Abstract
We describe a system to analyze the individual contribution of a single physical DNA end on intramolecular recombination between partially homologous sequences. We took advantage of this partial sequence divergence to measure the distance separating the DNA end from the final recombination event. We show that a single physical DNA end stimulates recombination when located in a region of homology. Recombination frequency decreases gradually with the distance from the DNA end. A recombinational hot spot is found at the end of the region of homology. A large insertion of unrelated DNA interferes asymmetrically with this process, suggesting that a recombinogenic signal propagates along the region of homology.
Full text
PDF
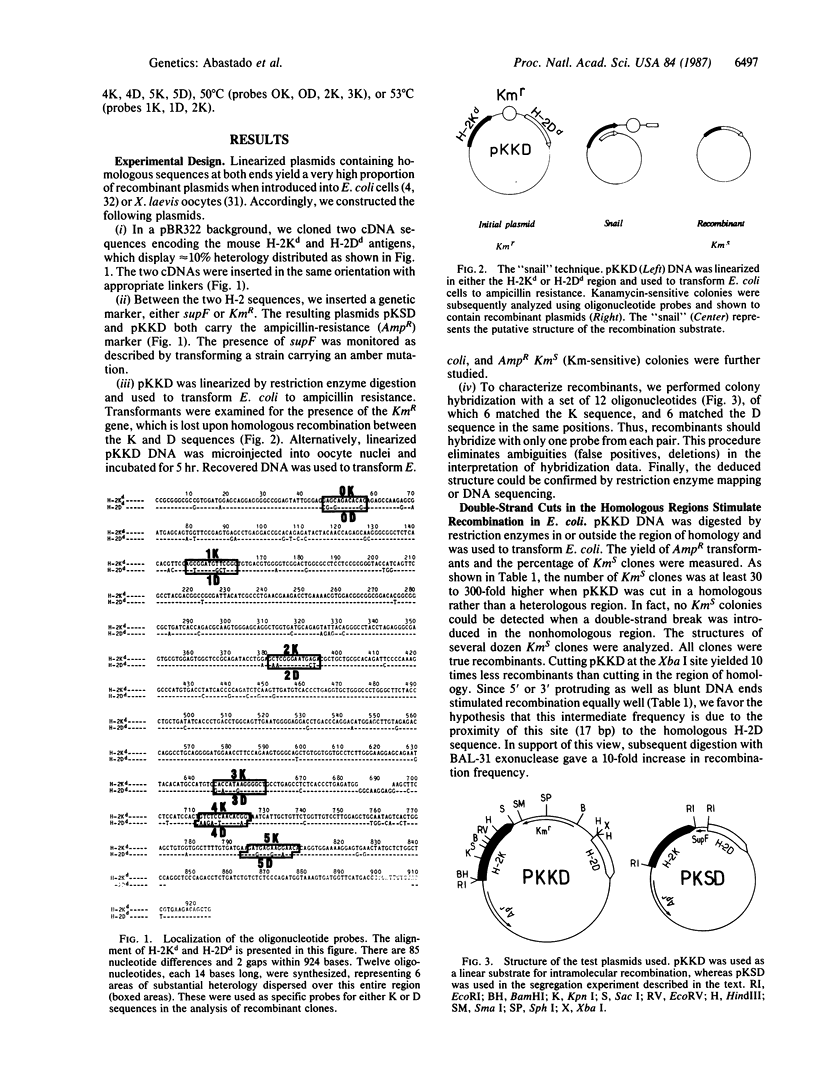
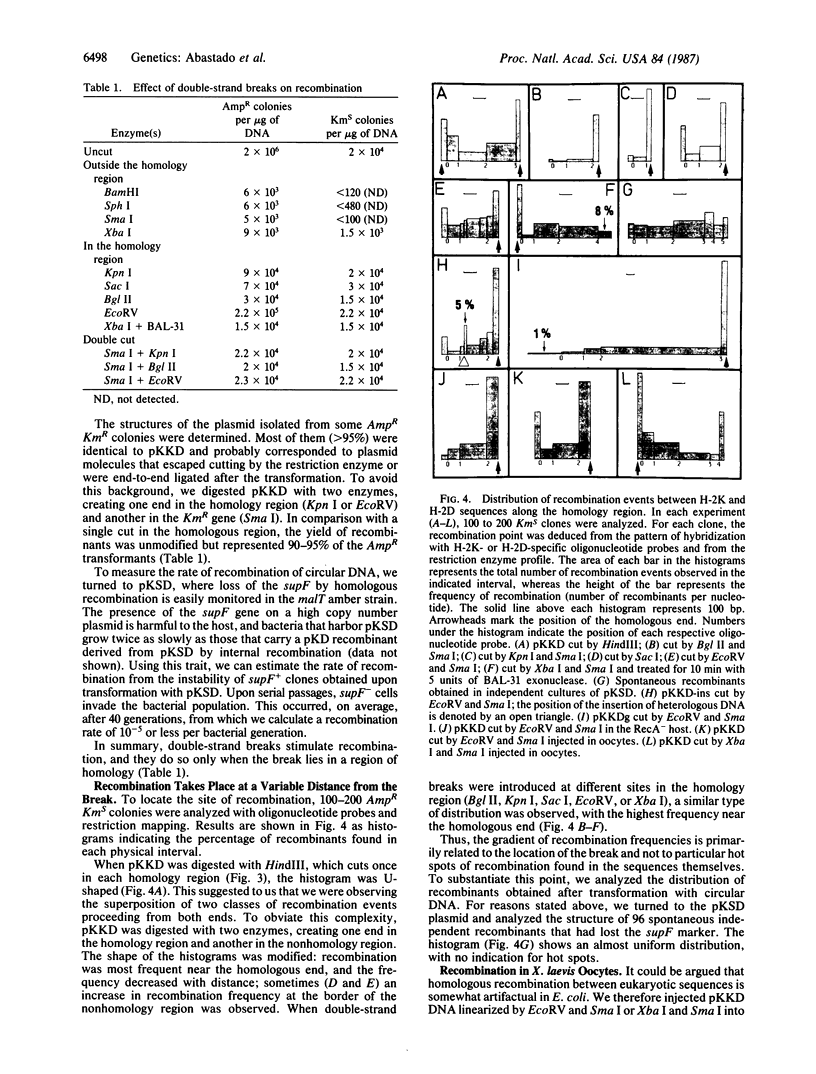


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Cami B., Dinh T. H., Igolen J., Kourilsky P. Processing of complex heteroduplexes in Escherichia coli and Cos-1 monkey cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5792–5796. doi: 10.1073/pnas.81.18.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M. E., Radding C. M. Insertions, deletions and mismatches in heteroduplex DNA made by recA protein. Cell. 1983 Dec;35(2 Pt 1):511–520. doi: 10.1016/0092-8674(83)90185-x. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Smigocki A. C., Camerini-Otero R. D. Double-strand gap repair results in homologous recombination in mouse L cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1762–1766. doi: 10.1073/pnas.83.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto E., West S. C., Mursalim J., Conlon S., Howard-Flanders P. Initiation of genetic recombination: homologous pairing between duplex DNA molecules promoted by recA protein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3962–3966. doi: 10.1073/pnas.77.7.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Sandler S. J., Willis D. K., Chu C. C., Blanar M. A., Lovett S. T. Genes of the RecE and RecF pathways of conjugational recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1984;49:453–462. doi: 10.1101/sqb.1984.049.01.051. [DOI] [PubMed] [Google Scholar]
- Cohen A., Laban A. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol Gen Genet. 1983;189(3):471–474. doi: 10.1007/BF00325911. [DOI] [PubMed] [Google Scholar]
