Summary
In visual cortex monocular deprivation (MD) during a critical period (CP) reduces the ability of the deprived eye to activate cortex, but the underlying cellular plasticity mechanisms are incompletely understood. Here we show that MD reduces the intrinsic excitability of layer 5 (L5) pyramidal neurons and enhances long-term potentiation of intrinsic excitability (LTP-IE). Further, MD and LTP-IE induce reciprocal changes in Kv2.1 current, and LTP-IE reverses the effects of MD on intrinsic excitability. Taken together these data suggest that MD reduces intrinsic excitability by preventing sensory-drive induced LTP-IE. The effects of MD on excitability were correlated with the classical visual system CP, and (like the functional effects of MD) could be rapidly reversed when vision was restored. These data establish LTP-IE as a novel candidate mechanism mediating loss of visual responsiveness within L5, and suggest that intrinsic plasticity plays an important role in experience-dependent refinement of visual cortical circuits.
Introduction
In mammals, visual deprivation early in life sets in motion a cascade of events that can result in dramatic impairment of visual function (Fagiolini et al., 1994; Hubel et al., 1977; LeVay et al., 1980; Shatz and Stryker, 1978). Even periods of visual deprivation as short as two days during an early critical period (CP) result in amblyopia –loss of visual acuity and cortical responsiveness to stimulation of the deprived eye (Frenkel and Bear, 2004; Kaneko et al., 2008). These changes have largely been ascribed to synaptic plasticity mechanisms (Smith et al., 2009; Tropea et al., 2009), but cortical neurons also possess plasticity mechanisms that regulate their intrinsic excitability (Beck and Yaari, 2008; Cudmore and Turrigiano, 2004; Daoudal and Debanne, 2003; Fan et al., 2005; Marder and Prinz, 2002; Nelson et al., 2003; Sourdet et al., 2003; Zhang and Linden, 2003). Whether intrinsic plasticity contributes to the loss of visual cortical responsiveness induced by visual deprivation, and by extension to the normal experience-development refinement of cortex, has not been investigated.
The rodent visual system is an important model system for investigating the cellular and molecular underpinnings of CP plasticity. Many visual response properties are influenced or degraded by visual deprivation during a critical window beginning around postnatal day (P) 19/20, including visual acuity and the ability of the deprived eye to activate cortex (amblyopia), and ocular dominance (OD) (Gordon and Stryker, 1996; Tagawa et al., 2005; Huberman et al., 2008). In rodents much of the visual fields of the two hemispheres do not overlap and are mapped to a purely monocular region, with a smaller binocular region receiving input from both eyes (Gordon and Stryker, 1996; Mrsic-Flogel et al., 2007). It has recently become clear that OD shifts in binocular cortex involve two mechanistically distinct processes; first a rapid (within 2 d) loss of responsiveness to the deprived eye, followed after 5–6 days by a potentiation of responsiveness to the non-deprived eye. Visual responsiveness to the deprived eye depresses with an identical time course in monocular and binocular cortex (Frenkel and Bear, 2004; Kaneko et al., 2008), indicating that the cellular plasticity mechanisms that underlie deprivation-induced amblyopia are present and can be studied in both cortical areas.
Many forms of plasticity are present within visual cortical circuits, and their expression is cell-type and layer-specific (Crozier et al., 2007; Daw et al., 1992; Desai et al., 2002; Jiang et al., 2007; Maffei et al., 2004; Maffei et al., 2006; Maffei and Turrigiano, 2008). The canonical view of information flow in visual cortical columns is that thalamic information enters the cortex primarily in layer 4 (L4), is relayed from L4 to layers 2/3 (L2/3) where there are extensive lateral connections with local and distant cortical areas, and from there passes to layer 5 (L5) (Bannister, 2005; Gilbert and Wiesel, 1983; Thomson and Bannister, 2003). L5 pyramidal neurons in turn project to a number of cortical and subcortical targets, and constitute a major output pathway of cortex (Hattox and Nelson, 2007; Kawamura et al., 1974; Thomson and Bannister, 2003). Each cortical layer thus has specific input and output connections, as well as specialized local circuitry (Martin, 2002), and layer-specific plasticity mechanisms are likely tuned to the particular functional requirements of each layer (Nelson and Turrigiano, 2008).
In keeping with this layer-specific view of cortical plasticity, the cellular plasticity mechanisms that underlie deprivation-induced amblyopia appear to differ in different layers (Daw et al., 2004; Liu et al., 2008; Maffei et al., 2006), and include potentiation of inhibition within L4 but not L2/3 (Maffei et al., 2006, Maffei and Turrigiano, 2008; Maffei et al., 2010) as well as distinct forms of LTD in L4 and L2/3 (Crozier et al., 2007; Liu et al., 2008). Using experience to generate a functional cortical column thus involves the well-orchestrated engagement of a distributed set of plasticity mechanisms, and our ability to understand and manipulate CP plasticity requires a full understanding of the layer- and cell type-specific mechanisms that underlie it. Currently, there is no candidate mechanism for the loss of visual responsiveness within L5, the major output pathway of cortex.
Changes in intrinsic excitability that alter the input-output function of a neuron can strongly affect network behavior, by enhancing or suppressing the effectiveness of individual neurons in driving postsynaptic partners. Like synaptic plasticity, intrinsic plasticity comes in homeostatic forms (Brager and Johnston, 2007; Desai et al., 1999; Pratt and Aizenman, 2007; Turrigiano et al., 1994) and in non-homeostatic forms, where high frequency firing drives an increase in intrinsic excitability (Aizenman et al., 2003; Cudmore and Turrigiano, 2004; Nelson et al., 2003; Sourdet et al., 2003). We recently found that a brief period of repetitive high frequency firing induces a long-term potentiation of intrinsic excitability (LTP-IE) in L5 visual cortical pyramidal neurons, characterized by a decreased excitability in the evoked firing rate vs. current curves (F-I curves). This LTP-IE can be induced by postsynaptic firing alone in the presence of synaptic blockers, and requires calcium influx and the activation of PKA for its expression (Cudmore and Turrigiano, 2004). The possibility that LTP-IE might play a role in experience-dependent changes in cortical function has not been investigated.
Here we investigated the effects of brief MD on intrinsic excitability of L5 pyramidal neurons in acute slices derived from the control and deprived hemispheres of monocular visual cortex. We found that neurons from the deprived hemisphere were less excitable than those from the control hemisphere, and were able to undergo a greater degree of potentiation during LTP-IE, suggesting that MD moves L5 pyramidal neurons further from LTP-IE saturation, perhaps by preventing LTP-IE induction. These excitability changes occurred through alterations in both a leak conductance and a persistent voltage-dependent K+ (Kv) conductance, and involved changes in surface expression of Kv2.1 channels, suggesting that they are induced by activity-dependent changes in Kv channel trafficking. The effects of MD on intrinsic excitability were evident during a critical window that corresponded well with the classical visual system CP, and could be rapidly reversed when vision was restored. These data show that LTP-IE plays an important role in regulating the excitability of a major output pathway of cortex, and establishes LTP-IE as a candidate mechanism mediating loss of visual responsiveness within L5. More generally, our data suggest that LTP-IE serves a “use it or lose it” function within L5, that gates cortical output by keeping active neurons responsive, while suppressing the output of inactive neurons.
