Abstract
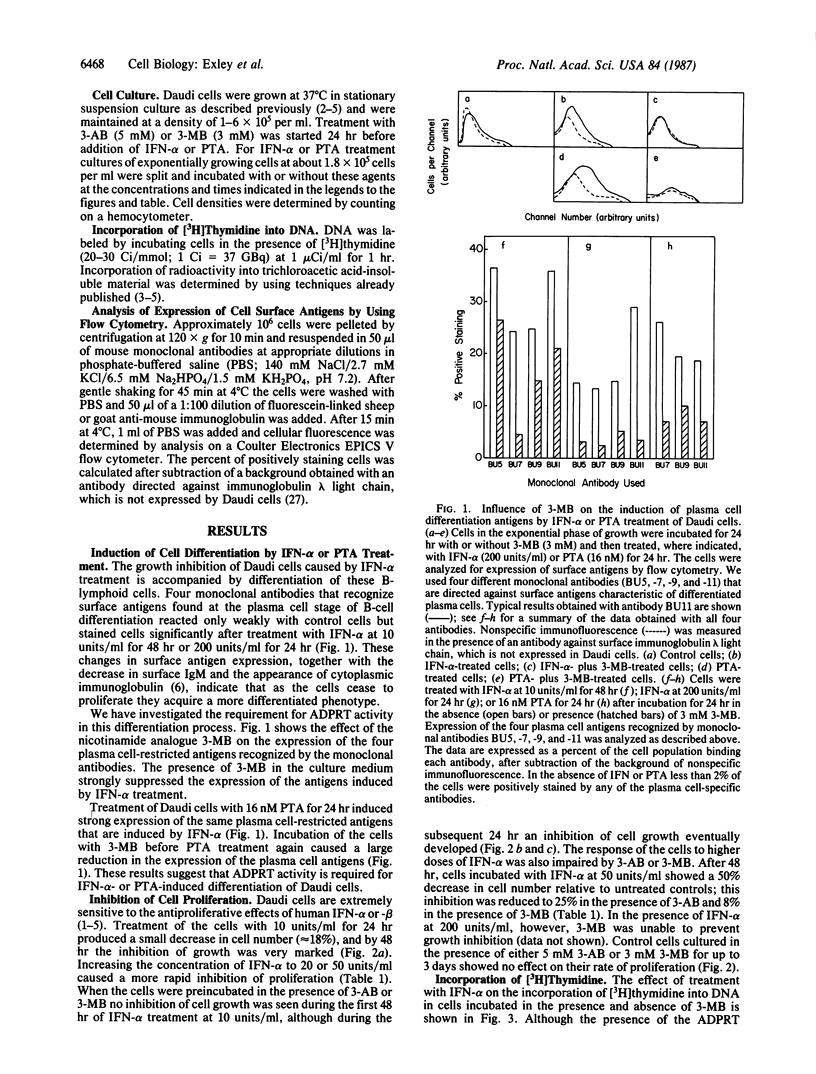
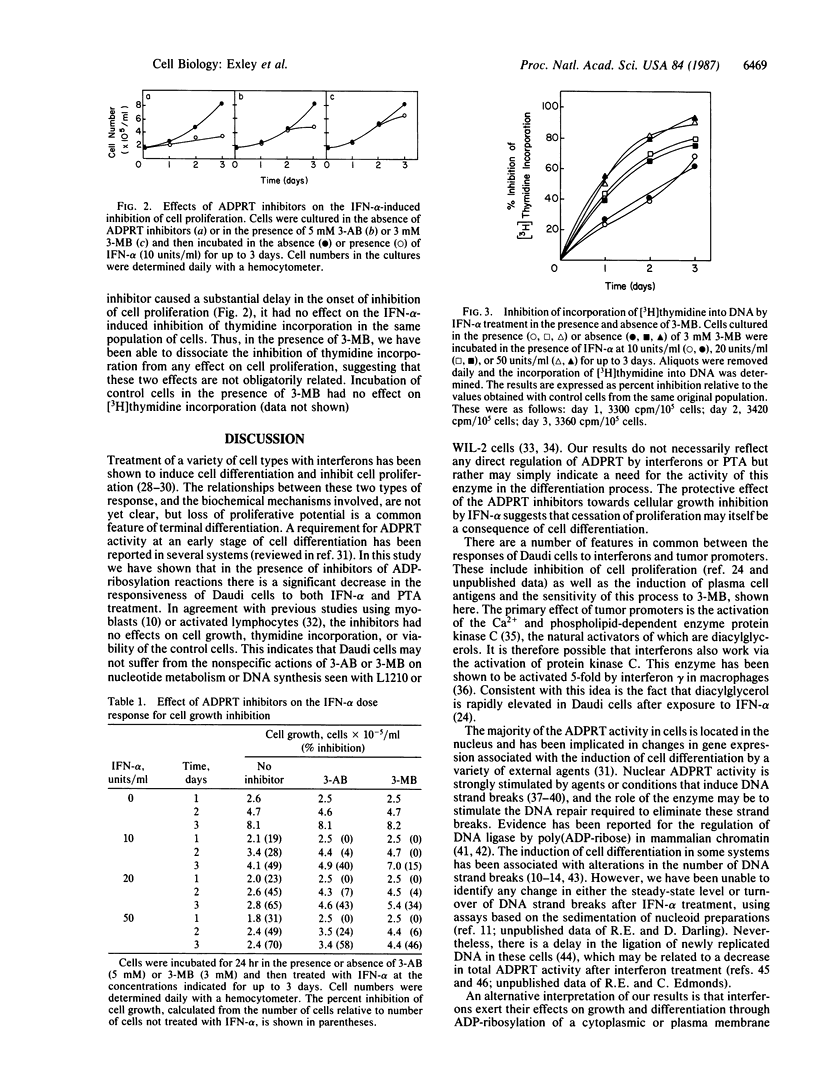
Treatment of the Daudi Burkitt lymphoma-derived cell line with human interferon alpha, which inhibits cell proliferation in this system, induces differentiation of these B-lymphoid cells into cells with a plasmacytoid phenotype. This differentiation, quantified by the appearance of surface antigens characteristic of mature plasma cells, is impaired by addition to the culture medium of the ADP-ribosyltransferase (ADPRT; EC 2.4.2.30) inhibitors 3-methoxybenzamide or 3-aminobenzamide. These agents also protect the cells against the inhibition of proliferation induced by low doses of interferon alpha. In contrast, the large inhibition of thymidine incorporation into DNA caused by interferon treatment is not affected by the ADPRT inhibitors. The phorbol ester phorbol 12-tetradecanoate 13-acetate induces the same plasma cell surface antigens that are induced by interferon treatment, and this effect is also impaired by the ADPRT inhibitors. These results suggest that interferons and phorbol esters share a mechanism of action that requires ADPRT activity. Protection of the cells against the antiproliferative effect of interferons by the ADPRT inhibitors suggests that growth inhibition may be a consequence of cell differentiation. In contrast, the inhibition of thymidine incorporation alone is not sufficient for the cessation of cell proliferation and is not a true reflection of the rate of DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. ADP-ribosylation in mammalian cell ghosts. Dependence of poly(ADP-ribose) synthesis on strand breakage in DNA. J Biol Chem. 1980 Nov 10;255(21):10493–10501. [PubMed] [Google Scholar]
- Butt T. R., Sreevalsan T. Interferon and sodium butyrate inhibit the stimulation of poly(ADP-ribose) synthetase in mouse cells stimulated to divide. Exp Cell Res. 1983 Oct 15;148(2):449–459. doi: 10.1016/0014-4827(83)90166-0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Shall S., Johnstone A. P. The dynamic nature of DNA-strand breaks present in differentiating muscle cells and quiescent lymphocytes. FEBS Lett. 1985 Sep 9;189(1):62–66. doi: 10.1016/0014-5793(85)80842-5. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Zalin R., Brill D., Shall S. DNA strand breaks and ADP-ribosyl transferase activation during cell differentiation. Nature. 1982 Nov 25;300(5890):362–366. doi: 10.1038/300362a0. [DOI] [PubMed] [Google Scholar]
