Abstract
Listeria monocytogenes escapes from the phagosome of macrophages and replicates within the cytosolic compartment. The macrophage responds to L. monocytogenes through detection pathways located on the cell surface (TLRs) and within the cytosol (Nod-like receptors) to promote inflammatory processes aimed at clearing the pathogen. Cytosolic L. monocytogenes activates caspase 1, resulting in post-translational processing of the cytokines IL-1β and IL-18 as well as caspase 1-dependent cell death (pyroptosis). We demonstrate that the presence of L. monocytogenes within the cytosolic compartment induces caspase 1 activation through multiple Nod-like receptors, including Ipaf and Nalp3. Flagellin expression by cytosolic L. monocytogenes was detected through Ipaf in a dose-dependent manner. Concordantly, detection of flagellin promoted bacterial clearance in a murine infection model. Finally, we provide evidence that suggests cytosolic L. monocytogenes activates caspase 1 through a third pathway, which signals through the adaptor protein ASC. Thus, L. monocytogenes activates caspase 1 in macrophages via multiple pathways, all of which detect the presence of bacteria within the cytosol.
The innate immune system detects pathogens through pattern recognition receptors (PRRs),5 which initiate proinflammatory responses. Pathogens present in extracellular, vacuolar, or cytosolic spaces shed pathogen-associated molecular patterns (PAMPs) that activate PRRs located in each compartment. Combined signals from these PRRs dictate a pattern of cytokine/chemokine release that activates general inflammatory responses and shapes the adaptive response to the particular pathogen. TLRs are transmembrane signaling proteins that detect PAMPs in the extracellular or vacuolar spaces and induce transcriptional responses (1). Nod-like receptors (NLRs) and RIG-I-like receptors respond to PAMPs found within the mammalian cell cytosol, and promote either transcriptional (e.g., Nod1, Nod2, and RIG-I) or post-translational responses (e.g., Nalp3 and Ipaf) (2, 3).
Listeria monocytogenes is a flagellated Gram-positive bacterium that can be detected by multiple receptors in different cellular compartments. Infection with L. monocytogenes can cause meningitis and septicemia in newborn, elderly, or immunocompromised individuals. Pregnant women are particularly susceptible to L. monocytogenes, leading to potentially fatal fetal infection. Infection with L. monocytogenes occurs through ingestion of contaminated food. In the gut, L. monocytogenes invades epithelial cells via interaction of bacterial virulence protein InternalinA with E-cad-herin, resulting in bacterial internalization within a membrane bound vacuole (4). Decreased pH in the vacuole activates listeriolysin O (LLO) to degrade the vacuolar membrane and the bacterium subsequently escapes into the cytosol (5). Once in the cytosol, the bacterium expresses ActA, which induces host-cell actin polymerization to promote cell to cell spread (6, 7). In vivo, L. monocytogenes replicates within epithelial cells, endothelial cells, hepatocytes, and macrophages. The adaptive response to L. monocytogenes is mediated by IFN-γ release from NKDCs and Th1 CD4+ T lymphocytes, which are activated by the synergistic signaling of IL-12 and IL-18 (8, 9) and by CD8+ T lymphocyte-mediated clearance of infected cells (10).
L. monocytogenes infection is detected by several cell surface and cytosolic sensors. Lipoteichoic acid shed by extracellular L. monocytogenes is detected through TLR2, while extracellular flagellin activates TLR5 (1). These TLRs induce transcription of many cytokines and chemokines, including TNF, IL-6, IL-12, KC/MIP-2, proIL-1β, and proIL-18 (11). Once in the cytosol, L. monocytogenes activates inflammatory transcriptional responses (12) mediated by Nod1- and Nod2-dependent detection of peptidoglycan components (13, 14). Additionally, other members of the NLR family detect L. monocytogenes and subsequently activate caspase 1, which promotes post-translational processing of proIL-1β and proIL-18 (2, 15).
IL-18 and IL-1β are translated with a leader sequence (the pro form) that is cleaved before secretion of the cytokine (16, 17). Initiation of this processing event is controlled by the inflammasome, a multiprotein complex in which NLRs direct activation of caspase 1 (18). These NLRs are characterized by common structural motifs—specifically an amino-terminal signaling domain (typically a caspase activation and recruitment domain (CARD) or pyrin domain), a central oligomerization domain, and a C-terminal regulatory domain. The signaling domain recruits procaspase 1 through its CARD via homotypic interactions; for example, the CARD of Ipaf directly interacts with the CARD of procaspase 1. Alternatively, Nalp family proteins contain a pyrin domain that interacts with the pyrin domain of the small adaptor protein ASC. ASC, in turn, recruits procaspase 1 through CARD-CARD interactions. Clustering of CARD/pyrin domains after NLR activation results in the processing of procaspase 1 by inducing close proximity of the procaspase 1 molecules to each other. Several NLRs have been shown to induce caspase 1 activation, the best studied of which are Nalp3 and Ipaf (19).
