic exposure model is more likely to reveal the presence (or absence) of Arg1 staining. It is plausible that in more chronic allergen exposure models (or different mouse strains) and established human asthma, the airway epithelium expresses Arg1 more consistently than that seen in more acute models such as ours[43].
The mechanism of simvastatin’s inhibition of Arg1 protein expression and enzyme activity is not clear from our experiments. One possible explanation is a reduction in IL-13 which is known to be a strong inducer of Arg1 in asthma[42]. In addition to IL-13, the Th2 cytokine IL-4 is also known to induce arginase expression[44]. This is supported by positive linear correlations between these two cytokines and Arg1 protein expression (Figure 5). We and others have previously shown strong inhibition of lung lavage IL-4 and IL-13 concentration by simvastatin[15, 45]. However, it is likely that more complex mechanisms are involved where the MA pathway may also play a role. In endothelial cells, the RhoA/ROCK pathway induces arginase activity[46, 47]. Signaling via the RhoA/ROCK pathway is a critical step for Arg1 and Arg2 mRNA and protein expression in gut endothelial cells[48]. In a breast cancer cell line (MCF-7), both simvastatin and fluvastatin inhibited Arg2 expression which was reversed with MA co-treatment[49]. Other mechanisms could involve inhibition of asymmetric dimethylarginine (ADMA)[50, 51] or modulation of transcription factors such as CCAAT/enhancer-binding protein (C/EBP) [52–57].
The cytokine TGFβ1 is a complex cytokine that plays a key role in asthma, and serves as an important link between inflammation and fibrosis. In addition to inducing Arg1, TGFβ1 can also stimulate varied cytokines important in airway remodeling[58], and induce goblet cell hyperplasia, fibroblast proliferation and myofibroblast differentiation[59]. Our data suggest that TGFβ1 is unlikely to be the prime mediator of simvastatin’s inhibition of goblet cell hyperplasia or Arg1 expression/function at the 2-week time-point (Figure 3, Figure 7, and Figure 8). We conclude that other mechanisms are likely at play, and that a more chronic model of OVA exposure (i.e. 4–6 weeks) may yield different results with respect to statins and TGFβ1.
There are several important limitations in this study. First, although our model manifests some early hallmarks of airway remodeling, it is not a chronic OVA exposure model and therefore not all aspects of airway remodeling and potential statin effect(s) can be thoroughly investigated. This is of special concern for testing whether simvastatin inhibits collagen synthesis and airway fibrosis in asthma, by analogy with previously reported effects in other animal models of lung fibrosis[13, 60–62]. Second, although for some features of remodeling the simvastatin effect appears to be MA-dependent (i.e. goblet cell hyperplasia), the detailed mechanism remains unknown. For the MA-independent observations, we did not confirm whether MA was actually present or absent in the tissue of interest after i.p. injection; the presumption is that MA was ubiquitously distributed. Third, we assessed whole lung tissue for the majority of endpoints, restricting some of our observations to the whole lung rather than the specific lung compartment or cell types. Fourth, extrapolation of these results to human disease would be premature, as more animal studies are needed to understand mechanisms and show consistent efficacy.
Simvastatin likely has many cellular targets and future studies must utilize a chronic exposure model with special focus on smooth muscle cell hyperplasia/hypertrophy, fibroblast-to-myofibroblast differentiation, and airway fibrosis, while exploring the underlying mechanism(s) of action. Given the potential for innovative therapy in asthma and airway remodeling, such investigations are urgently needed.
Acknowledgements
We would like to thank Lisa M. Franzi and Michelle Rabowsky for their technical assistance and input.
Grant/Funding Support: NIH HL07013 (T32), NCRR UL1 RR024146 (CTSC), HL-076415 (K08), NCHCS VA Medical Center, and American Thoracic Society (ATS) Fellows Career Development Award, CTSC K12 Award.