Results
MD selectively decreases the intrinsic excitability of L5 pyramidal neurons
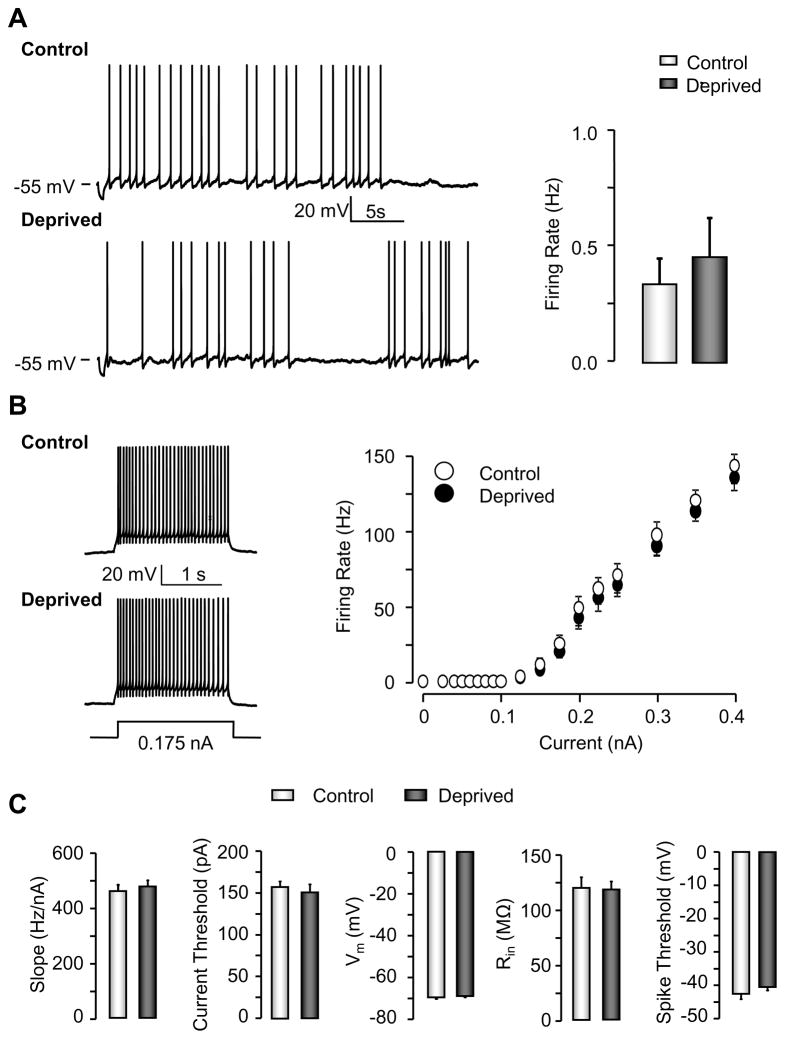
To assess the effects of MD on excitability of L5 pyramidal neurons, we first compared the spontaneous action potential (AP) firing rates of L5 pyramidal neurons from control and deprived hemispheres in slices from the monocular portion of primary visual cortex (V1M). Monocular lid suture was performed between P18–P21 (the very beginning of the classical visual system CP), and slices were prepared from the control and deprived hemispheres. To initiate spontaneous AP activity and compare firing rates across conditions, we used ACSF in which the Ca2+/Mg2+ ratio was modified to enhance spontaneous activity, and injected a small constant bias current to keep the interspike membrane potential (Vm) close to −60 mV, as described previously (Maffei et al., 2004; Maffei and Turrigiano, 2008). Under these conditions L5 pyramidal neurons fired APs spontaneously in response to stochastic synaptic events (Figure 1A), and MD caused a five-fold decrease in spontaneous firing (Figure 1A, middle panel; control: n = 14; deprived: n = 14; p < 0.01).
Figure 1. MD decreases spontaneous firing and intrinsic excitability of L5 pyramidal neurons.
(A) Examples of spontaneous L5 pyramidal neuron firing in slices from the control (left upper panel) or deprived (left lower panel) hemisphere, average firing rates (middle panel), and camera lucida reconstruction of biocytin fill (right panel). (B) Examples of evoked responses of neurons from the control (left upper panel) or deprived (left lower panel) hemispheres, in the presence of synaptic blockers. Right panel: average F-I curves from the two conditions. (C) Average values (left to right) of F-I curve slope, current threshold, membrane potential (Vm), input resistance (Rin), and voltage threshold for spiking. * = p < 0.05 here an in subsequent figures.
This reduction in spontaneous firing could reflect changes in synaptic drive and/or changes in intrinsic excitability. To directly assess the intrinsic excitability of L5 pyramidal neurons we compared F-I curves in neurons from the control and deprived hemispheres, measured in the presence of synaptic blockers from a membrane potential of −65 mV. MD significantly reduced the number of spikes over the full range of current injections (ANOVA, p < 0.01), (Figure 1B), significantly decreased the slope of the linear portion of the F-I curve (Figure 1C; control; n = 21; deprived: n = 21; p < 0.01), increased the current threshold for evoking spikes (Figure 1C; control; n = 21; deprived: n = 21; p < 0.05), and significantly reduced Rin (Figure 1C; control: n = 21; deprived: n = 21; p < 0.01). MD did not affect Vm (Figure 1C; control; n = 21; deprived: n = 21; p = 0.81), or the voltage threshold for AP generation, defined as the membrane voltage (Vm) where dVm/dt is ≥ 10 V/s (Figure 1C; control, n = 14; deprived: n = 14; p = 0.35). These data suggest that a major contributor to the reduced spontaneous firing of L5 pyramidal neurons is a reduction in intrinsic excitability.
Pyramidal and GABAergic interneurons serve different functions within the cortical microcircuit. To ask whether excitability changes were specific to pyramidal neurons we recorded from FS cells within L5, using the G42 transgenic mouse line, in which PV-positive interneurons express GFP, to target FS cells (Chattopadhyaya et al., 2004). First, we confirmed that, as for rat, L5 pyramidal neurons in mouse show decreased spontaneous activity and intrinsic excitability following MD from P18–P21 (Figure S1). In contrast, neither the spontaneous activity (Figure 2A, control: n = 23; deprived: n = 23; p = 0.78) nor intrinsic excitability (Figure 2B; control: n = 13; deprived: n = 12; p = 0.36) were altered in FS cells following MD, nor were there significant changes in Rin, Vm, or spike threshold (Figure 2C). There are several classes of L5 pyramidal neurons with different targets, morphologies, and firing properties (Hattox and Nelson, 2007); MD reduced intrinsic excitability in both thick- and thin-tufted neurons as determined from cell fills, suggesting that this effect generalized across L5 pyramidal neuron types. MD thus reduces neuronal excitability in a cell-type specific manner, and selectively targets the principle excitatory output neurons of L5.
Figure 2. MD does not affect excitability of L5 FS cells.
(A) Examples of spontaneous FS firing from neurons from the control (left upper panel) or deprived (left lower panel) hemisphere. Right panel: average firing rates for the two conditions. (B) Examples of evoked responses from neurons from the control (left upper panel) or deprived (left lower panel) hemisphere. Right panel: F-I curves of control and deprived FS cells. (C) Average values (left to right) of F-I curve slope, current threshold, membrane potential (Vm), input resistance (Rin), and voltage threshold for spiking. See also Figure S1.