Nalp3 (also called cryopyrin or NLRP3) is activated in response to numerous stimuli, both by microbe associated molecules, including nigicerin (15) and ssRNA homologues R837 and R848 (20), and by endogenous danger-associated molecules, including extracellular ATP (15). Many of the stimuli result in loss of membrane integrity; thus, it has been suggested that Nalp3 is activated in response to a depletion of intracellular potassium (21). Nalp3 has been shown to be activated in response to L. monocytogenes (15), Staphylococcus aureus, and Escherichia coli (19). Ipaf (also called NLRC4) responds to the presence of flagellin within the macrophage cytosol during infection by Salmonella typhimurium (22–24) and Legionella pneumophila (25–27) (reviewed in Ref. 28). Ipaf also responds to Shigella flexneri independently of flagellin expression (29). Prior studies have reported conflicting data concerning the relative importance of Nalp3 and Ipaf to caspase 1 activation in response to L. monocytogenes (15, 30).
Caspase 1 is required for the clearance of L. monocytogenes in murine infection (31). However, the NLRs activated by L. monocytogenes are not completely defined. In this report, we provide evidence that L. monocytogenes activates caspase 1 through multiple NLRs including Nalp3, Ipaf, and an unidentified receptor.
Materials and Methods
Mice
C57BL/6 mice were obtained from The Jackson Laboratory. Nalp3−/−, Ipaf−/−, and ASC−/− mice were a gift from Vishva Dixit (Genentech, South San Francisco, CA) and were backcrossed onto the C57BL/6 background eight times. All mice were housed in specific pathogen-free conditions and with approval of the Institutional Animal Care and Use Committee at Institute for Systems Biology (Seattle, WA).
Bacterial and mammalian cell cultures
Bone marrow-derived macrophages (BMM) from wild-type (WT) or mutant mice backcrossed onto the C57BL/6 background were cultured in L cell media consisting of 50% DMEM with 30% FBS, 20% L929 cell supernatant, penicillin, and streptomycin for 6 days before infection. WT L. monocytogenes 10403s was a gift from Dan Portnoy (University of California, Oakland, CA), as was the LLO-deficient (ΔLLO) strain. Flagellin-deficient (flaA) L. monocytogenes in the 10403 background was a gift from Chris Wilson (University of Washington, Seattle, WA) (32). We generated flgK-deficient L. monocytogenes by cloning the 5′ and 3′ ends of flgK from L. monocytogenes genomic DNA into vector pKSV7. This was then transformed into competent L. monocytogenes 10403s and grown at 40°C to induce chromosomal insertion followed by passage at 30°C to induce recombination and plasmid excision. For infections, all bacterial strains were grown at 37°C overnight with shaking, the diluted 1/5 and grown for 3 h at ambient temperature on a rotator.
Macrophage infections
BMM were plated in 96-well cell culture plates at 5 × 104 cells/well. The following day, BMM were stimulated with 50 ng/ml ultrapure LPS (List Biological Laboratories) for 3 h at 37°C to induce proIL-1β expression. L. monocytogenes was then added at a multiplicity of infection (MOI) of 6:1 and cells were spun 1000 rpm (250 × g) for 5 min to increase contact between BMM and amotile L. monocytogenes. BMM were then incubated for 1 h at 37°C before supernatant collection, or being washed and returned to media containing 50 ng/ml LPS and 15 μg/ml gentamicin to kill all extracellular bacteria. Cells were then incubated for 3 h before supernatants were harvested.
Protein transfections
Macrophages were seeded at 5 × 104 c/well into 96-well culture dishes. The following day, cells were stimulated with 50 ng/ml ultrapure LPS for 3 h to induce proIL-1β transcription then purified GST-tagged flagellin or hook protein was delivered by the protein transfection reagent Profect P1 (Targeting Systems). Cells were spun 5 min 250 rpm (15 × g). Supernatants were harvested 1 h later and ELISAs performed.