- Conley E. C., Saunders J. R. Recombination-dependent recircularization of linearized pBR322 plasmid DNA following transformation of Escherichia coli. Mol Gen Genet. 1984;194(1-2):211–218. doi: 10.1007/BF00383519. [DOI] [PubMed] [Google Scholar]
- DasGupta C., Radding C. M. Polar branch migration promoted by recA protein: effect of mismatched base pairs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):762–766. doi: 10.1073/pnas.79.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbouille M., Schwartz M. The use of gene fusions to study the expression of malT the positive regulator gene of the maltose regulon. J Mol Biol. 1979 Aug 15;132(3):521–534. doi: 10.1016/0022-2836(79)90273-0. [DOI] [PubMed] [Google Scholar]
- Fox M. S. Some features of genetic recombination in procaryotes. Annu Rev Genet. 1978;12:47–68. doi: 10.1146/annurev.ge.12.120178.000403. [DOI] [PubMed] [Google Scholar]
- Klein H. L. Lack of association between intrachromosomal gene conversion and reciprocal exchange. 1984 Aug 30-Sep 5Nature. 310(5980):748–753. doi: 10.1038/310748a0. [DOI] [PubMed] [Google Scholar]
- Kourilsky P. Genetic exchanges between partially homologous nucleotide sequences: possible implications for multigene families. Biochimie. 1983 Feb;65(2):85–93. doi: 10.1016/s0300-9084(83)80178-3. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Delarbre C., Gachelin G., Kourilsky P. A cDNA clone containing the entire coding sequence of a mouse H-2Kd histocompatibility antigen. Nucleic Acids Res. 1983 Mar 11;11(5):1567–1577. doi: 10.1093/nar/11.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M., Fox M. S. Evidence for inclusion of regions of nonhomology in heteroduplex products of bacteriophage lambda recombination. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7180–7184. doi: 10.1073/pnas.81.22.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matfield M., Badawi R., Brammar W. J. Rec-dependent and Rec-independent recombination of plasmid-borne duplications in Escherichia coli K12. Mol Gen Genet. 1985;199(3):518–523. doi: 10.1007/BF00330768. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Méjean V., Claverys J. P. Use of a cloned DNA fragment to analyze the fate of donor DNA in transformation of Streptococcus pneumoniae. J Bacteriol. 1984 Jun;158(3):1175–1178. doi: 10.1128/jb.158.3.1175-1178.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J. L., Nicolas A., Hamza H., Langin T. Origins of gene conversion and reciprocal exchange in Ascobolus. Cold Spring Harb Symp Quant Biol. 1984;49:13–21. doi: 10.1101/sqb.1984.049.01.004. [DOI] [PubMed] [Google Scholar]
