Abstract
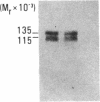
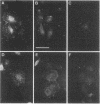
Human umbilical vein endothelial cells express a heterodimeric adhesion receptor complex consisting of noncovalently associated alpha and beta subunits that under reducing conditions have molecular masses of 135 kDa and 115 kDa, respectively. This complex can be isolated in pure form from an affinity matrix consisting of an Arg-Gly-Asp-containing heptapeptide and is specifically immunoprecipitated with monoclonal antibodies (mAbs) directed against the vitronectin receptor of human melanoma cells. These data suggest that this complex is one member of a large family of cell adhesion receptors. One of the mAbs, LM609, inhibits the attachment of human endothelial cells to fibrinogen, von Willebrand factor, and vitronectin yet has no effect on the attachment of these cells to fibronectin, collagen, or laminin. In addition, mAb LM609 inhibits attachment of endothelial cells to an immobilized synthetic peptide containing the Arg-Gly-Asp sequence. This adhesion receptor appears structurally similar to the IIb/IIIa glycoprotein complex expressed on platelets yet is antigenically distinct, since mAb LM609 fails to recognize IIb/IIIa glycoproteins. This receptor organizes in clusters on endothelial cells during their attachment to von Willebrand factor, vitronectin, or the Arg-Gly-Asp-containing heptapeptide. The data presented in this report suggest that Arg-Gly-Asp recognition may play a significant role in biological events associated with vascular proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Harper J. R. Arg-Gly-Asp recognition by a cell adhesion receptor requires its 130-kDa alpha subunit. J Biol Chem. 1987 Feb 5;262(4):1434–1437. [PubMed] [Google Scholar]
- Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986 Mar;102(3):688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Polentarutti N., Balconi G., Ryckewaert J. J., Larrieu M. J., Donati M. B., Mantovani A., Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985 Jan;75(1):11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Gabius H. J., Springer W. R., Barondes S. H. Receptor for the cell binding site of discoidin I. Cell. 1985 Sep;42(2):449–456. doi: 10.1016/0092-8674(85)90102-3. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. A 135,000 molecular weight plasma membrane glycoprotein involved in fibronectin-mediated cell adhesion. Immunofluorescence localization in normal and RSV-transformed fibroblasts. Exp Cell Res. 1986 Mar;163(1):47–62. doi: 10.1016/0014-4827(86)90557-4. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Hasegawa E., Chen W. T., Yamada K. M. Characterization of a membrane-associated glycoprotein complex implicated in cell adhesion to fibronectin. J Cell Biochem. 1985;28(4):307–318. doi: 10.1002/jcb.240280409. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Hawiger J. Localization of a site interacting with human platelet receptor on carboxy-terminal segment of human fibrinogen gamma chain. Biochem Biophys Res Commun. 1982 Jul 16;107(1):181–187. doi: 10.1016/0006-291x(82)91686-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lombardo V. T., Hodson E., Roberts J. R., Kunicki T. J., Zimmerman T. S., Ruggeri Z. M. Independent modulation of von Willebrand factor and fibrinogen binding to the platelet membrane glycoprotein IIb/IIIa complex as demonstrated by monoclonal antibody. J Clin Invest. 1985 Nov;76(5):1950–1958. doi: 10.1172/JCI112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro. 1982 Jun;18(6):538–549. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Urban-Pickering M., Marder V. J. Von Willebrand protein binds to extracellular matrices independently of collagen. Proc Natl Acad Sci U S A. 1984 Jan;81(2):471–475. doi: 10.1073/pnas.81.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]