Western blots
To measure flagellin localization by Western blot, overnight cultures of the three L. monocytogenes strains (WT, flaA, and flgK) were diluted 1/5 and grown for 3 h to early logarithmic phase at ambient temperature on a rotator. Bacteria were pelleted, and proteins in the supernatant were precipitated with 10% trichloroacetic acid. Bacteria were resuspended in sample buffer. Pellet-associated and supernatant-associated proteins were run on a 4–20% gradient gel, transferred to polyvinylidene difluoride, and probed with the rabbit anti-L. monocytogenes flagellin Ab (Virostat; Cat. no. 4201) at 1/1000 for 1 h, then probed with a mouse anti-rabbit L chain specific HRP-conjugated secondary Ab at 1/5000 3 h and visualized by ECL (Pierce). Picture shown is 1-min exposure.
Processed caspase 1 was assessed from pooled lysates and supernatants of macrophages seeded in 6-well dishes and infected as described above. Trichloroacetic acid was used to precipitate secreted proteins, which were combined with the soluble cytosolic extract fraction and protein samples were run on 4–20% gradient gel, transferred to polyvinylidene difluoride, and probed with rabbit anti-caspase 1 p10 specific Ab (Santa Cruz Bio-technology; Cat. no. sc-514) at 1/200 for 1 h followed by goat-anti-rabbit H chain specific HRP-conjugated secondary Ab at 1/1000 overnight at 4°C. Pictures shown are at 1 h exposure.
Cytokine analysis
Secreted IL-1β was assessed from macrophages infected as described here with the DuoSet mouse IL-1β ELISA detection system from R&D Systems (Cat. no. DY401).
Mouse infection
The 8-wk-old C57BL/6 mice were injected i.v. with 1 × 104 live L. monocytogenes. Mice were sacrificed 5 days post infection and CFUs in the livers and spleen were determined by dilutional plating on Brain Heart Infusion plates. Significance was determined using Student’s t test with p < 0.05 defining significance.
Results
Cytosolic L. monocytogenes activates multiple NLRs
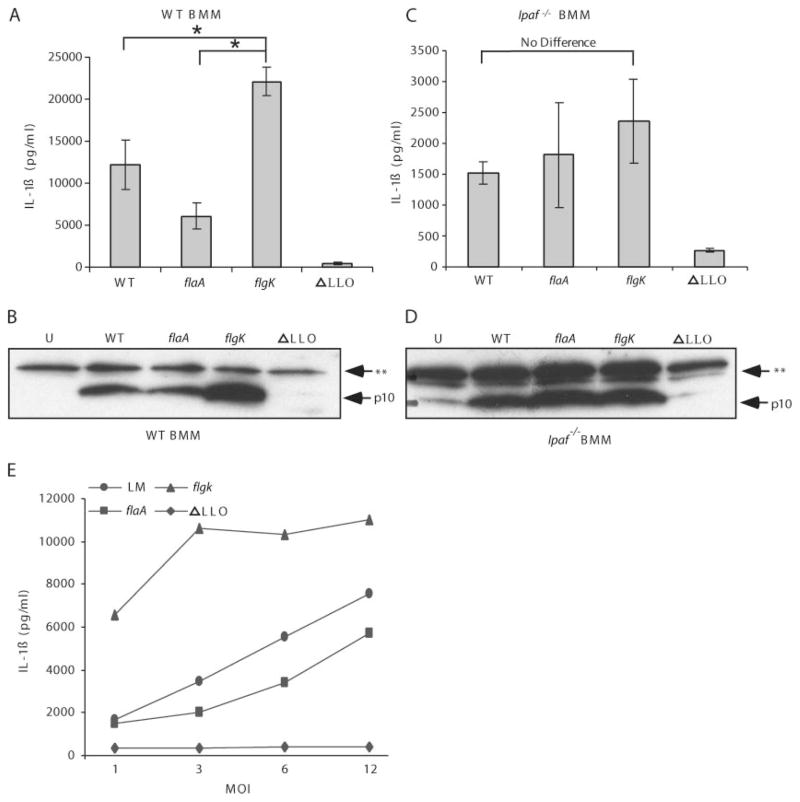
We hypothesized that cytosolic L. monocytogenes activates Ipaf as L. monocytogenes 10403s expressed flagellin at 37°C (32) and Ipaf responds to cytosolic flagellin delivered through virulence factor secretion systems by S. typhimurium and L. pneumophila (see Ref. 28). To characterize the relative contributions of Nalp3 and Ipaf to caspase 1 activation in response to L. monocytogenes, we infected macrophages deficient in various components of the inflammasome with L. monocytogenes and measured IL-1β secretion (Fig. 1). BMM were stimulated with LPS for 3 h to induce expression of proIL-1β and then infected with either WT L. monocytogenes or LLO-deficient L. monocytogenes (ΔLLO), which cannot access the cytosol. After 1 h of infection, Nalp3 played a dominant role in promoting IL-1β secretion, while Ipaf played a lesser role (Fig. 1A). In contrast, after 4 h of infection, total IL-1β secretion was enhanced and the contribution of Nalp3 and Ipaf were similar (Fig. 1B). Thus, both Nalp3 and Ipaf activate caspase 1 in response to cytosolic L. monocytogenes. Both Nalp3 and Ipaf-mediated responses detect cytosolic bacteria, as ΔLLO L. monocytogenes failed to induce IL-1β processing.