MD increases the magnitude of LTP-IE
L5 pyramidal neurons exhibit a form of intrinsic plasticity (LTP-IE) in which high frequency firing produces a persistent increase in intrinsic excitability (Cudmore and Turrigiano, 2004). This raised the interesting possibility that MD might reduce intrinsic excitability by preventing normal, sensory drive-induced LTP-IE. If so, then deprived neurons should be further from LTP-IE saturation, and should exhibit a greater degree of LTP-IE, than control neurons. To examine this we induced LTP-IE using a protocol modified from Cudmore and Turrigiano, 2004. We elicited precisely-timed trains of APs (15 spikes at 40 Hz) every 4 seconds for 10 minutes (Figure 3A, upper panel). Neuronal excitability was probed with a 500 ms long DC pulse of an amplitude that evoked 3–5 APs during the baseline period (Figure 3A, upper panel); once the appropriate current amplitude was determined it was fixed throughout the experiment.
Figure 3. LTP-IE is enhanced by MD.
(A) Example of LTP-IE in a neuron from the control hemisphere, showing evoked spike number vs. time (top) and Rin (bottom). Dotted vertical lines indicate the onset and end of induction protocol in this and subsequent figures. The insets (upper panel) illustrate the stimulus (grey) and evoked responses (black) during pre-induction, induction, and post-induction (not shown at same time scale). (B) Example of LTP-IE from the deprived hemisphere. (C) Average time course of LTP-IE from neurons from the control or deprived hemisphere, with and without induction. (D) F-I curves, (E) Rin, and (F) current threshold for control and deprived neurons before and after LTP-IE induction. See also Figure S2.
This induction protocol potentiated firing in responses to a DC current pulse in neurons from both control (Figure 3A) and deprived (Figure 3B) hemispheres, but strikingly, the magnitude of potentiation was approximately twice as large for deprived neurons (Figure 3C; control: 48.20±1.60 %, n = 14; deprived: 94.42±0.88 %, n = 15). This protocol is supra-saturating for LTP-IE induction, and additional periods of induction using the same protocol did not induce further potentiation (Figure S2). LTP-IE did not alter the input resistance (Rin) of control neurons (Figure 3E; n = 14; 0.1% change p = 0.98), yet caused a small but significant increase (~8%) in Rin for neurons from the deprived hemisphere (Figure 3E; n = 15; p < 0.01). In control recordings where no induction stimulus was delivered, the response remained stable for the duration of the recording from neurons in both control and deprived hemispheres (Figure 3C, triangles).
Next, we generated F-I curves before and after LTP-IE induction. As in the previous data set (Figure 1B), MD reduced excitability over the whole supra-threshold voltage range tested (Figure 3D), while LTP-IE increased excitability for neurons in both control and deprived hemispheres (p < 0.05), but produced a much larger change in the F-I curve in deprived neurons. Interestingly, after LTP-IE induction there was no significant difference in the F-I curves (ANOVA, p = 0.27) or current threshold (Figure 3F; control: n = 10; deprived: n = 12; p = 0.30) for control and deprived neurons. Although LTP-IE induced a small increase in the Rin of deprived neurons, Rin was still significantly lower in deprived neurons after LTP-IE induction (Figure 3E), which might account for the persistent small (but not significant) difference in F-I curves after induction (Figure 3D). Taken altogether, these results suggest that neurons from the deprived hemisphere are farther from saturation of LTP-IE than control neurons, and thus can undergo more LTP-IE.
In contrast to L5, where lid suture reduces the intrinsic excitability of L5 pyramidal neurons (Figure 1B), two days of lid suture at the same developmental stage increases the intrinsic excitability of L2/3 pyramidal neurons (Maffei and Turrigiano, 2008), suggesting that these two classes of pyramidal neuron express different forms of intrinsic plasticity. To determine whether L2/3 pyramidal neurons express LTP-IE we attempted to induce intrinsic plasticity using the same protocol that is maximally effective in L5 pyramidal neurons, and found no potentiation (Figure 4A, n = 9, p = 0.24), no significant change in the F-I curves (n = 5, p = 0.10), and no change in current threshold (Figure 4B, n = 5, p = 0.37). These data indicate that LTP-IE is not a general property of all pyramidal neurons.
Figure 4. L2/3 pyramidal neurons do not express LTP-IE.
(A) Example (upper left) and average (lower left) time course following delivery of LTP-IE protocol to L2/3 pyramidal neurons. Right: change in excitability 50 minutes after the LTP-IE induction protocol for L2/3 and L5 pyramidal neurons. (B) Average F-I curves before and after LTP-IE induction protocol in L2/3 pyramidal neurons. There was no significantly change in the F-I curve (left panel) or the current threshold for action potential firing (right panel).
MD reduces excitability by increasing a persistent, TEA-sensitive Kv current
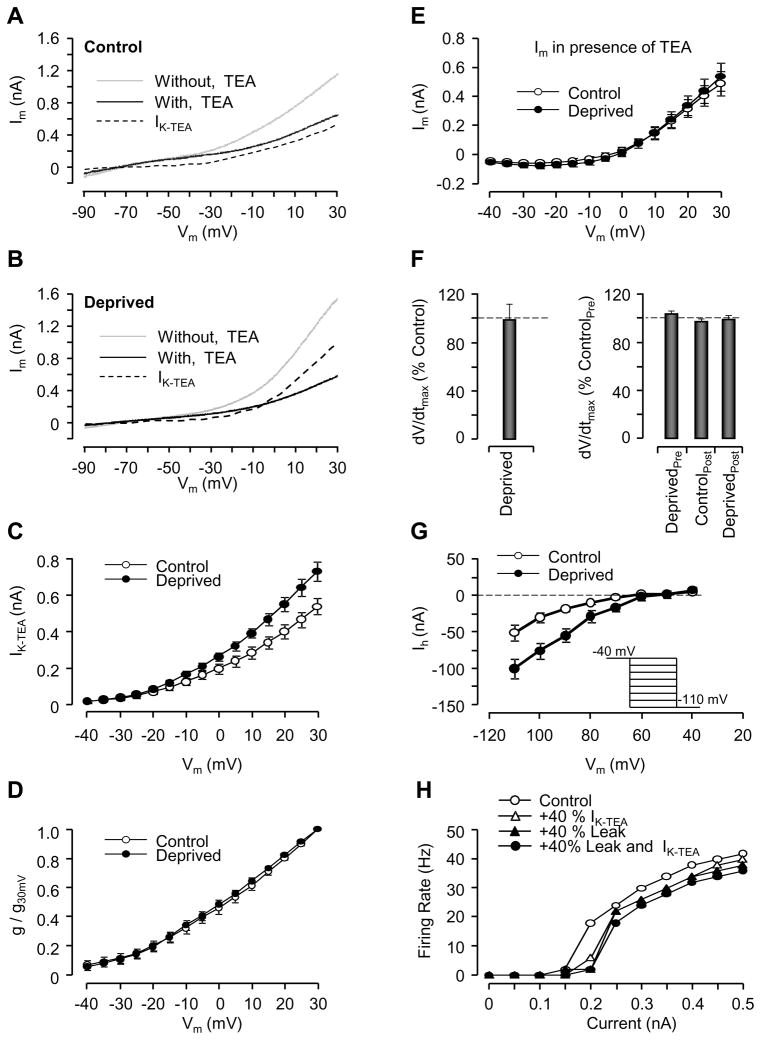
Part of the reduction in intrinsic excitability of L5 pyramidal neurons is likely due to the MD-induced reduction in Rin. To determine if changes in voltage-dependent currents also contribute, we performed a series of additional experiments. First, we compared the magnitude of a persistent, TEA-sensitive delayed-rectifier type K+ current (IK-TEA) in L5 pyramidal neurons from the control and deprived hemispheres. In pyramidal neurons the major somatic delayed-rectifier type K+ current is thought to be generated by Kv2.1 channels, and is TEA-sensitive but relatively 4-AP insensitive (Coetzee et al., 1999; Lien et al., 2002; Misonou et al, 2005). TEA blocks both transient and persistent delayed rectifier-type potassium currents, as well as some calcium-dependent potassium currents (Hille, 2001; Latorre et al., 1989). To isolate the persistent, 4-AP insensitive component of IK-TEA we performed voltage clamp recordings in the presence of TTX and 4-AP and used a ramp stimulus from −90 mV to +30 mV with different velocities (10, 30, and 50 mV/s), delivered before and after washing in TEA (Figures 5A, B). To minimize the contribution of calcium and calcium-dependent currents, we removed all calcium from the external solution and added 5 mM BAPTA to the recording micropipette. Ramp currents were leak subtracted, and then the current in TEA was subtracted from the control current to generate a TEA-sensitive current (Figures 5A,B, IK-TEA). This procedure isolated a voltage-dependent outward current, IK-TEA that activated above −40 mV (Figure 5C).
