Abstract
A cross-regulation between type I IFN and TNFα has been proposed recently, where both cytokines are hypothesized to counteract each other. According to this model, different autoimmune diseases can be viewed as disequilibrium between both cytokines. As this model may have important clinical implications, the present review summarizes and discusses the currently available clinical evidence arguing for or against the proposed cross-regulation between TNFα and type I IFN. In addition, we review how this cross-regulation works at the cellular and molecular levels. Finally, we discuss the clinical relevance of this proposed cross-regulation for biological therapies such as type I IFN or anti-TNFα treatment.
Type I IFN and TNFα: cytokines with pleiotropic functions
The family of type I IFN consists of multiple subtypes of IFNα, a single IFNβ and some less characterized family members, such as IFNε, IFNκ and IFNω. The difference in biological function between the multiple subtypes of type I IFN is unclear, especially since the induced genes downstream of the different types of IFN (the IFN response program) are highly similar between, for example, IFNα and IFNβ. In peripheral blood, plasmacytoid dendritic cells (pDC) are the main producers of type I IFN. All nucleated cells, however, can produce type I IFN upon activation by, for example, viral infections that trigger cytoplasmic nucleic acid sensors such as TLR-7 and MDA-5.
Binding of type I IFNs to their cognate receptor (a heterodimer of IFNAR1 and IFNAR2) leads to the phosphorylation of signal transducers and activators of transcription (STATs) and transcription of IFN response genes. This results in resistance to viral replication, enhanced MHC class I expression and differentiation of monocytes, all of which contribute to clear infection. Besides an essential role in the host antiviral state, type I IFN has immunoregulatory functions by affecting cell proliferation and differentiation and by inducing anti-inflammatory responses. Considering these important functions of type I IFN in normal homeostasis as well as host response, an aberrant function in type I IFN immunity may contribute to autoimmunity and chronic inflammation. This is illustrated by the observation that melanoma patients treated with IFNα2β developed clinical and serological signs of autoimmunity [1] and that patients with a trisomy of chromosome 9, which contains the type I IFN genes, develop high IFN levels and lupus-like disease [2].
TNFα is a pivotal pro-inflammatory cytokine produced by macrophages, activated T cells, natural killer cells and mast cells. Also non-immune, stromal cells are able to produce significant amounts of TNFα. TNFα is produced as a 26 kDa transmembrane protein, which can be cleaved by TNFα converting enzyme to form the 17 kDa soluble form. Upon binding to TNFR1 (which is constitutively expressed on most cell types) or TNFR2 (which is expressed on immune cells, endothelial cells and fibro-blasts), TNFα activates the mitogen-activated protein kinase and NF-κB signaling pathways [3] - which in turn can lead to an amplification of the proinflammatory response by increased production of chemokines and cytokines, including TNFα itself. Endothelial cells respond to TNFα by expressing adhesion molecules to facilitate tracking of immune cells to the inflamed tissue. Macrophages and neutrophils are attracted to the site, increase their cytokine production, and enhance phagocytic capacities. Taken together, TNFα initiates and orchestrates different mechanisms that lead to an effective immune response in the case of infection.
Besides its role in host defense, however, TNFα is recognized to play a key role in many immune-mediated inflammatory diseases (IMIDs), such as rheumatoid arthritis (RA), spondyloarthritis, psoriasis, and inflammatory bowel disease [4]. Accordingly, anti-TNFα treatment is very effective in these conditions. Interestingly, a recent animal study showed that the primary cell target of TNF in chronic inflammatory joint and intestinal diseases is mesenchymal cells [5], a cell type that can produce large amounts of type I IFN.
Cross-regulation of TNFα and type I IFN: the hypothesis
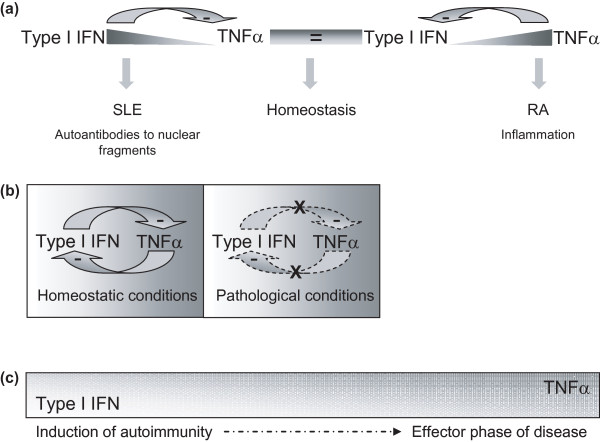
The relative contribution of TNFα and type I IFN to different types of autoimmunity and inflammatory disease is not well understood. An even more complex and intriguing picture emerged from the recently proposed hypothesis of an intimate interplay between both pleiotropic cytokines [6,7]. This hypothesis proposes that, similarly to Th1 and Th2 cytokines in T-cell biology, both cytokines can be regarded as opposite vectors in many innate immune responses. If both vectors are in balance, the sum normally yields an equilibrium point allowing protective immunity. Disturbance of this balance beyond a certain threshold may contribute to pathological conditions such as autoimmunity and inflammation (Figure 1a). A shift towards the TNFα arm may create a permissive environment for TNF-mediated autoimmunity such as RA. In contrast, when the type I IFN arm prevails, IFN-driven autoimmunity such as systemic lupus erythematosus (SLE) may occur.
Figure 1.
Cross-regulation between type I IFN and TNFα. (a) The original hypothesis proposes that both cytokines can be regarded as opposite vectors. Whereas the sum of both vectors normally yields an equilibrium point allowing protective immunity, disturbance of this balance beyond a certain threshold may contribute to a pathological state promoting autoimmunity, allergy, or inflammation. A shift towards the TNFα arm may create a permissive environment for TNF-mediated autoimmunity in rheumatoid arthritis (RA). In contrast, when the type I IFN arm prevails, IFN-driven autoimmunity as observed in systemic lupus erythematosus (SLE) may occur. (b) An alternative hypothesis: in homeostatic conditions, type I IFN and TNFα are influencing each other's levels but this balance is lost in a pathological condition. (c) An alternative hypothesis: type I IFN plays an important role in the initiation of autoimmunity, while the role of TNFα increases during the secondary inflammatory phase.