FIGURE 1.
Cytosolic Listeria monocytogenes in macrophages induces IL-1β secretion, which is partially dependent upon the NLRs Nalp3 and Ipaf. WT, Nalp3−/−, or Ipaf−/− BMM were stimulated with LPS to induce proIL-1β expression before infection with WT or LLO-deficient L. monocytogenes (MOI 6). IL-1β secretion was assessed by ELISA as a measure of caspase 1 activation at (A) 1 h or (B) 4 h post infection. Error bars, SD. Representative of three independent experiments.
L. monocytogenes flagellin activates Ipaf
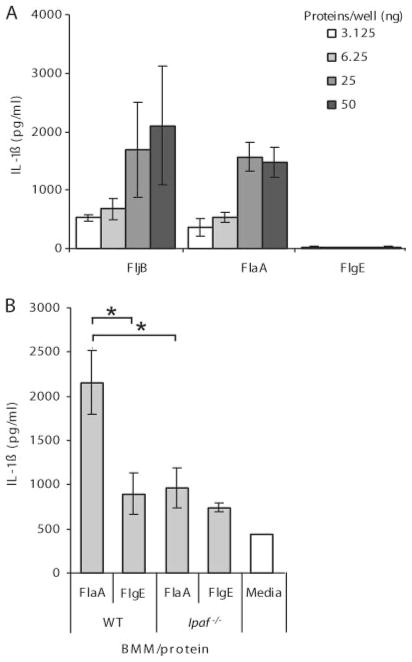
To establish whether Ipaf was responding to flagellin expressed by cytosolic L. monocytogenes, we delivered purified flagellin to the BMM cytosol independently of bacterial infection. In WT BMM, but not Ipaf−/− BMM, purified flagellin induced IL-1β secretion in a dose-dependent manner, whereas a negative control, the flagellar hook, did not (Fig. 2, A and B). TLR5−/− BMM behave like WT (data not shown), which is expected because TLR5 is not detected on mouse BMM (33, 34). Thus, cytosolic L. monocytogenes flagellin was detected through Ipaf but not the extracellular flagellin sensor TLR5.
FIGURE 2.
Ipaf is activated in response to cytosolic L. monocytogenes flagellin. A, LPS stimulated macrophages were transfected with purified flagellin from S. typhimurium (FljB) or L. monocytogenes (FlaA) or hook protein (FlgE) (negative control). IL-1β secretion was assessed after 1 h by ELISA. B, LPS stimulated WT or Ipaf−/− BMM were transfected with 31 ng of either FlaA or FlgE and IL-1β secretion was assessed after 2 h. Representative of three independent experiments.*, p < 0.05.
Flagellin hypersecreting mutant L. monocytogenes induces greater caspase 1 processing through Ipaf
To further characterize the relationship between inflammasome activation and flagellin expression by L. monocytogenes, we wished to compare amotile L. monocytogenes that expresses flagellin (flgK mutant) to L. monocytogenes that does not express flagellin (flaA mutant). Based on sequence homology to other flagellar structures, the FlgK gene product is predicted to act as an adaptor molecule between the flagellar hook and the flagellar filament, which is composed of FlaA monomers (Fig. 3A). S. typhimurium deficient in FlgK continually expresses and secretes flagellin monomers, but is unable to polymerize the monomers into a functional flagella (22). We verified that the L. monocytogenes flgK and flaA mutants were amotile by inoculation and overnight incubation in 0.3% agar LB motility agar (Fig. 3B) and that flgK bacteria have reduced cell-associated flagellin and secrete more flagellin to the supernatant, whereas the flaA mutant does not express flagellin (Fig. 3C). flaA and flgK mutant L. monocytogenes invasion of macrophages was similar to invasion by WT L. monocytogenes if contact was promoted by centrifugation (data not shown).
