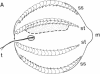
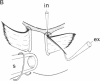
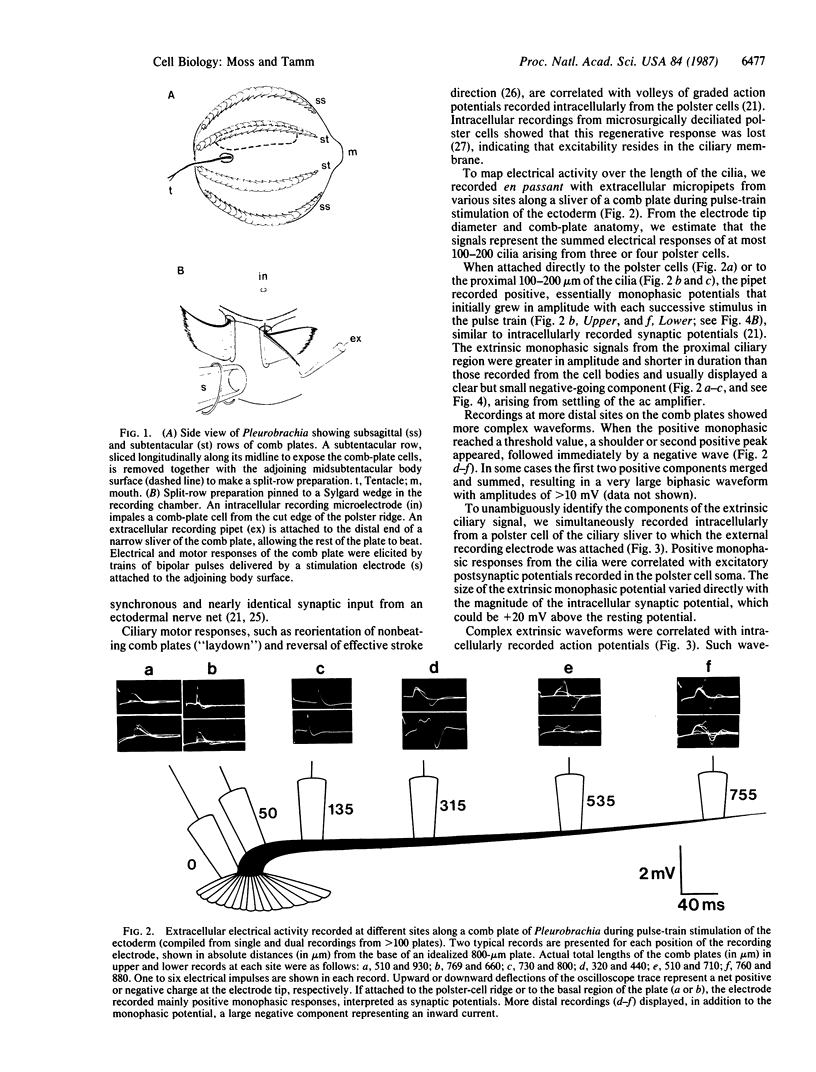
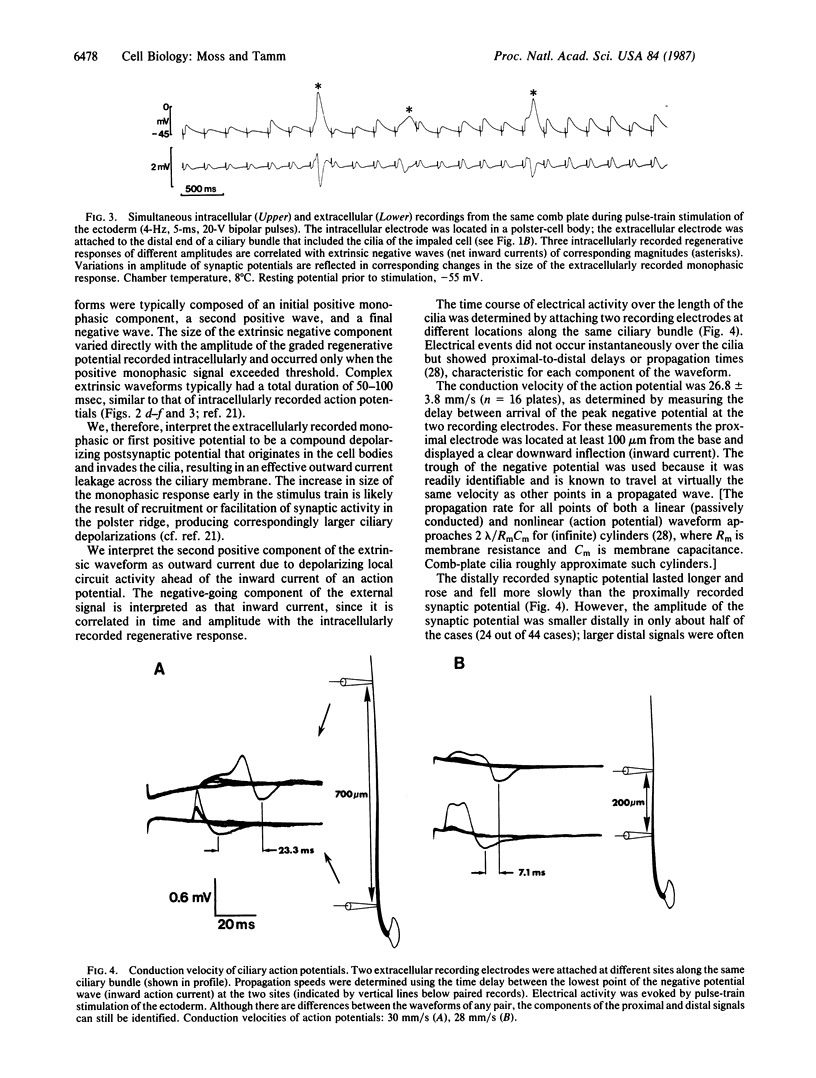
Abstract
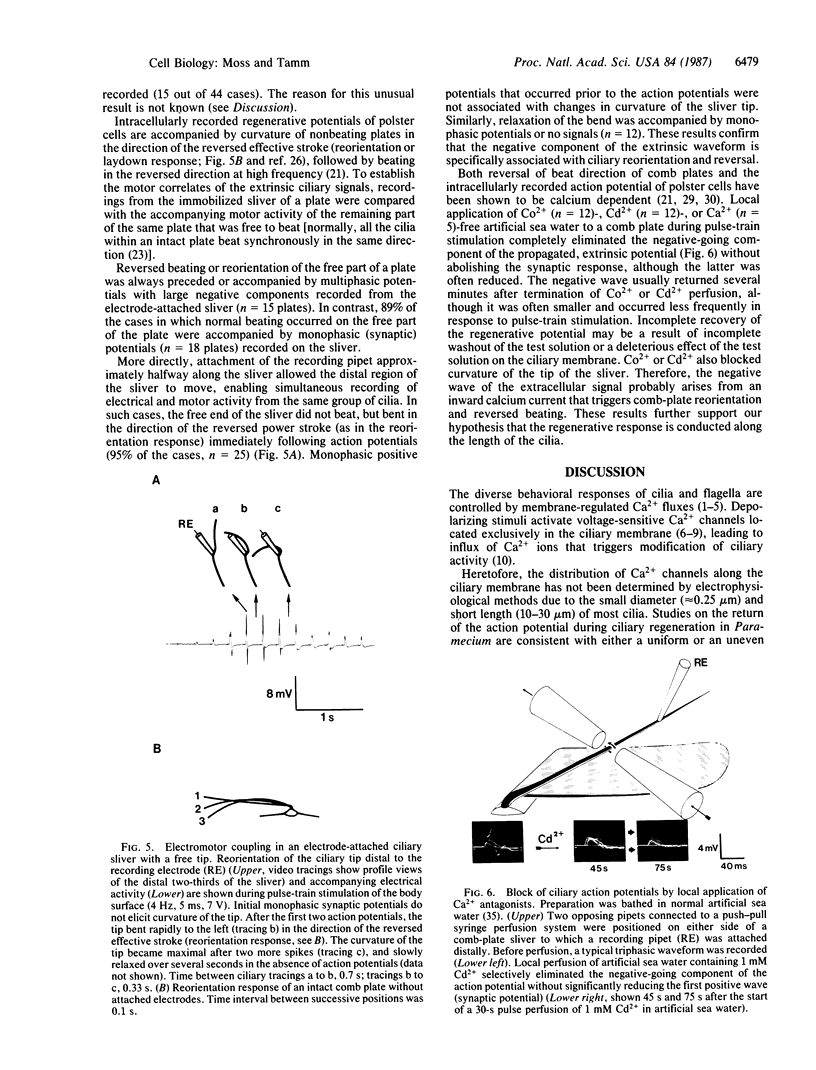
We have used the giant ciliary comb plates of ctenophores to record electrical activity directly from cilia. A compound action potential was recorded extracellularly over most of the length of the comb plate cilia in response to electrical stimulation of the ectodermal nerve net. The ciliary action potential was correlated with intracellularly recorded action potentials, selectively blocked by Ca2+-channel antagonists, and correlated with ciliary reorientation and reversed beating. Dual-electrode recording from different sites on the same comb plate showed that, unlike protistan cilia, the approximately 1-mm-long cilia of comb plates are not isopotential. Rather, action potentials are generated 150-200 microns from the base and propagate to the tip of the cilia. These results indicate that voltage-dependent channels that mediate increases in intraciliary Ca2+ concentration are distributed over most of the length of the cilia. Consequently, the Ca2+-sensitive machinery controlling ciliary motor responses is also likely to be located along the length of the axoneme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W. Evidence for two voltage-dependent calcium currents in the membrane of the ciliate Stylonychia. J Physiol. 1984 Oct;355:137–159. doi: 10.1113/jphysiol.1984.sp015411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W. Voltage dependence of two inward currents carried by calcium and barium in the ciliate Stylonychia mytilus. J Physiol. 