This concept was first formulated by Ivashkiv in 2003 based on the clinical observation that a fraction of RA patients treated with anti-TNFα therapy develop antinuclear antibodies and even sometimes lupus-like syndromes that reverse with the cessation of the therapy [7]. Banchereau and colleagues further established this hypothesis after the observation that five juvenile chronic arthritis patients treated with anti-TNFα therapy showed overexpression of IFNα-regulated genes in their peripheral blood mononuclear cell (PBMC) compartment compared with untreated control patients [6,8]. In addition, they showed that IFNα production by virally stimulated pDC is inhibited by TNFα through induction of maturation [8]. As this conceptual model may have important clinical implications for treatment with TNFα blockers or with type I IFN, the present review summarizes and discusses the currently available clinical evidence for the proposed cross-regulation between TNFα and type I IFN at the cellular level as well as in vivo in experimental models and in IMID patients. A summary of all studies cited is presented in Tables 1 and 2.
Table 1.
Complex relation between TNFα and type I IFN in human studies
| Cross- regulation | Cell type | Activation state | Experimental model | Results | Reference |
|---|---|---|---|---|---|
| TNF↓ ⇒ IFN ↑ | PBMCs | JIA | Anti-TNFα-treated vs. untreated patients | Patients treated with anti-TNFα showed higher IFNα-regulated genes | [8] |
| PBMCs | Healthy | In vitro culture with etanercept | Dose-dependent increase in transcription of IFNα inducible genes |
[9] | |
| Blood | RA | Infliximab-treated RA patients | Upregulation of type I IFN response genes only in patients with a poor clinical response | [52] | |
| Serum | SpA | Etanercept-treated SpA patients (all good clinical response) | Small increase in IFNα activity after 12 weeks of treatment | [53] | |
| Plasma | SS | Etanercept-treated SS patients (poor clinical response) | Increase plasma in IFNα activity after 12 weeks of treatment | [9] | |
| Plasma | Inflammatory myopathy | Infliximab-treated patients (no clinical response) | Increase in serum type I IFN activity | [55] | |
| TNF ↓ ⇒ IFN ↓ | Serum | SpA | Infliximab-treated SpA patients (all good clinical response) | Slightly decrease in IFNα activity after 2 weeks that returns to baseline after 12 weeks | [53] |
| TNF ↑⇒ IFN ↓ | pDC | Influenza virus | Incubation of virus-activated pDC with TNFα | TNFα inhibited IFNα, probably due to pDC maturation | [8] |
| TNF ↑ ⇒ IFN ↑ | Fibroblasts | Healthy | In vitro stimulation with TNFα | TNFα induced IFNβ mRNA levels | [10] |
| Macrophages | Healthy | In vitro stimulation with TNFα | TNFα induced type I IFN response program through IFN regulatory factor-1, leading to an IFNβ-mediated autocrine loop | [11] | |
| Serum | Juvenile DM | TNF-308 promotor polymorphism | Only in untreated patients: increased levels IFNα in carriers of minor allele, which is associated with increased TNFα production | [43] | |
| PBMCs | RRMS | Concanavalin A-stimulated PBMCs obtained from IFNβ-treated MS patients | More production of TNFα in concanavalin A-stimulated PBMCs after IFNβ treatment | [57] | |
| Monocytes | Healthy | Pre-incubation (30 min) with IFNβ, subsequent stimulation with LPS | IFNβ pretreatment enhanced LPS-induced TNFα production by monocytes | [17] | |
| INF ↑ ⇒ TFN ↓ | Macrophages | Healthy | In vitro pretreatment with IFNα (100 U/ml) and subsequent immune complexes, Fc receptor or TLR stimulation | IFNα suppressed FcγR-induced, TLR2-induced and TLR4-induced TNFα production through induction of Axl, a repressor of TNFα promoter | [15] |
| PBMCs | RRMS | Anti-CD3-stimulated PBMCs obtained from IFNβ-treated MS patients | IFNβ therapy decreased the production of TNFα by anti-CD3-stimulated PBMCs | [57] | |
| Synovial tissue | RA | Type I IFN treatment of RA patients | Decreased levels of TNFα in synovial tissue in some patients | [58] | |
| PBMCs | Healthy | PHA and IFNβ-treated PBMCs | IFNβ decreased PHA-induced TNFα production by PBMC | [12] | |
| Co-cultures of T lymphocytes and monocytes | Healthy | Co-cultures of T lymphocytes and monocytes stimulated by PHA in the presence of IFNβ | IFNβ inhibits the ability of stimulated T lymphocytes to induce cell contact-mediated TNFα production in monocytes | [13] | |
| THP-1 | Cell line | Pre-incubation (24 hours) with IFNβ1b, subsequent stimulation with LPS in the presence or absence of dexamethasone | LPS-induced TNFα production by THP-1 cells was suppressed by dexamethasone. This suppressive effect was augmented by pre-incubation with IFNβ | [14] | |
| Monocytes | Healthy | Pre-incubation (30 min) with IFNβ, subsequent stimulation with plasma membranes of PHA + PMA-stimulated HUT-78 cells | Pretreatment with IFNβ decreased TNFα production by contact-activated monocytes | [17] | |
| PBMCs | Healthy | IFNβ administration and ex vivo mitogen stimulation of PBMCs | IFNβ induced a transient decrease of inflamatory cytokines including TNFα | [56] | |
| INF ↓ ⇒ TFN ↓ | Blood and skin lesions | SLE | Treatment with an anti-IFNα antibody in SLE patients | Downmodulation of TNFα mRNA levels | [59] |
DM, dermatomyositis; HUT-78, human T-cell line; JIA, juvenile idiopathic arthritis; LPS, lipopolysaccharide; MS, multiple sclerosis; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cells; PHA, phytohemagglutinin; PMA, phorbol myristate acetate; RA, rheumatoid arthritis; RRMS, relapsing-remitting multiple sclerosis; SLE, systemic lupus erythematosus; SpA, spondyloarthritis; SS, Sjögren's syndrome; THP-1, human monocytic cell line.