- Schulze D. H., Pease L. R., Geier S. S., Reyes A. A., Sarmiento L. A., Wallace R. B., Nathenson S. G. Comparison of the cloned H-2Kbm1 variant gene with the H-2Kb gene shows a cluster of seven nucleotide differences. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2007–2011. doi: 10.1073/pnas.80.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher B. T., Nairn R., Coligan J. E., Hood L. E. DNA sequence of the mouse H-2Dd transplantation antigen gene. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1175–1179. doi: 10.1073/pnas.82.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W. Roles of double-strand breaks in generalized genetic recombination. Prog Nucleic Acid Res Mol Biol. 1986;33:169–194. doi: 10.1016/s0079-6603(08)60023-9. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Sugawara N., Szostak J. W. Recombination between sequences in nonhomologous positions. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5675–5679. doi: 10.1073/pnas.80.18.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Morrison P., Kolodner R. Intramolecular recombination of linear DNA catalyzed by the Escherichia coli RecE recombination system. J Mol Biol. 1985 Dec 5;186(3):515–525. doi: 10.1016/0022-2836(85)90126-3. [DOI] [PubMed] [Google Scholar]
- Symington L. S., Morrison P., Kolodner R. Plasmid recombination intermediates generated in a Saccharomyces cerevisiae cell-free recombination system. Mol Cell Biol. 1985 Sep;5(9):2361–2368. doi: 10.1128/mcb.5.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Kiefer H., Schulze R., Fischer-Lindahl K., Steinmetz M. Molecular characterization of a meiotic recombinational hotspot enhancing homologous equal crossing-over. EMBO J. 1986 Sep;5(9):2123–2129. doi: 10.1002/j.1460-2075.1986.tb04475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Weissmann C. Formation of genes coding for hybrid proteins by recombination between related, cloned genes in E. coli. Nucleic Acids Res. 1983 Aug 25;11(16):5661–5669. doi: 10.1093/nar/11.16.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