- Ferro A. M., Higgins N. P., Olivera B. M. Poly(ADP-ribosylation) of a DNA topoisomerase. J Biol Chem. 1983 May 25;258(10):6000–6003. [PubMed] [Google Scholar]
- Francis G. E., Gray D. A., Berney J. J., Wing M. A., Guimaraes J. E., Hoffbrand A. V. Role of ADP-ribosyl transferase in differentiation of human granulocyte-macrophage progenitors to the macrophage lineage. Blood. 1983 Nov;62(5):1055–1062. [PubMed] [Google Scholar]
- Francis G. E., Ho A. D., Gray D. A., Berney J. J., Wing M. A., Yaxley J. J., Ma D. D., Hoffbrand A. V. DNA strand breakage and ADP-ribosyl transferase mediated DNA ligation during stimulation of human bone marrow cells by granulocyte-macrophage colony stimulating activity. Leuk Res. 1984;8(3):407–415. doi: 10.1016/0145-2126(84)90080-8. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Moore G., Clemens M. J. Inhibition of cell division by interferons. The relationship between changes in utilization of thymidine for DNA synthesis and control of proliferation in Daudi cells. Biochem J. 1983 Sep 15;214(3):983–990. doi: 10.1042/bj2140983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewert D. R., Moore G., Tilleray V. J., Clemens M. J. Inhibition of cell proliferation by interferons. 1. Effects on cell division and DNA synthesis in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1984 Mar 15;139(3):619–625. doi: 10.1111/j.1432-1033.1984.tb08049.x. [DOI] [PubMed] [Google Scholar]
- Gewert D. R., Shah S., Clemens M. J. Inhibition of cell division by interferons: Changes in the transport and intracellular metabolism of thymidine in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1981 Jun 1;116(3):487–492. doi: 10.1111/j.1432-1033.1981.tb05362.x. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Johannsen R. Sensitivity of various human lymphoblastoid cells to the antiviral and anticellular activity of human leukocyte interferon. Arch Virol. 1977;54(3):271–277. doi: 10.1007/BF01314795. [DOI] [PubMed] [Google Scholar]
- Hunting D. J., Gowans B. J., Henderson J. F. Specificity of inhibitors of poly(ADP-ribose) synthesis. Effects on nucleotide metabolism in cultured cells. Mol Pharmacol. 1985 Aug;28(2):200–206. [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. R., Lehmann A. R. Role of poly(adenosine diphosphate ribose) in deoxyribonucleic acid repair in human fibroblasts. Biochemistry. 1982 Aug 17;21(17):4007–4013. doi: 10.1021/bi00260a016. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Ling N. R., Nathan P. D., Hardie D. L. Use of monoclonal antibodies reactive with secretory epithelial cells for the immunocytochemical identification of plasma cells. Immunol Lett. 1986 Jun;12(5-6):295–300. doi: 10.1016/0165-2478(86)90033-7. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P. Rejoining of DNA strand breaks is an early nuclear event during the stimulation of quiescent lymphocytes. Eur J Biochem. 1984 Apr 16;140(2):401–406. doi: 10.1111/j.1432-1033.1984.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Johnstone A. P., Williams G. T. Role of DNA breaks and ADP-ribosyl transferase activity in eukaryotic differentiation demonstrated in human lymphocytes. Nature. 1982 Nov 25;300(5890):368–370. doi: 10.1038/300368a0. [DOI] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Ittel M. E., Niedergang C., Vosberg H. P., Mandel P. DNA topoisomerase I from calf thymus is inhibited in vitro by poly(ADP-ribosylation). Eur J Biochem. 1983 Nov 2;136(2):391–396. doi: 10.1111/j.1432-1033.1983.tb07754.x. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Mandel P., Okazaki H., Niedergang C. Poly(adenosine diphosphate ribose). Prog Nucleic Acid Res Mol Biol. 1982;27:1–51. doi: 10.1016/s0079-6603(08)60596-6. [DOI] [PubMed] [Google Scholar]
- Milam K. M., Cleaver J. E. Inhibitors of poly(adenosine diphosphate-ribose) synthesis: effect on other metabolic processes. Science. 1984 Feb 10;223(4636):589–591. doi: 10.1126/science.6420886. [DOI] [PubMed] [Google Scholar]
- Moore G., Gewert D. R., Clemens M. J. Inhibition of cell proliferation by interferons. 2. Changes in processing and stability of newly synthesized DNA in human lymphoblastoid (Daudi) cells. Eur J Biochem. 1984 Mar 15;139(3):627–635. doi: 10.1111/j.1432-1033.1984.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Nathan P. D., Walker L., Hardie D., Richardson P., Khan M., Johnson G. D., Ling N. R. An antigenic study of human plasma cells in normal tissue and in myeloma: identification of a novel plasma cell associated antigen. Clin Exp Immunol. 1986 Jul;65(1):112–119. [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Ueda K., Kawaichi M., Hayaishi O. Activation of DNA ligase by poly(ADP-ribose) in chromatin. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3604–3607. doi: 10.1073/pnas.80.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. L., Sikorski G. W., Catino D. M., Berger S. J., Berger N. A. Poly(adenosinediphosphoribose) polymerase inhibitors stimulate unscheduled deoxyribonucleic acid synthesis in normal human lymphocytes. Biochemistry. 1982 Apr 13;21(8):1813–1821. doi: 10.1021/bi00537a017. [DOI] [PubMed] [Google Scholar]