Table 2.
Complex relation between TNF and type I IFN in murine studies
| Cross- regulation | Cell type | Activation state | Experimental model | Results | Reference |
|---|---|---|---|---|---|
| IFN ↑ ⇒ TNF ↓ | Embryonic fibroblasts (MEF) and macrophages | p38 MAPK stimulus | In vitro stimulation with IFNβ and p38 MAPK stimulus simultaneously | In the presence of a p38 MAPK stimulus, IFNβ induces - via STAT1 activation - TTP, which destabilizes mRNA of several proinflamatory genes including TNFα | [16] |
| Macrophages | IFNγ and LPS | Priming by IFNγ, stimulation by LPS in the presence of IFNβ-EF supernatant | IFNβ suppressed LPS/IFNγ induced TNFα production | [26] | |
| Synovial tissue | CIA | Daily treatment of CIA using recombinant IFNβ injection (7 days) | FNβ treatment reduced TNFα production in the synovial tissue | [28] | |
| IFN ↑ ⇒ TNF ↑ | Macrophages | Healthy | In vitro stimulation with IFNβ | IFNβ mediated upregulation of TNF mRNA | [18] |
| IFN ↓ ⇒ TNF ↑ | Macrophages | LPS and IFNγ | EAE in IFNβ KO mice | Increased TNFα production compared with wild-type controls | [23] |
| Spleen-derived macrophages | LPS and IFNγ | Cells isolated from IFNβ-deficient mice. Priming by IFNγ with subsequent stimulation with LPS | Increased TNFα production compared with control mice | [24] | |
| Synovial tissue | CIA | CIA in IFNβ-deficient mice | Increased TNFα production in synovial of arthritic IFNβ-deficient mice | [24] | |
| IFN ↓ ⇒ TNF ↓ | Liver | TNFα-induced lethal shock | IFNAR1 or IFNβ KO mice | Lack of type I IFN signaling protects against TNFα-induced inflammation | [25] |
| TNF↓ ⇒ IFN ↑ | Serum | Poly I:C | NZB/W mouse (defect in TNF) injected with poly I:C | NZB/W mice produce more poly I:C- induced IFNα | [22] |
CIA, collagen-induced arthritis; EAE, experimental autoimmune encephalomyelitis; EF, expressing fibroblasts; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MEF, muse embryonic fibroblast; poly I:C, polyinosinic-polycytidylic acid; STAT, signal transducers and activators of transcription; TTP, tristetraprolin.
Cross-regulation of TNFα and type I IFN at the cellular level
At the cellular level, the hypothesis of cross-regulation between TNFα and type I IFN is based on the observation that TNFα inhibits the generation of pDC as well as the secretion of type I IFN by immature pDC upon viral triggering [8]. Incubation of influenza-virus activated pDC with TNFα inhibited the IFNα production by 40%, which was due to maturation of the pDC by TNFα rather than a direct inhibition or cross -regulation. In vitro culture of healthy PBMCs with the soluble TNFα receptor etanercept resulted in a dose-dependent increase in the expression of IFNα and IFNα-inducible genes [9]. These studies specifically focused on IFNα and not on IFNβ, but similar genes are induced by IFNβ. In contrast with these findings in PBMCs, studies on human fibro-blasts indicated that stimulation with TNFα induced an approximately 16-fold increase in the steady-state level of IFNβ mRNA [10]. Moreover, it has been shown more recently that TNFα induces a type I IFN response program in macrophages through IFN regulatory factor-1 activation, leading to an IFNβ-mediated autocrine loop [11]. The TNFα canonical pathway and the IFNβ pathway may thereby synergize in the expression of downstream response genes. Taken together, these data suggest that the suppressive effect versus stimulating effect of TNFα on the synthesis of type I IFN is not universal but, rather, is cell-type dependent.
The opposite question related to the presumed cross-regulation is whether type I IFN suppresses TNFα production. Indeed, several studies have shown suppressive effects of type I IFN. Stimulation of peripheral blood cells with IFNβ decreases the production of TNFα [12,13]. In addition, IFNβ augmented dexamethasonemediated suppression of TNFα in a human monocytic cell line [14]. A study on human macrophages indicated that IFNα can suppress TNFα production after immune complex, Fc receptor or Toll-like receptor stimulation by induction and activation of Axl, a receptor tyrosine kinase that induces the expression of a transcriptional repressor of the TNFα promoter [15]. Moreover, IFNβ can induce the expression of tristetraprolin, an RNA-binding protein that destabilized the mRNA of proinflammatory cytokines including TNFα [16]. Accordingly, IFN-induced tristetraprolin limits lipopolysaccharide (LPS)-induced expression of several proinflammatory cytokines, including TNFα, by macrophages.
Of interest, the inhibitory effect of IFNβ on TNFα production by human monocytes was shown to be stimulus dependent. IFNβ diminishes TNFα production in T-cell contact-activated monocytes, while IFNβ enhances TNFα production in LPS-activated monocytes [17]. Another study showed that direct stimulation of murine macrophages with IFNβ does not suppress TNFα but, on the contrary, induces a fourfold upregulation of TNFα mRNA expression [18]. Whether the same holds true for IFNα awaits further investigation. Besides these direct effects, low constitutive expression of type I IFN in many cell types contributes to boost the responsiveness towards other cytokines. This phenomenon - called cross-priming - implicates that previous exposure to low doses of the pleiotropic type I IFN enhances subsequent response to proinflammatory cytokines such as TNFα [19]. Taken together, these cellular studies yield conflicting results going from cross-priming to cross-regulation of TNFα production by type I IFN. This may be partially due to differences in experimental settings mimicking homeostasis versus inflammatory conditions.