1986 Nov;380:551–574. doi: 10.1113/jphysiol.1986.sp016302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Gitelman S. E., Witman G. B. Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol. 1980 Dec;87(3 Pt 1):764–770. doi: 10.1083/jcb.87.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Saimi Y. The physiological basis of taxes in Paramecium. Annu Rev Physiol. 1982;44:519–534. doi: 10.1146/annurev.ph.44.030182.002511. [DOI] [PubMed] [Google Scholar]
- Machemer H., Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol. 1979 Nov;296:49–60. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihle N. J., Dedman J. R., Means A. R., Chafouleas J. G., Satir B. H. Presence and indirect immunofluorescent localization of calmodulin in Paramecium tetraurelia. J Cell Biol. 1981 Jun;89(3):695–699. doi: 10.1083/jcb.89.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. G., Tamm S. L. Electrophysiological control of ciliary motor responses in the ctenophore Pleurobrachia. J Comp Physiol A. 1986 Apr;158(3):311–330. doi: 10.1007/BF00603615. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Tamm S. L. Calcium control of ciliary reversal in ionophore-treated and ATP-reactivated comb plates of ctenophores. J Cell Biol. 1985 May;100(5):1447–1454. doi: 10.1083/jcb.100.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature. 1976 Nov 11;264(5582):170–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Suzuki Y., Watanabe Y. Studies on calmodulin isolated from Tetrahymena cilia and its localization within the cilium. Exp Cell Res. 1982 Jan;137(1):217–227. doi: 10.1016/0014-4827(82)90022-2. [DOI] [PubMed] [Google Scholar]