Abbreviations
- AHR
Airway hyperreactivity
- MA
mevalonate
- Sim
simvastatin
- OVA
ovalbumin
- Cdyn
dynamic lung compliance
- Rrs
respiratory system resistance
- FeNO
fraction of exhaled nitric oxide
- NO
nitric oxide
- NOS
nitric oxide synthase
- Th1 or Th2
T-helper 1 or 2 cell
- FPP
farnesylpyrophosphate
- GGPP
geranylgeranylpyrophosphate
- HMG-CoA reductase
3-hydroxy-3-methyl-glutaryl-CoA reductase
- GTPases
guanosine triphosphatases
- i.p.
intraperitoneal
- PBS
phosphate-buffered saline
- EtOH
ethanol
- FA
filtered air
- MCh
methacholine
- ppb
parts per billion
- H&E
hematoxylin and eosin
- NS
not significant
- COPD
chronic obstructive pulmonary disease
- PAS
Periodic Acid-Schiff
- ProxEpi
proximal airway epithelial cells
- DistEpi
distal airway epithelial cells
- BM
basement membrane
- SMC
airway and vascular smooth muscle cells
- TGFβ1
transforming growth factor-β1
- RLU
Relative Light Units
- Arg1
arginase-1
- Arg2
arginase-2
- IHC
immunohistochemistry
- LPS
lipopolysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
References
- 1.Vignola AM, Mirabella F, Costanzo G, et al. Airway Remodeling in Asthma. Chest. 2003;123:417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 2.Holgate S. Pathogenesis of Asthma. Clinical and Experimental Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 3.Royce SG, Tang M. The effects of current therapies on airway remodeling in asthma and new possibilities for treatment and prevention. Curr Mol Pharmacol. 2009;2(2):169–181. doi: 10.2174/1874467210902020169. [DOI] [PubMed] [Google Scholar]
- 4.Payne DNR, Rogers AV, Adelroth E, et al. Early Thickening of the Reticular Basement Membrane in Children with Difficult Asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 5.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol. 2006;35(6):722–729. doi: 10.1165/rcmb.2006-0034OC. [DOI] [PubMed] [Google Scholar]
- 6.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexeeff SE, Litonjua A, Sparrow D, Vokonas PS, Schwartz J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda S, Park K, FitzGerald JM, Etminan M, Swiston J. Statins in COPD: A Systematic Review. Chest. 2009;136:734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 9.Camoretti-Mercado B. Targeting the airway smooth muscle for asthma treatment. Translational Research. 2009;154:165–174. doi: 10.1016/j.trsl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hothersall EJ, Chaudhuri R, McSharry C, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 12.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328–335. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Ou XM, Wen FQ, Uhal BD, et al. Simvastatin attenuates experimental small airway remodelling in rats. Respirology. 2009;14:734–745. doi: 10.1111/j.1440-1843.2009.01549.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Lee DS, Kim EK, et al. Simvastatin Inhibits Cigarette Smoking-induced Emphysema and Pulmonary Hypertension in Rat Lungs. Am J Respir Crit Care Med. 2005;172:987–993. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- 15.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin Inhibits Airway Hyperreactivity: Implications for the Mevalonate Pathway and Beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenyon NJ, Last J. Reversible and irreversible airway inflammation and fibrosis in mice exposed to inhaled ovalbumin. Inflamm Res. 2005;54:57–65. doi: 10.1007/s00011-004-1325-6. [DOI] [PubMed] [Google Scholar]
- 17.Maarsingh H, Zuidhof A, Bos IST, et al. Arginase Inhibition Protects against Allergen-induced Airway Obstruction, Hyperresponsiveness, and Inflammation. Am J Respir Crit Care Med. 2008;178:565–573. doi: 10.1164/rccm.200710-1588OC. [DOI] [PubMed] [Google Scholar]
- 18.Mabalirajan U, Aich J, Agrawal A, Ghosh B. Mepacrine inhibits subepithelial fibrosis by reducing the expression of arginase and TGF-b1 in an extended subacute mouse model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:411–419. doi: 10.1152/ajplung.00138.2009. [DOI] [PubMed] [Google Scholar]
- 19.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:911–920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 20.Takemoto K, Oginko K, Shibamori M, et al. Transiently, paralleled upregulation of arginase and nitric oxide synthase and the effect of both enzymes on the pathology of asthma. Am J Physiol Lung Cell Mol Physiol. 2007;293:1419–1426. doi: 10.1152/ajplung.00418.2006. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA. Pirfenidone Inhibits Lung Allograft Fibrosis through L-Arginine-Arginase Pathway. American Journal of Transplantation. 2005;5:1256–1263. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 22.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol. 1995;155(4):2077–2084. [PubMed] [Google Scholar]