Counterbalance of TNFα and type I IFN in experimental models of inflammation and autoimmunity
The cellular studies indicated that the proposed cross-regulation of type I IFN and TNFα may depend on both cell type and inflammatory conditions, thereby emphasizing the need for additional information on this cross-regulation in vivo in the context of tissue inflammation and autoimmunity. The NZB/W mouse, a model for SLE, bears a genetic defect in the TNFα gene that leads to reduced levels of TNFα [20]. These mice develop anti-nuclear antibodies and nephritis. In accordance, treatment of the mice with TNFα resulted in attenuation of the disease.
An IFNα signature has been characterized in the splenic mononuclear cells of pre-autoimmune NZB/W mice that is not observed in BALB/c control mice [21]. Also, whereas IFNα serum levels are undetectable in both Balb/c control mice and NZB/W mice under homeostatic conditions, NZB/W mice but not Balb/c control mice produced IFNα after poly I:C stimulation [22]. On the other hand, IFNβ KO mice, which have an increased susceptibility to experimental autoimmune encephalomyelitis, display extensive microglia activation and TNFα production in the effector phase of the disease [23]. Macrophages isolated from these mice after experimental autoimmune encephalomyelitis induction produced increased amounts of TNFα after stimulation with LPS and IFNγ, compared with wild-type controls [23]. In addition, these IFNβ KO mice are also more susceptible to collagen-induced arthritis and develop an exacerbated disease compared with control mice, with a greater production of TNFα [24]. Of interest, mice lacking the receptor for type I IFN (IFNAR1) or IFNβ are protected against TNFα-induced lethal shock [25] - showing that the absence of type I IFN signaling may not only impact TNFα production, but also the outcome of the TNFα-induced inflammation.
These observations also raise the reverse question: does increased type I IFN signaling downregulate TNFα production and/or TNF-induced inflammation? IFNβ treatment has a significant therapeutic effect in collagen-induced arthritis in mice and rhesus monkeys as well as in adjuvant arthritis in rats [26-28]. In these models, IFNβ was shown to have an inhibitory effect on the production of TNFα by LPS -stimulated macrophages [26]. Indirect upregulation of type I IFN also showed beneficial effects in mouse models of arthritis [29,30]. In addition, mice treated with IFNβ had a 50% lowered expression of TNFα in the synovial tissue [28].
Taken together, these animal models demonstrate the presence and functionality of the cross-regulation between TNFα and type I IFN, but also indicate that this cross-regulation occurs mainly in a context-dependent manner during inflammatory conditions (Table 2). These observations in turn raise the question of whether and to what extent the inflammatory conditions seen in these experimental models are relevant for human IMIDs.
Cross-regulation of TNFα and type I IFN in immune-mediated inflammatory diseases
Upregulation of type I IFN-response genes has now been observed in peripheral blood cells and/or target tissue in many different IMIDs - for example, RA [31], SLE [32], systemic scleroderma [33], multiple sclerosis (MS) [34,35], psoriasis [36,37], Sjögren's syndrome (SS) [38], dermatomyositis [39] and type 1 diabetes [40]. These findings suggest that an activated type I IFN gene expression program may be a common denominator in chronic inflammatory diseases in general. If cross-regulation is present and effective, this activated type I IFN response program should lead to a repressed TNFα profile. Most of these diseases, however, also have an elevated expression of TNFα both systemically and locally in the target tissues. For example, upregulation of both TNFα and type I IFN has been shown in lesional skin in psoriasis [37] as well as in synovial tissues of RA [41] and juvenile idiopathic arthritis patients [42]. The question therefore arises of whether cross-regulation might be insufficient in specific pathological conditions.
The relative balance between IFNα and TNFα has been studied in more detail in juvenile dermatomyositis. By measuring serum IFNα activity, higher serum IFNα levels were shown to be associated with the presence of the TNFα-308 promotor polymorphism [43]. This polymorphism leads to increased production of TNFα in 50% of the carriers of the minor allele [44]. In early untreated patients, serum IFNα activity and TNFα are positively correlated. As the disease progresses, however, serum IFNα activity levels go down while TNFα levels remain stable [43]. This observation indicates that type I IFN might be more important in the earliest phases of the autoimmune phase of disease, while TNF plays a more prominent role in the secondary effector phase of the disease (Figure 1b). Collectively, the relationship between both cytokines is influenced by timing and disease progression.
The relationship between type I IFN and TNFα also appears to be complex in SLE. Patients with SLE display a strong type I IFN signature but also systemic overexpression of TNFα.Moreover, serum TNFα levels correlate with disease activity [45]. Recently, serum levels of both TNFα and IFNα were measured by ELISA in 171 SLE patients. The patients showed elevated levels of both cytokines, and the correlation between both was highly significant [46]. Another study, however, indicated that clustering of SLE patients according to serum IFNα activity and TNFα levels resulted in three groups: a group in which IFNα levels were much higher than TNFα levels, a group in which IFNα and TNFα levels were correlated, and a group in which TNFα levels were much higher than IFNα levels [47]. The latter group had a weaker association with PTPN22 SNPs than the former two groups. This study suggests that the relative balance between both cytokines may also be heterogeneous within one single disease.
This heterogeneity was also confirmed in MS, where a subgroup of patients displayed increased expression levels of type I IFN response genes in the peripheral blood [34]. The extent of this type I IFN signature before treatment was inversely associated with the biological and clinical response to IFNβ treatment [35,48]. Elevated TNFα levels have been detected in the peripheral blood and brain lesions of MS patients, and correlated with disease activity [49], but it remains unknown whether there is a relationship with the type I IFN signature.