FIGURE 3.

flgK L. monocytogenes are amotile and secrete increased amounts of flagellin into the environment. A, Schematic of the structure of L. monocytogenes flagella. CM, Cell membrane; and PGN, peptidoglycan. B, Motility plate demonstrating that WT and LLO L. monocytogenes are motile but flaA and flgK L. monocytogenes are not. C, Proteins were precipitated from bacterial pellets and supernatants and run on western blot to assess flagellin expression and secretion. P, Pellet (bacteria associated); and S, supernatant (secreted). **, Nonspecific band.
To investigate the effect of flgK mutation on IL-1β secretion in BMM, WT macrophages were infected with four strains of L. monocytogenes (WT, flaA, flgK, and ΔLLO). IL-1β processing was proportional to the amount of flagellin secreted by the bacteria and dependent upon bacterial presence in the cytosol (Fig. 4A). Inflammasome activation was confirmed by Western blot for processed caspase 1, which revealed slight decreases in processing after infection with flaA mutant and significant increases in processing after infection with flgK mutant (Fig. 4B). We hypothesized that Ipaf was required for flagellin detection during L. monocytogenes infection. When we infected Ipaf−/− macrophages with the four strains of L. monocytogenes (WT, flaA, flgK, and ΔLLO), the flagellin-dependent variation in IL-1β secretion was eliminated (Fig. 4C), but not the requirement for cytosolic bacteria. Western blots confirmed no differences in caspase 1 processing in Ipaf−/− macrophages following infection with the L. monocytogenes flagellar mutants (Fig. 4D). Note that caspase 1 processing and IL-1β secretion were not eliminated in these macrophages as the Nalp3/ASC detection pathway remains intact in the absence of Ipaf. In an examination of the dose response to WT and flagellin mutant L. monocytogenes, we noted that the threshold for flagellin detection was reached between MOIs 1 and 3 after 4 h, whereas the response to flgK mutants was pronounced even at low doses (MOI 1) (Fig. 4E).
FIGURE 4.
Flagellin-dependent variation in caspase 1 processing in L. monocytogenes-infected macrophages is dependent on Ipaf. A, LPS-primed WT macrophages were infected with WT, flaA, flgK, or ΔLLO L. monocytogenes at MOI 6. IL-1β secretion from supernatants was assessed 4 h post infection. *, p < 0.05. B, WT macrophages were infected at MOI 6 for 4 h then proteins precipitated from supernatants and combined with cell lysates. Samples were Western blotted then probed for the p10 fragment of caspase 1. U, Uninfected; and **, nonspecific band. L. monocytogenes infected Ipaf−/− macrophages were analyzed for (C) IL-1β secretion and (D) caspase 1 processing as in A and B above. Error bars, SD. Representative of three experiments. E, LPS-primed WT macrophages were infected with WT, flaA, flgK, or ΔLLO L. monocytogenes at MOI 1, 3, 6, or 12. IL-1β secretion from supernatants was assessed 4 h post infection.
To investigate the role of flagellin detection in the context of the immune response, we examined bacterial clearance in the liver and spleen of C57BL/6 mice infected for 5 days with WT, flaA, or flgK L. monocytogenes (Fig. 5). L. monocytogenes that expresses and exports flagellin monomers (flgK mutant) was significantly attenuated in comparison to the flagellin-deficient strain (flaA mutant) in both organs. We also examined the effect of the polymerization state of flagellin on L. monocytogenes virulence. WT L. monocytogenes (which expresses polymerized flagellin) was more virulent than the flgK mutant strain (which expresses monomeric flagellin) in the spleen, and trended similarly in the liver. Thus, flagellin expression, especially in its monomeric form, is detrimental to bacterial survival in vivo.
FIGURE 5.
Detection of flagellin enhances bacterial clearance. C57BL/6 mice were injected with 104 L. monocytogenes i.v. Five days later, bacterial clearance was assessed by plating organ homogenates and counting CFUs. Geometric mean of 10 mice per strain is shown (−).*, p < 0.05 and **, p < 0.01. NS, Not significant. Ten mice per cohort.