- 23.Temelkovski J, Hogan S, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolized allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon NJ, Ward RW, Last JA. Airway fibrosis in a mouse model of airway inflammation. Toxicology and Applied Pharmacology. 2003;186:90–100. doi: 10.1016/s0041-008x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon NJ, Bratt J, Linderholm AL, Last MS, Last JA. Arginases I and II in lungs of ovalbumin-sensitized mice exposed to ovalbumin: sources and consequences. Toxicol Appl Pharmacol. 2008;230(3):269–275. doi: 10.1016/j.taap.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silkoff PE, Wakita S, Chatkin J, et al. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care Med. 1999;159:940–944. doi: 10.1164/ajrccm.159.3.9805044. [DOI] [PubMed] [Google Scholar]
- 27.McManus J. Histological and histochemical uses of periodic acid. Stain Techn. 1948;23:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- 28.Bratt JM, Franzi L, Linderholm AL, Last MS, Kenyon NJ, Last JA. Arginase enzymes in isolated airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2009;234(3):273–280. doi: 10.1016/j.taap.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Hilliard B, Carmody RJ, et al. Arginase and autoimmune inflammation in the central nervous system. Immunology. 2003;110(1):141–148. doi: 10.1046/j.1365-2567.2003.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophy. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 31.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103(1):113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 32.Hausding M, Witteck A, Rodriguez-Pascual F, von Eichel-Streiber C, Forstermann U, Kleinert H. Inhibition of small G proteins of the rho family by statins or clostridium difficile toxin B enhances cytokine-mediated induction of NO synthase II. Br J Pharmacol. 2000;131(3):553–561. doi: 10.1038/sj.bjp.0703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joyce M, Kelly CJ, Chen G, Bouchier-Hayes DJ. Pravastatin Attenuates Lower Torso Ischaemia-Reperfusioninduced Lung Injury by Upregulating Constitutive Endothelial Nitric Oxide Synthase. Eur J Vasc Endovasc Surg. 2001;21:295–300. doi: 10.1053/ejvs.2001.1318. [DOI] [PubMed] [Google Scholar]
- 34.Kreiselmeier NE, Kraynack N, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(6):L1286–L1295. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]
- 35.Laufs U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. Eur J Clin Pharmacol. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- 36.Kim DY, Ryu S, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. European Journal of Pharmacology. 2007;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 37.Ou XM, Wang BD, Wen FQ, Feng YL, Huang XY, Xiao J. Simvastatin attenuates lipopolysaccharide-induced airway mucus hypersecretion in rats. Chin Med J. 2008;121(17):1680–1687. [PubMed] [Google Scholar]
- 38.Curran DR, Cohn L. Advances in Mucous Cell Metaplasia: A Plug for Mucus as a Therapeutic Focus in Chronic Airway Disease. Am J Respir Cell Mol Biol. 2009 Jun 11; doi: 10.1165/rcmb.2009-0151TR. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeffery P. Remodeling in Asthma and Chronic Obstructive Lung Disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 40.Bratt JM, Franzi L, Linderholm AL, O’Roark EM, Kenyon NJ, Last JA. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicol Appl Pharmacol. 2010;242(1):1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maarsingh H, Zaagsma J, Meurs H. Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol. 2009;158(3):652–664. doi: 10.1111/j.1476-5381.2009.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann N, King N, Laporte J, et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergeron C, Boulet L, Page N, et al. Influence of cigarette smoke on the arginine pathway in asthmatic airways: Increased expression of arginase I. J Allergy Clin Immunol. 2007;119:391–397. doi: 10.1016/j.jaci.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Wei LH, Jacobs AT, Morris SM, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:248–256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 45.Imamura M, Okunishi K, Ohtsu H, et al. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitization, interleukin 17 production and antigen presentation in the lung. Thorax. 2009;64:44–49. doi: 10.1136/thx.2007.094540. [DOI] [PubMed] [Google Scholar]
