Abstract
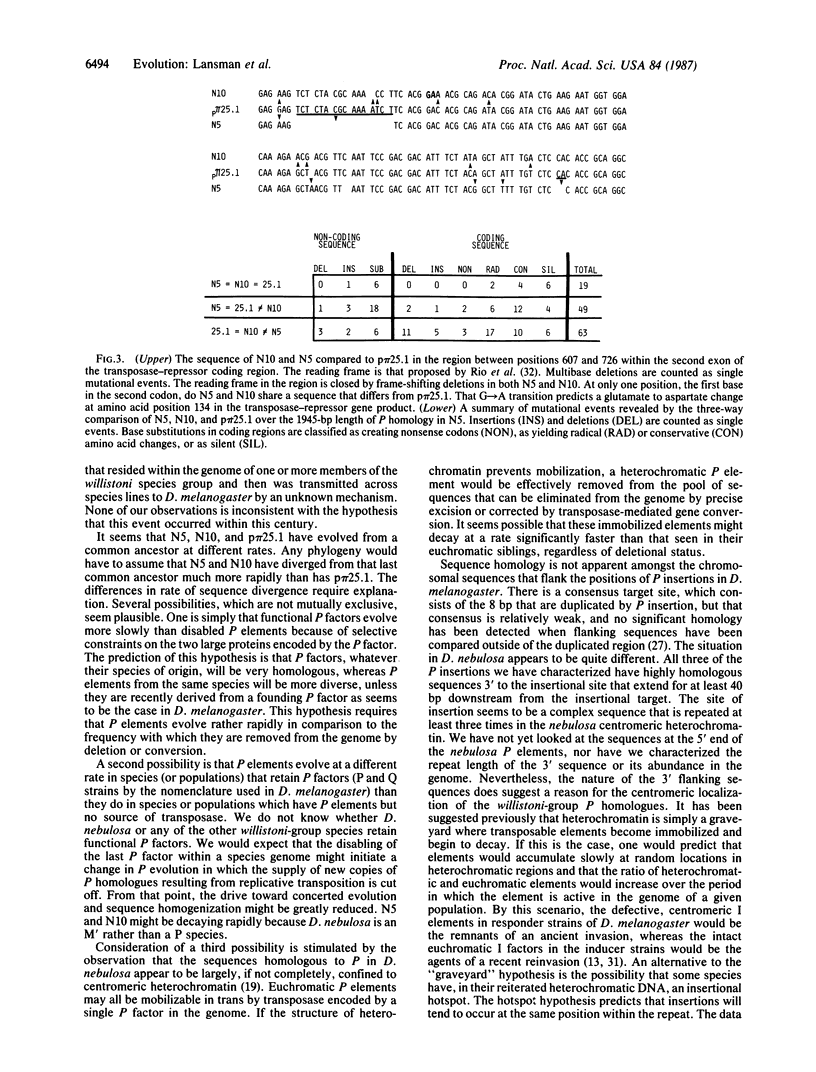
P elements have been cloned and sequenced from Drosophila nebulosa. Their sequences have diverged less than 6% from P elements of Drosophila melanogaster. However D. nebulosa P elements have nucleotide changes that close all four open reading frames found in the D. melanogaster P element. Microinjection experiments show that D. nebulosa P elements cannot provide transposase function for D. melanogaster P elements, nor are D. nebulosa P elements mobilized by the transposase provided by a D. melanogaster P factor. Three D. nebulosa P elements appear to have integrated into the same position of a complex, centromeric repeated sequence. Comparison of nucleotide sequences suggests that D. nebulosa P elements have diverged upon different pathways from a common ancestor that was 99% homologous to the P elements of D. melanogaster.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anxolabéhère D., Nouaud D., Périquet G., Tchen P. P-element distribution in Eurasian populations of Drosophila melanogaster: A genetic and molecular analysis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5418–5422. doi: 10.1073/pnas.82.16.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham P. M., Kidwell M. G., Rubin G. M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell. 1982 Jul;29(3):995–1004. doi: 10.1016/0092-8674(82)90463-9. [DOI] [PubMed] [Google Scholar]
- Brookfield J. F., Montgomery E., Langley C. H. Apparent absence of transposable elements related to the P elements of D. melanogaster in other species of Drosophila. 1984 Jul 26-Aug 1Nature. 310(5975):330–332. doi: 10.1038/310330a0. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Daniels S. B., Strausbaugh L. D., Ehrman L., Armstrong R. Sequences homologous to P elements occur in Drosophila paulistorum. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6794–6797. doi: 10.1073/pnas.81.21.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S. B., Strausbaugh L. D. The distribution of P-element sequences in Drosophila: the willistoni and saltans species groups. J Mol Evol. 1986;23(2):138–148. doi: 10.1007/BF02099908. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Germline hypermutability in Drosophila and its relation to hybrid dysgenesis and cytotype. Genetics. 1981 Jul;98(3):565–587. doi: 10.1093/genetics/98.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Identifying P factors in Drosophila by means of chromosome breakage hotspots. Cell. 1981 Nov;26(3 Pt 1):421–428. doi: 10.1016/0092-8674(81)90211-7. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Ginzburg L. R., Bingham P. M., Yoo S. On the theory of speciation induced by transposable elements. Genetics. 1984 Jun;107(2):331–341. doi: 10.1093/genetics/107.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kidwell M. G. Evolution of hybrid dysgenesis determinants in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1655–1659. doi: 10.1073/pnas.80.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Novy J. B. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: Sterility Resulting from Gonadal Dysgenesis in the P-M System. Genetics. 1979 Aug;92(4):1127–1140. doi: 10.1093/genetics/92.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T., Baba M., Akiyama M., Uowaki N., Kusakabe S., Tajima F. Rapid change in mutation rate in a local population of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7671–7675. doi: 10.1073/pnas.82.22.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Laski F. A., Rubin G. M. Identification and immunochemical analysis of biologically active Drosophila P element transposase. Cell. 1986 Jan 17;44(1):21–32. doi: 10.1016/0092-8674(86)90481-2. [DOI] [PubMed] [Google Scholar]
- Rose M. R., Doolittle W. F. Molecular biological mechanisms of speciation. Science. 1983 Apr 8;220(4593):157–162. doi: 10.1126/science.220.4593.157. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Sakoyama Y., Todo T., Ishiwa-Chigusa S., Honjo T., Kondo S. Structures of defective P transposable elements prevalent in natural Q and Q-derived M strains of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6236–6239. doi: 10.1073/pnas.82.18.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Stacey S. N., Lansman R. A., Brock H. W., Grigliatti T. A. Distribution and conservation of mobile elements in the genus Drosophila. Mol Biol Evol. 1986 Nov;3(6):522–534. doi: 10.1093/oxfordjournals.molbev.a040413. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