ASC not essential for cytosolic flagellin detection
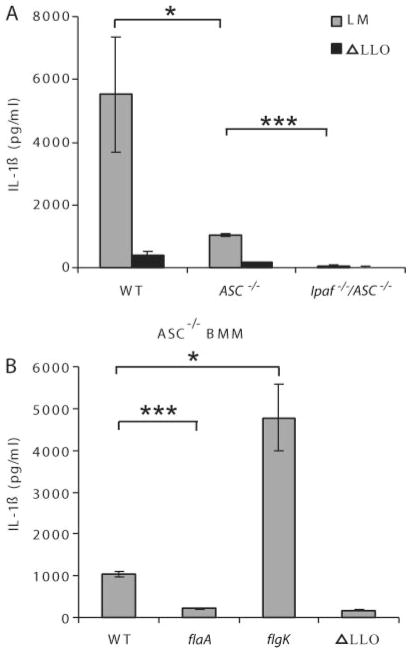
ASC is the adaptor protein used by pyrin domain-containing NLRs to recruit the CARD of procaspase 1 (35). Previous reports have suggested that Ipaf signaling is at least partially dependent on ASC (2, 22, 36). To determine whether the Ipaf signaling pathway can function in the absence of ASC, we compared ASC−/− macrophages to Ipaf−/−/ASC−/− macrophages. When infected with WT L. monocytogenes for 4 h, ASC−/− macrophages retained some ability to process IL-1β, whereas Ipaf−/−/ASC−/− macrophages had no IL-1β secretion, indicating that Ipaf can signal in the absence of ASC (Fig. 6A). To further investigate the role of ASC in IL-1β processing in response to cytosolic L. monocytogenes flagellin, we infected ASC−/− BMM with WT, flaA, flgK, or ΔLLO L. monocytogenes. Ipaf signaling in the absence of ASC was supported by the observation that IL-1β hypersecretion in response to flgK mutants is preserved in ASC−/− BMM (Fig. 6B). As predicted by our previous results, when both ASC and Ipaf signaling were ablated (ASC by macrophage gene deletion and Ipaf by deleting the flagellin agonist in flaA L. monocytogenes), IL-1β secretion was abrogated (Fig. 6B). Together, these data demonstrate that signaling through Ipaf in response to cytosolic flagellin can occur independently of the ASC adaptor.
FIGURE 6.
Ipaf signaling and flagellin detection is independent of ASC. Macrophages were infected with L. monocytogenes strains (MOI 6) for 4 h before IL-1β secretion was determined by ELISA. A) WT, ASC−/−, or Ipaf−/−/ASC−/− macrophages infected with WT or ΔLLO mutant L. monocytogenes B) ASC−/− macrophages infected with WT, flaA, flgK, or ΔLLO L. monocytogenes. Error bars, s.d. Representative of three experiments. *p = 0.05; ***p < 0.001.
Novel ASC-dependent pathway activated by cytosolic L. monocytogenes
The results above demonstrate that over the first 4 h after L. monocytogenes infection, all detectable inflammasome activity signals through the combined action of ASC and Ipaf. ASC acts as an adaptor molecule between caspase 1 and proteins containing pyrin domains; it is necessary for Nalp1-, Nalp2-, and Nalp3-driven caspase 1 activation and may play a similar role for other Nalp proteins (37–39). To determine whether signaling through ASC during L. monocytogenes infection originates entirely from Nalp3, we compared Nalp3−/− and ASC−/− macrophages infected with WT L. monocytogenes. ASC−/− BMMs were much more defective than Nalp3−/− macrophages in IL-1β secretion (Fig. 7A). This suggests that additional pyrin-containing signaling molecules activate caspase 1 through ASC during L. monocytogenes infection. One potential caveat to this hypothesis is that ASC may have minor effects on the magnitude of Ipaf signaling. We have shown that Ipaf can signal in the absence of ASC, but have not excluded the possibility that ASC enhances this signaling. Thus, IL-1β section may be lower in ASC−/− relative to Nalp3−/− BMM due to a partial inhibition of Ipaf-mediated signaling. To control for this possibility, we eliminated flagellin-dependent Ipaf signaling by infecting BMM with flaA mutant L. monocytogenes. Nalp3−/− BMM detected flaA mutant L. monocytogenes and responded by secreting IL-1β, whereas ASC−/− BMM did not (Fig. 7B). These results suggest the presence of a novel signaling pathway activated by cytosolic L. monocytogenes, which is dependent upon ASC for signaling to the inflammasome.