In RA, TNFα is overexpressed in the primary target tissue of the disease - the synovial membrane [50]. In addition, the expression of IFNβ as well as the number of IFNα-expressing and IFNβ-expressing pDC is significantly elevated in RA synovial tissue compared with synovial tissues from patients with osteoarthritis or reactive arthritis [41]. A similar picture emerges from peripheral blood, as about one-half of the RA patient population shows elevated expression levels of type I IFN response genes compared with healthy controls [31]. In the other half of the patients, the type I IFN response gene expression profile is similar to that of healthy controls. Of interest, the peripheral blood IFN gene signature can already be observed in the preclinical phase of the disease [51]. The clinical significance of this elevated type I IFN expression profile in blood is still unknown, as there is no difference in patient characteristics or disease severity between patients with elevated or normal expression levels of type I IFN response genes. Thus, in RA both cytokines appear to be elevated systemically as well as in the target tissue.
Together, these studies in different IMIDs clearly indicate that there is no straightforward balance between the levels of type I IFN and TNFα, and that factors such as the specific type of IMID, the disease phase, and patient-specific factors may contribute to create a complex picture. One also has to consider that it is not completely clear how the absolute levels of these cytokines relate to their functional activity and role in disease pathogenesis.
Cross-regulation of TNFα and type I IFN during targeted treatment
Targeted therapies aimed at regulating cytokine activity provide an experimental approach to study cross-regulation between TNFα and type I IFN in patients. In fact, the concept of TNFα/type I IFN cross-regulation proposed by Banchereau and colleagues was based on the observation that juvenile chronic arthritis patients treated with infliximab (anti-TNFα monoclonal antibody) displayed increased transcription of IFNα-regulated genes compared with untreated patients. However, the inter-individual variability in the expression of IFNα, the relative small number of patients, and the cross-sectional design warranted further translational confirmation of these findings in prospective studies.
Studying 33 RA patients during treatment with infliximab, we observed no overall modulation of the expression of type I IFN response genes by TNFα blockade. Further analysis, however, revealed that infliximab induced an upregulation of the type I IFN genes in a subset of patients with a poor clinical response to treatment [52]. In contrast, the type I IFN response genes were not affected in patients with a good response to TNFα blockade. In spondyloarthritis, a disease that responds very well to TNFα blockade, infliximab treatment induced a small decrease of type I IFN serum activity after 2 weeks but the levels returned to baseline after 12 weeks of treatment. TNFα blockade with the soluble TNFα receptor etanercept led to a small increase in type I IFN serum activity after 12 weeks of treatment in a comparable patient population [53]. Similar results have been reported for patients with SS and inflammatory myopathies. In SS patients, treatment with the soluble TNFα receptor construct etanercept, which is not clinically effective in SS [54], increased serum IFNα activity [9]. In patients with inflammatory myopathies, infliximab induced an increase in type I IFN serum activity without any clinical improvement and even disease exacerbation in some patients [55]. Collectively, these longitudinal studies indicate that the effect of TNFα blockade on type I IFN is not universal and may depend on the disease, the type of TNFα blocker, as well as the clinical response to treatment.
How are TNFα levels and/or activity aected by type I IFN treatment? In healthy volunteers, administration of IFNβ induced a transient decrease of the production of TNFα as well as other inflammatory cytokines such as IL-1β, IL-6 and lymphotoxin by PBMCs upon ex vivo stimulation [56]. In MS, IFNβ treatment was also associated with decreased production of TNFα by anti-CD3-stimulated PBMCs. In contrast, concanavalin A-stimulated PBMCs produced more TNFα after IFNβ treatment [57], indicating again that the proposed cross-regulation is not universal but is dependent on factors such as stimulus and cell type. In a proof-of-concept trial in RA patients [27], type I IFN treatment had an immunomodulatory effect on synovial tissue inflammation with decreased levels of synovial TNFα expression in some but not all patients [58]. Conversely, treatment with an anti-IFNα monoclonal antibody downmodulated TNFα mRNA expression in peripheral blood and skin lesions in SLE patients [59]. Before treatment TNFα levels were increased compared with healthy controls, but the levels returned to normal 1 day after anti-IFNα treatment. These data indicate that administration of type I IFNβ may lead to suppression of TNFα production in RA, whereas blocking IFNα does not directly entail elevation of TNFα levels. Consistent with preclinical studies, this experience with targeted interventions in patients highlights the dependence of the interaction between TNFα and type I IFN on the specific type I IFN subtype, the pathogenesis of the disease, and the intrinsic characteristics of the patient.
Clinical relevance of proposed cross-regulation between type I IFN and TNFα
The cellular, experimental, and human data reviewed here indicate that cross-regulation between type I IFN and TNFα may occur in homeostatic conditions but is certainly not a universal principle in IMIDs. The presence or absence of the cross-regulation seems to depend on many factors, including the exact cell type, the type and level of activation, the specific IMID and, within a single IMID, the individual patient. This complexity questions the potential clinical implications of the conceptual framework of type I IFN-TNFα cross-regulation. Three relevant questions in this context are as follows: Can the type I IFN signature in IMID contribute to prediction of response to TNFα blockade? Can successful TNFα blockade induce type I IFN-driven adverse effects? And would IFN treatment be a viable option in TNF-driven IMIDs?
Since not all IMID patients respond well to anti-TNFα therapy, it is very relevant to identify biomarkers predicting clinical efficacy. Could the type I IFN signature be such a biomarker contributing to the prediction of response? The expression of type I IFN response genes are upregulated after TNF blockade, especially in patients who have a poor clinical response to treatment [9,52]. In patients with a good response to treatment, the expression of type I IFN response genes seems unaffected by TNFα blockade.