- 46.Ming XF, Barandier C, Viswambharan H, et al. Thrombin Stimulates Human Endothelial Arginase Enzymatic Activity via RhoA/ROCK Pathway: Implications for Atherosclerotic Endothelial Dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 47.Romero MJ, Platt D, Tawfik HE, et al. Diabetes-induced Coronary Vascular Dysfunction Involves Increased Arginase Activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horowitz S, Binion D, Nelson VM, et al. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:1323–1336. doi: 10.1152/ajpgi.00499.2006. [DOI] [PubMed] [Google Scholar]
- 49.Kotamraju S, Williams C, Kalyanaraman B. Statin-induced breast cancer cell death: role of inducible nitric oxide and arginase-dependent pathways. Cancer Res. 2007;67(15):7386–7394. doi: 10.1158/0008-5472.CAN-07-0993. [DOI] [PubMed] [Google Scholar]
- 50.Ivashchenko CY, Bradley BT, Ao Z, Leiper J, Vallance P, Johns DG. Regulation of the ADMA-DDAH system in endothelial cells: a novel mechanism for the sterol response element binding proteins, SREBP1c and −2. Am J Physiol Heart Circ Physiol. 2010;298:251–258. doi: 10.1152/ajpheart.00195.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oguz A, Uzunlulu M. Short Term Fluvastatin Treatment Lowers Serum Asymmetric Dimethylarginine Levels in Patients With Metabolic Syndrome. Int Heart J. 2008;49:303–311. doi: 10.1536/ihj.49.303. [DOI] [PubMed] [Google Scholar]
- 52.Akiba T, Kuroiwa N, Shimizu-Yabe A, et al. Expression and Regulation of the Gene for Arginase I in Mouse Salivary Glands: Requirement of CCAAT/Enhancer-Binding Protein α for the Expression in the Parotid Gland. J Biochem. 2002;132:621–627. doi: 10.1093/oxfordjournals.jbchem.a003265. [DOI] [PubMed] [Google Scholar]
- 53.Albina JE, Mahoney EJ, Daley JM, Wesche DE, Morris SM, Reichner JS. Macrophage Arginase Regulation By CCAAT/Enhancer-binding protein β. Shock. 2005;23(2):168–172. doi: 10.1097/01.shk.0000148054.74268.e2. [DOI] [PubMed] [Google Scholar]
- 54.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPβ. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Dong J, Fujii S, Li H, et al. Interleukin-6 and Mevastatin Regulate Plasminogen Activator Inhibitor-1 Through CCAAT/Enhancer-Binding Protein-δ. Arterioscler Thromb Vasc Biol. 2005;25:1078–1084. doi: 10.1161/01.ATV.0000159701.24372.49. [DOI] [PubMed] [Google Scholar]
- 56.Mäuser W, Perwitz N, Meier B, Fasshauer M, Klein J. Direct adipotropic actions of atorvastatin: Differentiation state-dependent induction of apoptosis, modulation of endocrine function, and inhibition of glucose uptake. European Journal of Pharmacology. 2007;564:37–46. doi: 10.1016/j.ejphar.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Mayer C, Gruber H, Landl EM, et al. Rosuvastatin reduces interleukin-6-induced expression of C-reactive protein in human hepatocytes in a STAT3- and C/EBP-dependent fashion. Int J Clin Pharmacol Ther. 2007;45(6):319–327. doi: 10.5414/cpp45319. [DOI] [PubMed] [Google Scholar]
- 58.Murphy DM, Forrest IA, Corris PA, et al. Simvastatin attenuates release of neutrophilic and remodeling factors from primary bronchial epithelial cells derived from stable lung transplant recipients. Am J Physiol Lung Cell Mol Physiol. 2008;294:592–599. doi: 10.1152/ajplung.00386.2007. [DOI] [PubMed] [Google Scholar]
- 59.Makinde T, Murphy R, Agrawal DK. The regulatory role of TGF-β in airway remodeling in asthma. Immunology and Cell Biology. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 60.Ou XM, Feng LY, Wen FQ, et al. Simvastatin attenuates bleomycin-induced pulmonary fibrosis in mice. Chinese Medical Journal. 2008;121(18):1821–1829. [PubMed] [Google Scholar]
- 61.Williams JP, Hernady E, Johnston CJ, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161(5):560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 62.Chang SA, Kim YJ, Lee HW, et al. Effect of Rosuvastatin on Cardiac Remodeling, Function, and Progression to Heart Failure in Hypertensive Heart With Established Left Ventricular Hypertrophy. Hypertension. 2009;54:591–597. doi: 10.1161/HYPERTENSIONAHA.109.131243. [DOI] [PubMed] [Google Scholar]