FIGURE 7.
A third receptor activates inflammasome following L. monocytogenes infection. Macrophages were infected with L. monocytogenes strains (MOI 6) and IL-1β secretion determined after four hours. A) WT, Nalp3−/−, and ASC−/− macrophages infected with WT or ΔLLO mutant L. monocytogenes. B) WT, Nalp3−/−, and ASC−/− macrophages were infected with either flaA or ΔLLO mutant L. monocytogenes. Error bars, s.d. Representative of three experiments. *p = 0.05.
Discussion
We have examined the signaling molecules that contribute to caspase 1 activation in macrophages in response to infection by L. monocytogenes. Our data indicate that multiple NLR proteins independently respond to the presence of L. monocytogenes in the cytosol, including Nalp3, Ipaf, and potentially a third unidentified activator that signals through ASC.
During the first hour after infection, the dominant caspase 1 activator in response to L. monocytogenes is Nalp3. Nalp3 is activated by several stimuli, many of which are known to result in membrane disruption. For example, extracellular ATP triggers the opening of P2X7, a small-cation channel. Nalp3 is also activated by nigericin and maitotoxin, toxins which also induce membrane damage (40, 41). L. monocytogenes rapidly disrupts the vacuolar membrane after internalization though the virulence factor LLO, mediating bacterial escape from the vacuole into the cytosol, so it is possible that some event triggered by vacuolar disruption induces Nalp3 activation. However, in contrast to ATP, nigericin and maitotoxin which trigger pore formation in the plasma membrane, LLO activity is induced by low pH and, thus, specifically targets the vacuolar membrane for permeabilization.
L. monocytogenes is the first cytosolic pathogen that has been demonstrated to be detected by Ipaf through flagellin expression. Many pathogens that do not escape the vacuole are also detected by Ipaf through recognition of flagellin within the mammalian cell cytosol, including S. typhimurium (22, 24), L. pneumophila (25, 42), and Pseudomonas aeruginosa (43–45). Shigella flexneri, another pathogen that replicates in the cytosol, also activates Ipaf, but do not express flagellin (29), suggesting that Ipaf may integrate multiple signals.
Our data indicate that during L. monocytogenes infection, Ipaf responds to the presence of flagellin in the cytosol. A strain of L. monocytogenes that secretes flagellin monomers (flgK) induced increased capsase 1 activity and IL-1β secretion through Ipaf. This results in increased clearance of bacteria in a murine infection model. The importance of evading Ipaf detection can be inferred by the fact that 80% of clinical isolates of L. monocytogenes repress flagellin when grown at 37°C (32). Furthermore, we demonstrated that this detection of cytosolic flagellin did not require ASC, as ASC−/− macrophages still increased caspase 1 processing following infection with flgK L. monocytogenes.
Prior reports have presented conflicting data regarding the role of NLRs in caspase 1 activation induced by L. monocytogenes infection in macrophages. Mariathasan et al. (15) first reported that Nalp3 was required to induce caspase 1 processing and IL-1β secretion in response to L. monocytogenes in peritoneal macrophages. However, Franchi et al. (30) observed caspase 1 processing in L. monocytogenes-infected Nalp3−/− BMM as well as Ipaf−/− BMM. In light of our data, we hypothesize that the data of Franchi et al. may reflect strong activation of both signaling pathways, either of which is sufficient to induce caspase 1 cleavage. Under Mariathasan’s conditions, the role of Nalp3 accounted for the majority of IL-1β secretion; however, a lower level of detectable IL-1β secretion was observable in Nalp3−/− macrophages which our results suggest may be due to Ipaf signaling. Our data support a continuum of Nalp3 and Ipaf contributions during the course of an infection. Nalp3 provides the majority of the caspase 1 activating signal early in infection and the Ipaf contribution increases over time. The delayed Ipaf response may be attributed to a slow rate of shedding flagellin monomers from the polymerized flagella.
In addition to signaling from Nalp3 and Ipaf, the inflammasome may also be activated in response to cytosolic L. monocytogenes through a third receptor. Using ASC−/− and Nalp3−/− macrophages infected with flaA L. monocytogenes (to eliminate flagellin-dependent Ipaf signaling), we have shown that Nalp3−/− macrophages generate more IL-1β than ASC−/− macrophages 4 h after infection. This data suggests that there is at least one more receptor for L. monocytogenes that signals through ASC. There are over 20 identified NLR proteins in the mouse, and agonists have been identified for a few (46). Because this potential new receptor activates the inflammasome through ASC, we speculate that it contains a pyrin domain and thus could be one of the orphan Nalp receptors. Further studies are needed to identify this receptor and define its contribution to caspase 1 signaling in the context of L. monocytogenes infections.