Figure 5. MD reduces excitability by increasing a persistent Kv current (IK-TEA).
(A) Voltage clamp recording from the control hemisphere showing response to a voltage ramp (slope: 30 mV/s). The leak-subtracted response before (Without) and after (With) TEA, and the TEA-sensitive difference current (IK-TEA, obtained by subtracting the two) are shown. (B) Same as A, for the deprived hemisphere. (C) Average values of IK-TEA from neurons from the control or deprived hemisphere. MD significantly increased IK-TEA at voltages ≥+5 mVs. (D) Average TEA-sensitive K conductance normalized to the conductance at +30 mV. (E) Average values of remaining membrane current (Im) from neurons from the control and deprived hemispheres in the presence of TEA. (F) Ratio of the maximum rate of rise of the action potential (dV/dtmax) between control and deprived neurons, before and after LTP-IE. Left: from spontaneous AP; right: from evoked APs. (G) Average values of Ih (ZD-7288 sensitive currents) from control and deprived neurons. Inset shows step protocol used to isolate Ih. (H) Simulation of the effects of modifying leak current and IK-TEA on the F-I curve in model L5 pyramidal cell. Leak and IK-TEA were increased by 40% from starting values either separately or together and the effect on F-I curve plotted.
MD significantly increased IK-TEA (Figure 5C; ANOVA, p < 0.05). At +30 mV the average magnitude of IK-TEA increased by about 40% in cells from the deprived hemisphere (Figure 5C; control: n = 16; deprived: n = 13; p < 0.01). Similar results were obtained with all ramp velocities (data not shown). Converting current to conductance and normalizing to the conductance at +30 mV revealed that the increase in IK-TEA occurred without any change in the voltage dependence of activation (Figure 5D), indicating that MD enhanced IK-TEA by increasing the maximal conductance rather than modulating the voltage dependence, possibly by increasing channel number. Consistent with our current clamp data, MD also significantly increased the linear leak current by 27.3 % (control: n = 16; deprived: n = 13; p < 0.01). In the voltage range above −60 mV there was no difference between control and deprived neurons in the leak-subtracted Im in the presence of TEA (Figure 5E control: n = 16; deprived: n = 13; p = 0.71), indicating that MD did not affect additional sustained outward currents. These data indicate that MD increases the magnitude of both leak and IK-TEA in L5 pyramidal neurons, and these changes are in the right direction to contribute to the reduction in intrinsic excitability.
To assess a possible contribution from changes in inward sodium current, we took advantage of the fact that the AP rate of rise (dV/dt) is directly proportional to the sodium current density (Kole et al., 2007). Quantification of the maximum dV/dt revealed no difference between control and MD, or before and after LTP-IE (Figure 5F), indicating no measurable effect on somatic sodium currents. Finally, we examined the response of L5 pyramidal neurons to long hyperpolarizing current steps from −40 mV in the presence and absence of ZD-7288, a selective inhibitor of the hyperpolarization-activated inward current Ih. As described previously (Berger et al., 2001; Kole et al., 2006) Ih activated below −60 mV in these neurons (Figure 5G), and MD significantly increased Ih at all voltages below −70 mV (Figure 5G control: n = 10; deprived: n = 10; p = 0.11). Because of the voltage dependence of Ih this might contribute to changes in Rin but is unlikely to contribute to changes in the suprathreshold F-I curve. To assess the contribution of Ih we measured excitability in the presence of ZD-7288, and found that the difference in Rin and F-I curves between neurons from the control and deprived hemispheres persisted (difference in ZD-7288 was 103 ± 4% of difference without ZD-7288, N = 4).
The changes in leak current and IK-TEA are in the right direction to account for the MD-induced reduction in intrinsic excitability. To determine whether these changes are sufficient, we utilized a realistic conductance-based model of L5 pyramidal neurons (modified from Kampa and Stuart 2006, see Methods) to assess the effects of modifying leak and IK-TEA on the F-I curve. The initial values of leak and K+ conductances in the model were adjusted to better reproduce the shape of our measured F-I curves, and the delayed-rectifier type current was replaced with the TEA-sensitive current described in Korngreen and Sakmann (2000) to better model our measured TEA-sensitive current. We generated F-I curves in the model neuron in response to 0.5 second long DC current injections, and found that decreasing Rin and increasing IK-TEA by 40% each (the magnitude change observed experimentally) reduced firing over the whole F-I curve, and increased the voltage threshold (Figure 5H), similar to the effects of MD. At current injections of 0.4–0.45 nA, a 40% increase in leak alone produced a small decrease in firing (by 2 Hz); a 40% increase in IK-TEA alone reduced firing by 4 Hz, and when both were increased together firing was reduced by 6 Hz. This matches well the magnitude of effect observed in the experimental data (a reduction of 6–10 Hz, Figure 1B or 3D). Qualitatively similar results were obtained for the original unmodified Kampa and Stuart model, and for a simple single compartment pyramidal neuron model with a different balance of conductances (Pospischil et al., 2008), indicating that these results are not particular to specific model parameters. These modeling results demonstrate that the change in outward currents measured here are sufficient to account for the MD-induced reduction in intrinsic excitability, and that IK-TEA plays a larger role in modifying the supra-threshold F-I curve than does the reduction in leak.