- Suhadolnik R. J., Sawada Y., Gabriel J., Reichenbach N. L., Henderson E. E. Accumulation of low molecular weight DNA and changes in chromatin structure in HeLa cells treated with human fibroblast interferon. J Biol Chem. 1984 Apr 25;259(8):4764–4769. [PubMed] [Google Scholar]
- Tanuma S., Johnson G. S. ADP-ribosylation of nonhistone high mobility group proteins in intact cells. J Biol Chem. 1983 Apr 10;258(7):4067–4070. [PubMed] [Google Scholar]
- Thi Man N., Shall S. The alkylating agent, dimethyl sulphate, stimulates ADP-ribosylation of histone H1 and other proteins in permeabilised mouse lymphoma (L1210) cells. Eur J Biochem. 1982 Aug;126(1):83–88. doi: 10.1111/j.1432-1033.1982.tb06749.x. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M., Oku T., Kishida T. Prolongation by interferon preparation of the survival time of mice implanted with differentiation-inducible mouse myeloid leukemia cells. Leuk Res. 1983;7(3):439–441. doi: 10.1016/0145-2126(83)90108-x. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto Y., Hozumi M. Stimulation by interferon of induction of differentiation of human promyelocytic leukemia cells. Biochem Biophys Res Commun. 1982 Jan 15;104(1):30–37. doi: 10.1016/0006-291x(82)91936-2. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Wesierska-Gadek J., Sauermann G. Modification of nuclear matrix proteins by ADP-ribosylation. Association of nuclear ADP-ribosyltransferase with the nuclear matrix. Eur J Biochem. 1985 Dec 2;153(2):421–428. doi: 10.1111/j.1432-1033.1985.tb09319.x. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Johnstone A. P. ADP-ribosyl transferase, rearrangement of DNA, and cell differentiation. Biosci Rep. 1983 Sep;3(9):815–830. doi: 10.1007/BF01133780. [DOI] [PubMed] [Google Scholar]
- Yap W. H., Teo T. S., McCoy E., Tan Y. H. Rapid and transient rise in diacylglycerol concentration in Daudi cells exposed to interferon. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7765–7769. doi: 10.1073/pnas.83.20.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Murcia G., Huletsky A., Lamarre D., Gaudreau A., Pouyet J., Daune M., Poirier G. G. Modulation of chromatin superstructure induced by poly(ADP-ribose) synthesis and degradation. J Biol Chem. 1986 May 25;261(15):7011–7017. [PubMed] [Google Scholar]