If the regulation of type I IFN is impeded by successful TNFα blockade, does this subsequently lead to type I IFN-driven adverse events? Type I IFN is known to play an important role in B-cell activation and plasma cell differentiation, and the levels are associated with the presence of autoantibodies in SLE [60]. Accordingly, it is conceivable that modulation of type I IFN by TNF blockade may have an impact on autoantibodies. In RA patients, however, TNF blockade had similar effects on the levels of circulating autoantibodies such as rheumatoid factor or anti-citrullinated protein antibodies in type I IFNhigh patients and type I IFNlow patients [61]. Moreover, the induction of anti-nuclear antibodies by TNF blockade, a phenomenon that is frequently observed in both RA and spondyloarthritis [62], was not related to changes in the serum type I IFN activity [53]. From this we can conclude that there is no influence of the interplay between TNFα and type I IFN with respect to autoantibody production during TNF blockade.
Another intriguing side effect of TNF blockade is the induction of psoriasis-like disease in 3 to 5% of arthritis patients without pre-existing psoriasis, which was completely unexpected considering the excellent clinical response of psoriasis to TNF blockade [63]. Th is side effect was hypothesized to be due to the proposed cross-regulation between TNFα and type I IFN. Recent studies of human psoriatic tissue demonstrate that IFNα is present early in the disease process but is not detectable in the stable plaque, although downstream IFNα signaling continues to be upregulated [64]. Indeed, skin biopsies of four patients with anti-TNFα-induced psoriasis displayed increased expression of myxovirus-resistance protein A (a protein specifically induced by type I IFN) compared with biopsies from patients with psoriasis vulgaris [65]. It would be of interest to extend this cohort and analyze in more detail the type I IFN profile to provide formal evidence for the hypothesis that TNFα blockade can induce or enhance type I IFN, and thereby psoriasis, in these patients.
A third clinically relevant question based on the potential cross-regulation is whether type I IFN treatment could be a successful treatment strategy in TNF-driven IMIDs. In animal models for arthritis, a beneficial effect of IFNβ treatment on both swelling and joint destruction has consistently been observed [26,28]. Similar results have been obtained in a collagen-induced arthritis model in rhesus monkeys [27]. A multicenter, randomized, double-blind, placebo-controlled phase II study of subcutaneous IFNβ1a in 209 patients with active RA, however, did not indicate a clinical or radiological effect [66]. This discrepancy might relate to the mode of administration and the difference in IFNβ1a dosages used in man and mice. A successful example of IFNβ treatment is observed in MS, a disease in which TNFα has been shown to play an important role [49]. Further investigation of type I IFN therapy using innovative approaches is thus warranted in RA and other TNF-driven IMIDs.
Conclusion
The present review summarizes the currently available clinical evidence for the proposed cross-regulation between TNFα and type I IFN at the cellular level as well as in vivo in experimental models and in patients with IMIDs (Tables 1 and 2). Since both cytokines have pleiotropic effects that depend on the timing, dosage and cell type, the in vitro studies yielded conflicting results and indicated that the proposed cross-regulation is not as clear cut as anticipated. Moreover, the molecular mechanism of cross-regulation between both cytokines is completely unclear and might be an indirect result through the induction of other factors. Most experimental in vivo models support the concept of cross-regulation between both cytokines but again some studies yielded opposite results, confirming the fact that the cross-regulation may be context dependent.
The studies in patients with different IMIDs show there is not necessarily a direct balance between the levels of type I IFN and TNFα, and that factors such as the type of IMID, the disease phase, and patient heterogeneity may contribute to create a complex picture. It is also possible in patients with IMIDs that both cytokines are elevated and are still influencing each other's levels from rising even further.
An additional layer of complexity is added by the subtle differences in function of the different subtypes of type I IFN and the difficulty to directly measure these individual isoforms. The usage of type I IFN-induced genes is valuable, but this signature is not always a synonym for the presence of type I IFN specifically. Moreover, how the levels of these cytokines relate to their functional activity and role in disease pathogenesis is still to be investigated.
Abbreviations
ELISA: enzyme-linked immunosorbent assay; IFN: interferon; IL: interleukin; IMID: immune-mediated inflammatory disease; LPS: lipopolysaccharide; MS: multiple sclerosis; NF: nuclear factor; PBMC: peripheral blood mononuclear cell; pDC: plasmacytoid dendritic cells; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; SNP: single nucleotide polymorphism; SS: Sjögren's syndrome; Th: T-helper type; TNF: tumor necrosis factor.
Competing interests
TC, DB and LGMvB have no competing interests. PPT is Chief Scientific Officer of Arthrogen and holds shares in Arthrogen b.v. PPT is owner of the following patent: Method for prognosticating the clinical response of a patient to B-lymphocyte inhibiting or depelting therapy.
Contributor Information
Tineke Cantaert, Email: T.Cantaert@amc.uva.nl.
Dominique Baeten, Email: D.L.Baeten@amc.uva.nl.
Paul P Tak, Email: P.P.Tak@amc.uva.nl.
Lisa GM van Baarsen, Email: e.g.vanbaarsen@amc.uva.nl.