MD increases the number of Kv2.1 channels in the somatic membrane
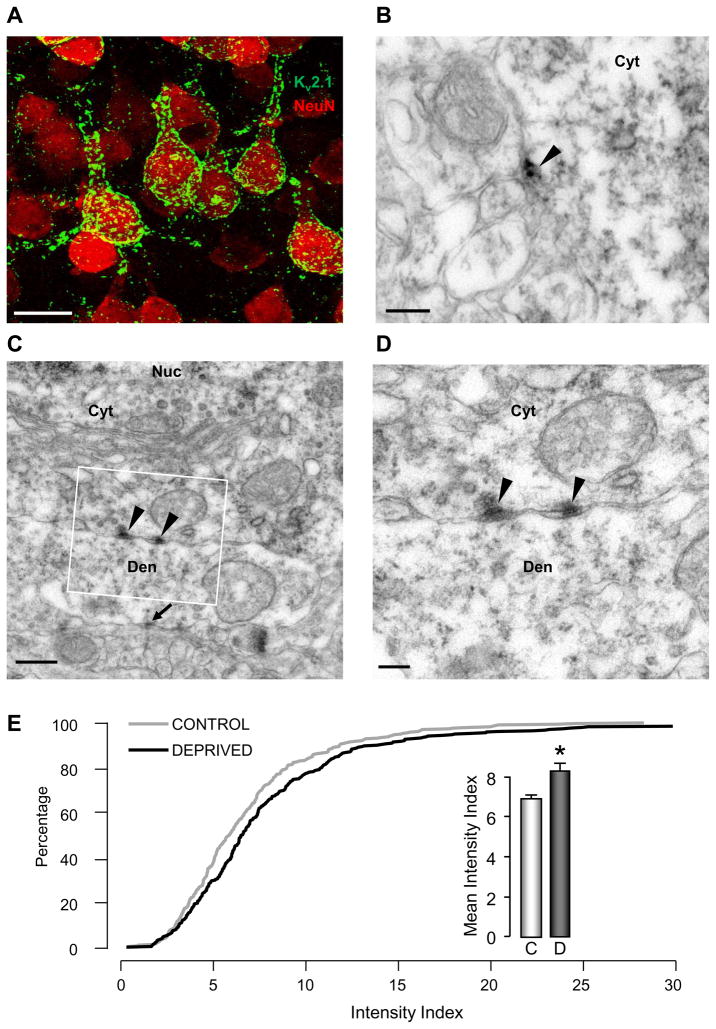
To directly determine whether MD increases the number of Kv2.1 channels in the membrane of L5 pyramidal neurons, we quantified the somatic expression of Kv2.1 by immuno-EM. L5 pyramidal neurons were identified by strong NeuN labeling (GABAergic interneurons show only weak NeuN labeling, Chattopadhyaya et al., 2004, Nahmani and Turrigiano, unpublished observations) and the characteristic size and shape of somata and proximal dendrites (Figure 6A). Confocal imaging after labeling with NeuN (red) and antibodies directed against Kv2.1 (green, Lim et al., 2000; Mohapatra et al., 2008 Muennich and Fyffe 2004, Trimmer 1991) revealed punctate accumulation of channels along the somata and proximal dendrites (Figure 6A). It was difficult to distinguish surface from internal channels at the light microscope level, so to quantify surface channels we went to the EM level, where the Kv2.1 accumulations could be visualized along the somatic membrane (Figure 6B,C,D). We quantified the amount of Kv2.1 signal/μm perimeter from cross-sections of L5 pyramidal somata from the control or deprived hemisphere (blind to condition) and found a significant increase in the Kv2.1 signal in deprived neurons, to 121 ± 6% of control (Figure 6E, n = 20 neurons from each condition, p = 0.005, Mann-Whitney test). This percentage increase is not as large as the change in IK-TEA, possibly because the signal-to-noise ratio is less favorable in the EM compared to voltage clamp experiments. These data strongly suggest that an increase in the surface accumulation of Kv2.1 channels following MD contributes to the changes in IK-TEA.
Figure 6. MD increases the accumulation of Kv2.1 at the somatic membrane of Layer 5 pyramidal neurons.
(A) Confocal z-projection showing Kv2.1 labeling (green) of NeuN-labeled layer 5 pyramidal neurons (red). (B) Representative electron micrograph showing Kv2.1-DAB labeling (arrowhead) of a layer 5 somatic membrane. Kv2.1 -DAB accumulations often appeared as electron-dense clusters on the extracellular surface of somatic membranes. (C) Lower magnification image and (D) higher magnification image (white box in C) showing Kv2.1 labeling on the extracellular surface of the somatic membrane (arrowheads) at a pyramidal somatic membrane. A large dendrite appears directly below the soma and receives a synapse (arrow in C) from an adjacent bouton. Nuc=nucleus, Cyt=cytoplasm, Den=dendrite. (E) Cumulative percentage plot of Kv2.1 label intensity on layer 5 somatic membranes for control and deprived neurons; inset shows mean intensity. C = Control, D = Deprived. Scale bars = 20 μm (A), 200 nm (B), 500 nm (C), 200 nm (D).
LTP-IE induction targets IK-TEA and requires endocytosis but not new protein synthesis
If MD reduces intrinsic excitability by modulating the induction of LTP-IE, then LTP-IE and MD should induce reciprocal changes in IK-TEA. To test this, we measured IK-TEA 30 minutes after “mock” induction (no firing) or actual LTP-IE induction in neurons from the deprived hemisphere. IK-TEA was measured as described above except BAPTA was omitted from the recording pipette to allow LTP-IE induction (Cudmore and Turrigiano, 2004). LTP-IE induction significantly enhanced excitability as in previous experiments, and reduced IK-TEA magnitude by about 40% (Figure 7A; No Induction: n = 6; LTP-IE without DYN: n = 9; p < 0.05), without affecting the voltage-dependence of IK-TEA activation (Figure 7B). This demonstrates that MD and LTP-IE have reciprocal effects on IK-TEA, as expected if MD reduces intrinsic excitability by preventing LTP-IE induction.
Figure 7. LTP-IE induction targets IK-TEA and requires endocytosis but not new protein synthesis.
(A) IK-TEA 30 minutes after mock induction (No Induction), or LTP-IE induction with or without DYN to block endocytosis. (B) Average TEA-sensitive K+ conductance normalized to the conductance at +5 mV. (C) Average time course of LTP-IE with or without protein synthesis blockade. (D) Average potentiation after LTP-IE protocol with and without anisomycin. (E) Average time course of LTP-IE with and without endocytosis blocker (DYN: 100 μM) in the recording pipette. (F) Average potentiation after LTP-IE protocol with and without DYN.
Our data suggest that LTP-IE is expressed through a rapid reduction in IK-TEA, likely through a reduction in the number of channels in the neuronal membrane. LTP-IE did not require new protein synthesis because it could still be induced in the presence of anisomycin (Figure 7C, D; Control: n = 14; Anisomycin: n = 9; p = 0.32, data from control hemisphere). To test the idea that channel endocytosis might underlie LTP-IE induction, we infused a dynamin inhibitory peptide DYN (QVPSRPNRAP) into neurons through the recording pipette (Kim et al., 2007). Dynamin is essential for vesicle endocytosis, and DYN has been shown to block Kv channel internalization in hippocampal pyramidal neurons (Jung and Hoffman, 2009). DYN by itself had no effect on baseline excitability over the time course of these experiments (Figure 7E), but was able to completely block the induction of LTP-IE (Figures 7E, F; Control: n = 14; DYN: n = 6; p < 0.001, data from the control hemisphere). DYN was also able to completely prevent the change in IK-TEA induced by LTP-IE induction (Figures 7A, B; LTP-IE with DYN: n = 5; LTP-IE without DYN: n = 9; p < 0.05, data from the deprived hemisphere). Taken together with the enhanced Kv2.1 channel accumulation during MD, these data suggest that LTP-IE depends critically on the dynamin-dependent endocytosis of channels underlying IK-TEA (likely Kv2.1 channels).