- Otter T., Satir B. H., Satir P. Trifluoperazine-induced changes in swimming behavior of paramecium: evidence for two sites of drug action. Cell Motil. 1984;4(4):249–267. doi: 10.1002/cm.970040404. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Garofalo R. S., Gilligan D. M., Maihle N. J. Possible functions of calmodulin in protozoa. Ann N Y Acad Sci. 1980;356:83–91. doi: 10.1111/j.1749-6632.1980.tb29602.x. [DOI] [PubMed] [Google Scholar]
- Satterlie R. A., Case J. F. Gap junctions suggest epithelial conduction within the comb plates of the ctenophore Pleurobrachia bachei. Cell Tissue Res. 1978 Oct 6;193(1):87–91. doi: 10.1007/BF00221603. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel E. W., Stephens R. E., Masure H. R., Head J. F. Specific localization of scallop gill epithelial calmodulin in cilia. J Cell Biol. 1982 Mar;92(3):622–628. doi: 10.1083/jcb.92.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm S. L. Mechanical synchronization of ciliary beating within comb plates of ctenophores. J Exp Biol. 1984 Nov;113:401–408. doi: 10.1242/jeb.113.1.401. [DOI] [PubMed] [Google Scholar]
- Tamm S. L., Moss A. G. Unilateral ciliary reversal and motor responses during prey capture by the ctenophore Pleurobrachia. J Exp Biol. 1985 Jan;114:443–461. doi: 10.1242/jeb.114.1.443. [DOI] [PubMed] [Google Scholar]
- Thiele J., Klumpp S., Schultz J. E., Bardele C. F. Differential distribution of voltage-dependent calcium channels and guanylate cyclase in the excitable ciliary membrane from Paramecium tetraurelia. Eur J Cell Biol. 1982 Aug;28(1):3–11. [PubMed] [Google Scholar]
- Thiele J., Schultz J. E. Ciliary membrane vesicles of paramecium contain the voltage-sensitive calcium channel. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3688–3691. doi: 10.1073/pnas.78.6.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]