References
- Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, Pectasides D, Fountzilas G, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Kosboth M, Lee P, Rice A, Driscoll DJ, Zori R, Narain S, Lyons R, Satoh M, Sobel E, Reeves WH. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54:1573–1579. doi: 10.1002/art.21800. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331–337. doi: 10.1084/jem.20070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. Type I interferon modulation of cellular responses to cytokines and infectious pathogens: potential role in SLE pathogenesis. Autoimmunity. 2003;36:473–479. doi: 10.1080/08916930310001605882. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-α in autoimmune diseases. Proc Natl Acad Sci USA. 2005;102:3372–3377. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren's syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LF, Ho LT, Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989;264:16351–16354. [PubMed] [Google Scholar]
- Yarilina A, Park-Min KH, Antoniv T, Hu X, Ivashkiv LB. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- Coclet-Ninin J, Dayer JM, Burger D. Interferon-beta not only inhibits interleukin-1β and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur Cytokine Netw. 1997;8:345–349. [PubMed] [Google Scholar]
- Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-β inhibits the ability of T lymphocytes to induce TNF-α and IL-1β production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272–282. doi: 10.1006/cyto.2001.0884. [DOI] [PubMed] [Google Scholar]
- Uitdehaag BM, Hoekstra K, Koper JW, Polman CH, Dijkstra CD. IFN-β1b augments glucocorticoid-induced suppression of tumor necrosis factor-alpha production by increasing the number of glucocorticoid receptors on a human monocytic cell line. J Interferon Cytokine Res. 2001;21:133–135. doi: 10.1089/107999001750133113. [DOI] [PubMed] [Google Scholar]
- Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer I, Schaljo B, Vogl C, Gattermeier I, Kolbe T, Muller M, Blackshear PJ, Kovarik P. Interferons limit inflammatory responses by induction of tristetraprolin. Blood. 2006;107:4790–4797. doi: 10.1182/blood-2005-07-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite effects of IFN beta on cytokine homeostasis in LPS- and T cell contact-activated human monocytes. J Neuroimmunol. 2004;146:76–83. doi: 10.1016/j.jneuroim.2003.10.035. [DOI] [PubMed] [Google Scholar]
- O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–386. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- Jacob CO, McDevitt HO. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988;331:356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Lu Q, Shen N, Li XM, Chen SL. Genomic view of IFN-α response in pre-autoimmune NZB/W and MRL/lpr mice. Genes Immun. 2007;8:590–603. doi: 10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofi lopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, Holmdahl R, Issazadeh-Navikas S. IFN-β gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- Treschow AP, Teige I, Nandakumar KS, Holmdahl R, Issazadeh-Navikas S. Stromal cells and osteoclasts are responsible for exacerbated collagen-induced arthritis in interferon-beta-deficient mice. Arthritis Rheum. 2005;52:3739–3748. doi: 10.1002/art.21496. [DOI] [PubMed] [Google Scholar]
- Huys L, Van HF, Dejager L, Dejonckheere E, Lienenklaus S, Weiss S, Leclercq G, Libert C. Type I interferon drives tumor necrosis factor-induced lethal shock. J Exp Med. 2009;206:1873–1882. doi: 10.1084/jem.20090213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphyllopoulos KA, Williams RO, Tailor H, Chernajovsky Y. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum. 1999;42:90–99. doi: 10.1002/1529-0131(199901)42:1<90::AID-ANR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tak PP, Hart BA, Kraan MC, Jonker M, Smeets TJ, Breedveld FC. The effects of interferon beta treatment on arthritis. Rheumatology (Oxford) 1999;38:362–369. doi: 10.1093/rheumatology/38.4.362. [DOI] [PubMed] [Google Scholar]
- van Holten J, Reedquist K, Sattonet-Roche P, Smeets TJ, Plater-Zyberk C, Vervoordeldonk MJ, Tak PP. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2004;6:R239–R249. doi: 10.1186/ar1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr M, Boyle DL, Ronacher L, Flores N, Firestein GS. Synergistic benefit in inflammatory arthritis by targeting IκB kinase epsilon and interferon beta. Ann Rheum Dis. 2009;68:257–263. doi: 10.1136/ard.2008.095356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. 2007;178:2204–2211. doi: 10.4049/jimmunol.178.4.2204. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Voskuyl AE, Rustenburg F, Baggen JM, Ibrahim SM, Fero M, Dijkmans BA, Tak PP, Verweij CL. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann Rheum Dis. 2007;66:1008–1014. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan FK, Zhou X, Mayes MD, Gourh P, Guo X, Marcum C, Jin L, Arnett FC Jr. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 2006;45:694–702. doi: 10.1093/rheumatology/kei244. [DOI] [PubMed] [Google Scholar]
- van Baarsen LG, van der Pouw Kraan TC, Kragt JJ, Baggen JM, Rustenburg F, Hooper T, Meilof JF, Fero MJ, Dijkstra CD, Polman CH, Verweij CL. A subtype of multiple sclerosis defined by an activated immune defense program. Genes Immun. 2006;7:522–531. doi: 10.1038/sj.gene.6364324. [DOI] [PubMed] [Google Scholar]
- Comabella M, Lunemann JD, Rio J, Sanchez A, Lopez C, Julia E, Fernandez M, Nonell L, Camina-Tato M, Deisenhammer F, Caballero E, Tortola MT, Prinz M, Montalban X, Martin R. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–3365. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- van der FL, van der Wel LI, Laman JD, Prens EP, Verschuren MC. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J Invest Dermatol. 2004;122:51–60. doi: 10.1046/j.0022-202X.2003.22113.x. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, de los RM, Higgs BW, Boutrin A, White B, Coyle A, Krueger J, Kiener PA, Jallal B. Type I interferon: potential therapeutic target for psoriasis? PLoS One. 2008;3:e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, Alm GV, Ronnblom L. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, Barohn RJ, Saperstein DS, Briemberg HR, Ericsson M, Park P, Amato AA. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57:664–678. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis. 2005;64:1780–1782. doi: 10.1136/ard.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattorno M, Chicha L, Gregorio A, Ferlito F, Rossi F, Jarrossay D, Lanzavecchia A, Martini A, Manz MG. Distinct expression pattern of IFN-α and TNF-α in juvenile idiopathic arthritis synovial tissue. Rheumatology (Oxford) 2007;46:657–665. doi: 10.1093/rheumatology/kel346. [DOI] [PubMed] [Google Scholar]
- Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60:1815–1824. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, Chen EH. TNFα-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43:2368–2377. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Studnicka-Benke A, Steiner G, Petera P, Smolen JS. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol. 1996;35:1067–1074. doi: 10.1093/rheumatology/35.11.1067. [DOI] [PubMed] [Google Scholar]
- Lopez P, Gomez J, Prado C, Gutierrez C, Suarez A. Influence of functional interleukin 10/tumor necrosis factor-alpha polymorphisms on interferon-alpha, IL-10, and regulatory T cell population in patients with systemic lupus erythematosus receiving antimalarial treatment. J Rheumatol. 2008;35:1559–1566. [PubMed] [Google Scholar]
- Kariuki SN, Crow MK, Niewold TB. The PTPN22 C1858T polymorphism is associated with skewing of cytokine profiles toward high interferon-alpha activity and low tumor necrosis factor alpha levels in patients with lupus. Arthritis Rheum. 2008;58:2818–2823. doi: 10.1002/art.23728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarsen LG, Vosslamber S, Tijssen M, Baggen JM, van d, Killestein J, van der Pouw Kraan TC, Polman CH, Verweij CL. Pharmacogenomics of interferon-beta therapy in multiple sclerosis: baseline IFN signature determines pharmacological differences between patients. PLoS One. 2008;3:e1927. doi: 10.1371/journal.pone.0001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Kitze B, Weber T, Tumani H, Broocks A, Luer W, Helwig A, Poser S. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann Neurol. 1995;37:82–88. doi: 10.1002/ana.410370115. [DOI] [PubMed] [Google Scholar]
- Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- van Baarsen LG, Bos WH, Rustenburg F, van der Pouw Kraan TC, Wolbink GJ, Dijkmans BA, van SD, Verweij CL. Gene expression profiling in autoantibody-positive patients with arthralgia predicts development of arthritis. Arthritis Rheum. 2010;62:694–704. doi: 10.1002/art.27294. [DOI] [PubMed] [Google Scholar]
- van Baarsen LG, Wijbrandts CA, Rustenburg F, Cantaert T, van der Pouw Kraan TC, Baeten DL, Dijkmans BA, Tak PP, Verweij CL. Regulation of IFN response gene activity during infliximab treatment in rheumatoid arthritis is associated with clinical response to treatment. Arthritis Res Ther. 2010;12:R11. doi: 10.1186/ar2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantaert T, De RL, Mavragani CP, Wijbrandts CA, Niewold TB, Niers T, Vandooren B, Veys EM, Richel D, Tak PP, Crow MK, Baeten D. Exposure to nuclear antigens contributes to the induction of humoral autoimmunity during tumour necrosis factor alpha blockade. Ann Rheum Dis. 2009;68:1022–1029. doi: 10.1136/ard.2008.093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E, Combe B, Puechal X, Pennec Y, Sauvezie B, Perdriger A, Hayem G, Janin A, Sibilia J. Inefficacy of infliximab in primary Sjogren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren's Syndrome (TRIPSS) Arthritis Rheum. 2004;50:1270–1276. doi: 10.1002/art.20146. [DOI] [PubMed] [Google Scholar]
- Dastmalchi M, Grundtman C, Alexanderson H, Mavragani CP, Einarsdottir H, Helmers SB, Elvin K, Crow MK, Nennesmo I, Lundberg IE. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008;67:1670–1677. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- Rothuizen LE, Buclin T, Spertini F, Trinchard I, Munafo A, Buchwalder PA, Ythier A, Biollaz J. Influence of interferon beta-1a dose frequency on PBMC cytokine secretion and biological effect markers. J Neuroimmunol. 1999;99:131–141. doi: 10.1016/s0165-5728(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Brod SA, Marshall GD Jr, Henninger EM, Sriram S, Khan M, Wolinsky JS. Interferon-beta 1b treatment decreases tumor necrosis factor-alpha and increases interleukin-6 production in multiple sclerosis. Neurology. 1996;46:1633–1638. doi: 10.1212/wnl.46.6.1633. [DOI] [PubMed] [Google Scholar]
- Smeets TJ, Dayer JM, Kraan MC, Versendaal J, Chicheportiche R, Breedveld FC, Tak PP. The effects of interferon-beta treatment of synovial inflammation and expression of metalloproteinases in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:270–274. doi: 10.1002/1529-0131(200002)43:2<270::AID-ANR5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Higgs BW, Morehouse CA, de los RM, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- Cantaert T, van Baarsen LG, Wijbrandts CA, Thurlings RM, van de Sande MG, Bos C, van der Pouw TK, Verweij CL, Tak PP, Baeten DL. Type I interferons have no major influence on humoral autoimmunity in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:156–166. doi: 10.1093/rheumatology/kep345. [DOI] [PubMed] [Google Scholar]
- De RL, Kruithof E, Van DN, Hoffman IE, Van den BN, Van den BF, Veys EM, De KF. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondylarthropathy. Arthritis Rheum. 2003;48:1015–1023. doi: 10.1002/art.10876. [DOI] [PubMed] [Google Scholar]
- Cuchacovich R, Espinoza CG, Virk Z, Espinoza LR. Biologic therapy (TNF-α antagonists)-induced psoriasis: a cytokine imbalance between TNF-α and IFN-α? J Clin Rheumatol. 2008;14:353–356. doi: 10.1097/RHU.0b013e318190dd88. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, Shojania K, Martinka M, Dutz JP. Psoriasis and pustular dermatitis triggered by TNF-α inhibitors in patients with rheumatologic conditions. Arch Dermatol. 2007;143:223–231. doi: 10.1001/archderm.143.2.223. [DOI] [PubMed] [Google Scholar]
- van Holten J, Pavelka K, Vencovsky J, Stahl H, Rozman B, Genovese M, Kivitz AJ, Alvaro J, Nuki G, Furst DE, Herrero-Beaumont G, McInnes IB, Musikic P, Tak PP. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:64–69. doi: 10.1136/ard.2003.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]