A critical period for MD-induced changes in intrinsic excitability
Rapid OD plasticity is confined to a developmental window that opens around P19/20 and closes near the end of the fifth postnatal week (around P34) (Fagiolini et al., 1994; Gordon and Stryker, 1996). If, as our data suggest, MD-induced reduction in excitability of L5 pyramidal neurons is a major contributor to the loss of visual responsiveness within L5, then it should exhibit a similar critical window. To test this we cut slices from animals of different ages and examined LTP-IE induction and the effects of MD. LTP-IE was absent in L5 pyramidal neurons just prior to eye opening (P14/15), was significant by P17/18, reached a peak by P20/21, and remained at a similar level until P34/35, the latest age tested. Thus LTP-IE turns on after eye opening and persists through the CP (Figure 8A). Next, we asked whether the ability of visual experience to reduce excitability and modulate the magnitude of LTP-IE was developmentally regulated, by performing 2D of MD at different developmental stages. The ability of MD to enhance the magnitude of LTP-IE was absent at P18/P19 (Control: n = 6; Deprived: n = 9; p = 0.82), present between P20/P21 (Control: n = 14; Deprived: n = 15; p < 0.001) and P27/P28 (Control: n = 9; Deprived: n = 8; p = 0.01), and completely absent again at P34/35 (Figure 8B; Control: n = 9; Deprived: n = 6; p = 0.64). The same pattern was observed for the ability of MD to modulate intrinsic excitability; we compared the ratio of deprived to control firing rates (from F-I curves at current injections from 0.25 to 0.4 nA), and found that excitability was reduced by MD at P20/21 and P27/28, but not earlier or later (Figure 8C). Thus, the ability of visual deprivation to reduce intrinsic excitability and enhance LTP-IE exhibits a critical window that mirrors the critical window for MD-induced loss of visual responsiveness.
Figure 8. Critical period for MD-induced changes in intrinsic excitability and LTP-IE induction.
(A) Magnitude of LTP-IE at different developmental ages. (B) The effects of MD at different developmental times on the magnitude of LTP-IE. (C) The effect of MD at different developmental ages on intrinsic excitability. Firing rates are expressed as the ratio of control to deprived firing rates (averaged over current injections ≥ 0.3 nA).
The effects of MD are rapidly reversed
The effects of brief (< 6 D) MD during the CP can be reversed by as little as 4 hr of visual experience where rapid reversal has been examined (ferrets, Krahe et al., 2005; cats, Mitchell et al., 2001). We wished to know if changes in intrinsic excitability (Figure 1D) and LTP-IE (Figure 3C) can also be reversed by restoring vision, and to determine how fast such a reversal might be. To test this we restored visual experience after 2 days of MD by removing the lid sutures under light isoflurane anesthesia. We tested the recovery at two time points: 6 hrs and 24 hrs after re-opening the eyelid. Both 6 and 24 hours of re-exposure to light returned the magnitude of LTP-IE to control levels (Figures 9A,C; Control: n = 14; Recovery, 6 Hrs: n = 11; Recovery, 24 Hrs: n = 10). As little as six hours of re-exposure to light also restored F-I curves (Figure 9B), Rin, and the current threshold for evoking spikes to control values (Figure 9C). Thus, reinstating visual experience can rapidly reverse both the effects of MD on L5 pyramidal neuron excitability, and the enhanced magnitude of LTP-IE.
Figure 9. The effects of MD on L5 pyramidal neuron intrinsic excitability are rapidly reversed by eye opening.
(A) Average time course of LTP-IE, and (B) average F-I curves for control neurons or neurons deprived from P18-P20 and then re-exposed to light for 6 or 24 hrs. (C) Examples of evoked responses for the various conditions. (D) Degree of potentiation (left), current threshold for evoking spikes (center), and input resistance (right) for control, deprived, and recovered neurons.
Discussion
The cellular mechanism by which MD induces a loss of visual responsiveness within L5 is unknown. Here we show that MD reduces the intrinsic excitability of L5 pyramidal neurons, and concomitantly increases the magnitude of LTP-IE. Interestingly, the absolute level of excitability of L5 pyramidal neurons following LTP-IE induction is similar for control and deprived neurons, suggesting that visual deprivation reduces L5 pyramidal neuron intrinsic excitability by reducing or preventing visually-driven LTP-IE. These changes in excitability were accomplished through changes in the surface expression of Kv2.1 channels, and exhibited a CP that mirrored the classical CP for visual cortical plasticity. These data suggest for the first time that intrinsic plasticity plays an important role in the experience-dependent refinement of visual cortical circuits.
Several forms of synaptic and intrinsic plasticity have been identified in L5 pyramidal neurons (Cudmore and Turrigiano, 2004; Kurotani et al., 2008; Majewska et al., 2006; Markram et al., 1997; Sjostrom et al., 2001), but none have been tied to experience-dependent plasticity. Here we show that brief visual deprivation (of a duration that reduces deprived-eye responses) dramatically reduces spontaneous firing and the intrinsic excitability of L5 pyramidal neurons. This decrease in excitability reduces the effectiveness of synaptic drive, and thus should contribute to reduced responsiveness to visual stimulation within L5. It should be noted that our experiments were confined to monocular cortex, so we do not know how intrinsic plasticity affects the responses in binocular cortex where neurons receive a graded mixture of inputs from the two eyes. In binocular cortex, excitability should presumably be strongly reduced in neurons receiving significant drive from the deprived eye and unaffected in neurons receiving strong or exclusive drive from the spared eye, while the impact on neurons receiving mixed drive will depend on the threshold for LTP-IE induction.
Further supporting the notion that intrinsic plasticity in L5 monocular cortex contributes to rapid MD-induced response depression, the susceptibility to MD-induced changes in excitability (and LTP-IE enhancement) is confined to a developmental window between P20/21 and P35. This timing coincides well with opening and closing of the CP for rapid OD plasticity in binocular rodent visual cortex (Fagiolini et al., 1994; Gordon and Stryker, 1996, Tagawa et al., 2005), although the exact CP for loss of visual responsiveness in monocular cortex has not been determined. The developmental acquisition of LTP-IE in L5 pyramidal neurons also coincides well with the opening of the classical visual system CP, with the magnitude of LTP-IE reaching its peak at the beginning of the CP. However, at P35 LTP-IE could still be induced in slice recordings, even though MD no longer triggered a reduction in excitability. A similar dissociation has been observed for LTD, a mechanism proposed to underlie loss of visual responsiveness in L4 and L2/3: developmental changes in the ability to induce LTD in slices does not correlate well with the OD CP in either L4 or L2/3 (Jiang et al., 2007). Why LTP-IE can still be induced in older slices even though MD no longer affects intrinsic excitability is unclear. One possibility is that the threshold for LTP-IE induction is lower in older animals, so the change in visual drive induced by lid suture is not sufficient to prevent LTP-IE induction. Alternatively, at older ages the excitability changes induced by LTP-IE may persist longer, so that two days of MD is no longer sufficient to reduce excitability. A third possibility is that lid suture has less impact on cortical activity in mature than in young animals, so that MD no longer withdraws excitation sufficiently to prevent LTP-IE induction. The persistence of LTP-IE in older animals suggests that in addition to contributing to CP plasticity, LTP-IE may play other important roles in the normal adult physiology of L5.
Our data strongly suggest that the MD-induced reduction in L5 intrinsic excitability arises in large part because visual deprivation prevents the induction of LTP-IE. The evidence for this is several-fold. First, visual deprivation enhances the magnitude of LTP-IE, and LTP-IE induces a similar absolute level of excitability in control and deprived neurons, suggesting that deprived neurons start further from saturation but reach the same saturation point as control neurons. Second, LTP-IE induction reverses many of the effects of visual deprivation, including the change in current threshold and F-I curves. Third, LTP-IE and MD target intrinsic plasticity by inducing reciprocal changes in IK-TEA. And finally, restoring vision completely reverses the changes in both intrinsic excitability and the magnitude of LTP-IE. Taken together, these data suggest that visual drive normally maintains L5 pyramidal neurons in an excitable state by inducing LTP-IE.