Acknowledgments
We thank Dr. Vishva Dixit (Genentech, South San Francisco, CA) for sharing the Ipaf−/−, ASC−/−, and Nalp3−/− mice. We also thank Dr. Daniel Portnoy (University of California, Oakland, CA) for the WT and ΔLLO L. monocytogenes strains and Dr. Christopher Wilson (University of Washington, Seattle, WA) for the flaA L. monocytogenes.
Footnotes
This work was supported by National Institutes of Health Grants AI065878, AI052286, AI032972, and AI025032, Howard Hughes Grant 56005710, and Grant T32CA009537 from the National Cancer Institute.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations used in this paper: PRR, pattern recognition receptor; PAMP, pathogen-associated molecular pattern; NLR, Nod-like receptor; LLO, listeriolysin O; CARD, caspase activation and recruitment domain; BMM, bone marrow-derived macrophages; WT, wild type; MOI, multiplicity of infection.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 2.Franchi L, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim YG, Nunez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10:1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 4.Seveau S, Pizarro-Cerda J, Cossart P. Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect. 2007;9:1167–1175. doi: 10.1016/j.micinf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Smith GA, Portnoy DA. How the Listeria monocytogenes ActA protein converts actin polymerization into a motile force. Trends Microbiol. 1997;5:272–276. doi: 10.1016/S0966-842X(97)01048-2. [DOI] [PubMed] [Google Scholar]
- 7.Pizarro-Cerda J, Cossart P. Subversion of cellular functions by Listeria monocytogenes. J Pathol. 2006;208:215–223. doi: 10.1002/path.1888. [DOI] [PubMed] [Google Scholar]
- 8.Plitas G, Chaudhry UI, Kingham TP, Raab JR, DeMatteo RP. NK dendritic cells are innate immune responders to Listeria monocytogenes infection. J Immunol. 2007;178:4411–4416. doi: 10.4049/jimmunol.178.7.4411. [DOI] [PubMed] [Google Scholar]
- 9.Biet F, Locht C, Kremer L. Immunoregulatory functions of inter-leukin 18 and its role in defense against bacterial pathogens. J Mol Med. 2002;80:147–162. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 10.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci USA. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kufer TA, Banks DJ, Philpott DJ. Innate immune sensing of microbes by Nod proteins. Ann NY Acad Sci. 2006;1072:19–27. doi: 10.1196/annals.1326.020. [DOI] [PubMed] [Google Scholar]
- 14.Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:12. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 16.Cameron P, Limjuco G, Rodkey J, Bennett C, Schmidt JA. Amino acid sequence analysis of human interleukin 1 (IL-1): evidence for biochemically distinct forms of IL-1. J Exp Med. 1985;162:790–801. doi: 10.1084/jem.162.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukocyte Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 21.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 22.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 24.Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica Serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 25.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 26.Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji NM, Tsutsui H, Seki E, Kuida K, Okamura H, Nakanishi K, Flavell RA. Roles of caspase-1 in Listeria infection in mice. Int Immunol. 2004;16:335–343. doi: 10.1093/intimm/dxh041. [DOI] [PubMed] [Google Scholar]
- 32.Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE, Wilson CB. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 2004;6:235–242. doi: 10.1046/j.1462-5822.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 33.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14:1065–1074. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- 34.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 35.Mariathasan S. ASC, Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect. 2007;9:664–671. doi: 10.1016/j.micinf.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 37.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukocyte Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 39.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 40.Perregaux D, Gabel CA. Interleukin-1 β maturation and release in response to ATP and nigericin: evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 41.Yokoyama A, Murata M, Oshima Y, Iwashita T, Yasumoto T. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marine dinoflagellate. J Biochem. 1988;104:184–187. doi: 10.1093/oxfordjournals.jbchem.a122438. [DOI] [PubMed] [Google Scholar]
- 42.Coers J, Vance RE, Fontana MF, Dietrich WF. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol. 2007;9:2344–2357. doi: 10.1111/j.1462-5822.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 43.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Nunez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 44.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinon F, Gaide O, Petrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]