LTP-IE in neocortical neurons requires postsynaptic calcium influx and PKA activation for its induction (Cudmore and Turrigiano, 2004), but how activation of this kinase cascade leads to an increase in excitability was unknown. Here we find that MD and LTP-IE-induced excitability changes were accompanied by reciprocal changes in a persistent voltage-dependent K+ current (IK-TEA) likely carried by Kv2.1 channels, without apparent changes in inward INa. MD also reduced Rin by increasing a linear leak current. Several forms of intrinsic plasticity in hippocampal pyramidal neurons are thought to involve Ih-driven changes in Rin (Brager and Johnston, 2007; Fan et al., 2005; van Welie et al., 2004), and we also observed a MD-induced enhancement of Ih in L5 pyramidal neurons. However the MD-induced increase in Ih we observed did not contribute significantly to the change in either Rin or the suprathreshold F-I curve, because these differences persisted in the presence of ZD-7288. This is consistent with dynamic clamp data showing that to significantly affect the suprahreshold F-I curve by modulating Ih requires 6 to 9 fold changes in Ih (van Welie et al., 2004), much larger than the doubling observed here. Further, although we cannot rule out additional changes in other currents, our modeling data show that the changes in IK-TEA and Rin can largely account for the effects of MD on the suprathreshold changes in the F-I curve, with IK-TEA playing the dominant role. The MD-induced change in IK-TEA was accompanied by an increase in somatic Kv2.1 channel number, and a dynamin-inhibitory peptide known to block Kv channel internalization (Kim et al., 2007) was able to block both LTP-IE induction and the resulting changes in IK-TEA. Taken together these data strongly suggest that MD and LTP-IE induce reciprocal changes in the surface expression of Kv2.1 channels.
LTP-IE is not a general feature of all cortical neurons; while it is strongly expressed within pyramidal neurons of L5, L2/3 pyramidal neurons have little or no LTP-IE, and within L5 the effects of MD on intrinsic excitability are restricted to pyramidal neurons. This raises the interesting question of what computational advantage is conferred on L5 pyramidal neurons by expressing this form of intrinsic plasticity. L5 pyramidal neurons are unique in that they constitute the major output pathways to cortical and subcortical structures such as the tectum, pontine nuclei, and contralateral cortex, and so are thought to be important modulators of numerous sensory and motor processes (Hattox and Nelson, 2007; Kawamura et al., 1974; Thomson and Bannister, 2003; Kurotani et al., 2008). Unlike Hebbian forms of synaptic plasticity that are thought to be synapse-specific, the change in excitability produced by LTP-IE will modify the responsiveness of the neuron to all synaptic inputs, and so could serve as a mechanism to regulate the overall gain of cortical output. Pyramidal neurons in L5 receive coordinated excitatory drive from L2/3, and lid suture both reduces correlated visual drive (Linden et al., 2009) and increases ex vivo spontaneous firing in layer 2/3 (Maffei et al., 2008). Functionally, loss of LTP-IE during visual deprivation and the resulting reduction in intrinsic excitability may help suppress the responsiveness of L5 pyramidal neurons to noisy input from L2/3, thus preventing the inappropriate activation of downstream sensory and motor centers. Further, enhanced LTP-IE in deprived neurons should help promote recovery of function if L5 begins to receive strong correlated input once again. More generally, by endowing L5 pyramidal neurons with the ability to transition between high and low gain states, LTP-IE may allow cortical output to be gated so that noisy pathways are suppressed and effective pathways are enhanced in a dynamic and reversible manner.
Experimental Procedures
All experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee at Brandeis University and followed the guidelines of the National Institute of Health.
Lid suture
Long-Evans rats or G42 mice were anesthetized with a cocktail of Ketamine, Xylazine, and Acepromazine and eyelids were sutured for 2–3 days as previously described (Maffei et al., 2004). Sutures were checked each day and if not intact, animals were not used. For suture removal animals were anesthetized using isoflurane, the stitches removed, and the eye flushed with sterile saline and examined; animals were excluded if any abnormalities were apparent.
Solutions
Whole cell recordings were obtained using regular ACSF or active ACSF and internal solution as described (Maffei et al., 2004; Maffei et al., 2006; Maffei and Turrigiano, 2008). Synaptic blockers were: 50 μM APV, 20 μM DNQX, 20 μM picrotoxin or bicuculline. To measure TEA-sensitive currents, the ASCF contained synaptic blockers, 0.3 μM TTX, 5 mM 4-AP, and 2 mM NiCl2. For bath application of TEA, the ASCF was modified by equimolar substitution of NaCl with TEA-Cl (30 mM). To measure Ih the ASCF contained 30 mM TEA-Cl, 1 mM BaCl2, 5 mM 4-AP, and 2 mM NiCl2. To block Ih, 20 μM ZD-7288 was added to ACSF (bath application). Anisomycin was bath applied at a concentration of 25 μM. DYN peptide (QVPSRPNRAP, 100 μM) was added to the internal solution. See supplemental data for details.
Slice Preparation and Electrophysiology
Coronal brain slices (300 μm) containing the monocular region of the primary visual cortex from control and deprived hemispheres were as described (Maffei et al., 2004; Maffei et al., 2006; Maffei and Turrigiano, 2008). Recordings were obtained using IR-DIC optics from both thick- and thin-tufted L5 pyramidal neurons, and firing types were a mixture of regular-spiking and weak burst firing; results were similar for both firing types so results were combined. FS cells from the G42 mouse line were identified under fluorescence optics and patched using IR-DIC. Morphology and location of recorded cells were verified after processing for biocytin; pyramidal cell morphologies included both slender and thick tufted architecture (Hattox and Nelson, 2007). Whole cell recordings were obtained as previously described using either an Axopatch 200B or a MultiClamp 700B (Molecular Devices, Sunnyvale, CA). Responses were filtered at 2–3 kHz and digitized at 10 kHz. Acquisition and analysis used in-house programs written in IgorPro (Wavemetrics, Lake Oswego, OR). Pyramidal and FS neurons were excluded if Vm > −60 mV, series resistances (Rs) > 25 MΩ, or if Rs varied by more than 25% of its initial value during the course of the experiment. All physiological recordings were performed at 33°–35° C.
To generate F-I curves a range of randomized depolarizing current injections (0 to 0.7 nA) were delivered with an inter-stimulus interval of 20 s; each current amplitude was repeated at least twice for each cell and responses averaged. To isolate IK-TEA, voltage ramps (−90 mV to +30 mV, slopes: 10, 30 and 50 mV/s) were presented before and after washing in TEA. Each ramp was presented at least twice (at 30 s intervals) for each condition and the responses averaged. Reported voltages were not adjusted for junction potential. Linear leak current was estimated around (±5 mV) resting membrane potential, where membrane current (Im) was zero. To isolate Ih, we measured responses to 1 s voltage steps to test potentials between −40 to −110 mV from a holding potential of −40 mV (see inset in Figure 3G). The responses were recorded before and after washing in ZD-7288, and Ih was obtained by subtracting the responses with and without ZD-7288. To measure the maximum rate of rise (dV/dtmax) of the AP, we differentiated Vm during the first AP in the spike train and extracted the peak dV/dt.
For LTP-IE experiments, only neurons with a stable baseline, stable recording parameters throughout, and with a post-induction duration of recording ≥ 40 minutes were included. LTP-IE was induced with short duration DC pulses (4±1 ms, 0.7–1.2 nA) to elicit precisely timed spikes. F-I curves were obtained at the end of the 10 minute baseline and again at the end of the post-induction recording. The degree of LTP-IE was calculated as the ratio of firing during the last 10 minutes of the post-induction recording to the baseline.
Modeling
We tested the effect of modifying outward conductances on F-I curves in a model reconstructed layer 5 pyramidal neuron adapted from Kampa and Stuart, 2006. Simulations were run on NEURON 7.1 (Hines and Carnevale, 1997). All parameters of the model remained as published (Kampa and Stuart, 2006) except for the following: 1) membrane resistivity (Rm) was changed to 50,000 Ω.cm2; 2) non inactivating potassium conductance was replaced with a published TEA-sensitive potassium current that better matched our voltage clamp data (Korngreen and Sakmann, 2000), with a conductance density of 500, 40, 80 pS/cm2 in the axon, soma and the dendrites respectively; and 3) the initial fast-inactivating (A-type) potassium conductance was also uniformly raised by 20%. These modifications resulted in model F-I curves that more closely resembled our experimental data. Leak and TEA-sensitive conductances were increased homogeneously to model the experimental changes, and the effects on the model F-I curve determined. Simulations were executed at 35°C, with an initial resting potential of −70 mV.
Confocal and electron microscopy
After MD rats were transcardially perfused with Tyrode’s solution (pH 7.4) followed by 4% paraformaldehyde (PF)/0.5% glutaraldehyde, and 50μm coronal sections from control and deprived hemispheres were cut on a vibrating microtome. sections were incubated in mouse anti-NeuN monoclonal antibody (Millipore; MAB377) at 1:500 and rabbit polyclonal anti-Kv2.1 C-terminal primary antibody (Alomone Labs, APC-012; Muennich and Fyffe, 2004; Trimmer, 1991) 1μg/mL; double- labeling with this and the Kv2.1 monoclonal antibody described below showed an identical distribution. Confocal images were acquired on a Leica SP5 with a 63x oil immersion objective (n.a. 1.4). Immuno-EM was performed as described (Nahmani and Erisir 2005) in parallel on control and deprived tissue; all processing, data collection, and analysis were done blind to condition. Sections were immersed in mouse monoclonal N-terminal anti-Kv2.1 antibody (NeuroMab, 75–159; Lim et al., 2000; Mohapatra et al., 2008) at 5μg/mL for 48 hours, then biotinylated anti-mouse IgG (Vector Labs; BA-9200) 1:100 for 2 hrs, avidin-biotin complex (Vector Labs; PK-6100) 2 hrs, 0.002% DAB/0.003% H2O2 (8 minutes), reacted with 1% OsO4 for 45 min., immersed in 4% uranyl acetate overnight, then dehydrated and resin-embedded. Ultrathin sections of L5 were cut to 70–80nm and collected on 200-mesh copper grids. EM analysis was performed at a uniform distance (25–100 μm) from the tissue to resin interface. Pyramidal somata were photographed as encountered and without regard to label intensity using a Morgagni 268 electron microscope with a 12-bit CCD camera and 24μm pixel size at 1280 x 1024. Images were obtained at 5–8K and analyzed at approximately 60–80K. Oblong oval-shaped regions of interest (ROIs), approximately 80 nm in radius from the somatic membrane, were made along the entire perimeter of identified somata. Background intensities within each image were calculated from unlabeled ROIs and an Intensity Index was calculated as: Mean Membrane ROI Intensity - Mean Background ROI Intensity/ROI Perimeter, for all ROIs.
Statistical Analysis
All data are expressed as mean± standard error of mean (SEM) for the number of neurons indicated. Unless otherwise indicated, paired two-tailed Student’s t-tests were used for within cell comparisons; for comparisons across conditions unpaired two-tailed Student’s t-tests, analysis of variance (ANOVA) followed by a posthoc Tukey test, or the non-parametric Kolmogorov-Smirnov and Mann-Whitney tests were used, as appropriate.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. doi: 10.1016/s0896-6273(03)00527-0. [DOI] [PubMed] [Google Scholar]
- Bannister AP. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci Res. 2005;53:95–103. doi: 10.1016/j.neures.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Daw NW. Layer differences in the effect of monocular vision in light- and dark-reared kittens. Vis Neurosci. 2001;18:811–820. doi: 10.1017/s0952523801185147. [DOI] [PubMed] [Google Scholar]
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore RH, Turrigiano GG. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J Neurophysiol. 2004;92:341–348. doi: 10.1152/jn.01059.2003. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- Daw N, Rao Y, Wang XF, Fischer Q, Yang Y. LTP and LTD vary with layer in rodent visual cortex. Vision Res. 2004;44:3377–3380. doi: 10.1016/j.visres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Freeman RD. Effects of brief uniocular 'patching' on kitten visual cortex. Trans Ophthalmol Soc U K. 1979;99:382–385. [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Functional organization of the visual cortex. Prog Brain Res. 1983;58:209–218. doi: 10.1016/S0079-6123(08)60022-9. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Hille B. IIon channels of excitable membranes. 3. Sinauer Associates, Incorporated; 2001. [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–9652. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Hoffman DA. Biphasic somatic A-type K channel downregulation mediates intrinsic plasticity in hippocampal CA1 pyramidal neurons. PLoS One. 2009;4:e6549. doi: 10.1371/journal.pone.0006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60:657–671. doi: 10.1016/j.neuron.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci. 2006;9:1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Stuart GJ. Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts. J Neurosci. 2006;26:7424–7432. doi: 10.1523/JNEUROSCI.3062-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Sprague JM, Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1974;158:339–362. doi: 10.1002/cne.901580308. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54:933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;525(Pt 3):621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, Medina AE, de Bittencourt-Navarrete RE, Colello RJ, Ramoa AS. Protein synthesis-independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron. 2005;48:329–343. doi: 10.1016/j.neuron.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron. 2008;57:905–916. doi: 10.1016/j.neuron.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K(+) channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol. 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden ML, Heynen AJ, Haslinger RH, Bear MF. Thalamic activity that drives visual cortical plasticity. Nat Neurosci. 2009;12:390–392. doi: 10.1038/nn.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Heynen AJ, Shuler MG, Bear MF. Cannabinoid receptor blockade reveals parallel plasticity mechanisms in different layers of mouse visual cortex. Neuron. 2008;58:340–345. doi: 10.1016/j.neuron.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Maffei A, Lambo ME, Turrigiano GG. Critical period for inhibitory plasticity in rodent binocular V1. J Neurosci. 2010;30:3304–3309. doi: 10.1523/JNEUROSCI.5340-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Martin KA. Microcircuits in visual cortex. Curr Opin Neurobiol. 2002;12:418–425. doi: 10.1016/s0959-4388(02)00343-4. [DOI] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Gingras G, Kind PC. Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open. Proc Natl Acad Sci U S A. 2001;98:11662–11667. doi: 10.1073/pnas.201392698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil M, Toledo-Rodriguez M, Monier C, Piwkowska Z, Bal T, Fregnac Y, Markram H, Destexhe A. Minimal Hodgkin-Huxley type models for different classes of cortical and thalamic neurons. Biol Cybern. 2008;99:427–441. doi: 10.1007/s00422-008-0263-8. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17(1):167–73. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- van Welie I, van Hooft JA, Wadman WJ. Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci U S A. 2004;101:5123–5128. doi: 10.1073/pnas.0307711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.