Abstract
PURPOSE
To determine whether measurements obtained by partial coherence interferometry (PCI) correlate well with measurements obtained using immersion ultrasound (US) in children.
SETTING
Department of Ophthalmology, Emory University School of Medicine, Atlanta, Georgia, USA.
DESIGN
Evaluation of a diagnostic test or technology.
METHODS
The charts of pediatric patients who had cataract surgery from August 2008 to September 2009 were reviewed. Axial length (AL) measurements in the operative eye were obtained using PCI at the preoperative clinic visit and then using immersion US in the operating room before surgery. The data were compared to determine the degree of agreement.
RESULTS
The charts of 18 patients (27 eyes) were reviewed. Preoperative AL measurements by PCI were obtained in 21 eyes (78%). On average, the PCI-measured ALs were 0.1 mm less than the immersion US values (95% confidence interval, −0.2 to −0.1; P = .002). All eyes with an AL of 23.5 mm or less had lower PCI values than immersion US values. There was no systematic pattern of 1 measurement being greater or less than the other in eyes with an AL longer than 23.5 mm.
CONCLUSIONS
There is a systematic difference in AL measurement between PCI and immersion US, with PCI tending to give lower values, particularly in eyes with an AL longer than 23.5 mm. Depending on the length of the eye, a 0.1 mm error in AL measurement could result in a 0.25 to 0.75 diopter difference in intraocular lens calculation that could be clinically significant in some patients.
Most axial growth occurs in the human eye during the first 5 years of life.1 The effects of cataract surgery and intraocular lens (IOL) implantation on the axial growth in an infant’s eye are not well known.2 Immersion ultrasound (US) has been the gold standard for measuring axial length (AL) in children. Although this method can be easily performed with the child under general anesthesia, it is difficult when the child is awake. In contrast, partial coherence interferometry (PCI) does not require contact with the eye and can be performed without anesthesia. In a preschool vision screening setting, PCI had a 90% testability in children 3 years and older.3 Furthermore, PCI has been shown to have better reproducibility than A-scan ultrasonography in measuring AL in school-aged children.3 Studies of PCI alone show that it yields reproducible, highly precise AL measurements, even in young children.4
There are several advantages to obtaining AL measurements using PCI. In the clinic, PCI can be less time-consuming than immersion US performed in an operating room. Second, PCI allows AL measurement without the use of general anesthesia, saving operating room time and expense. Third, PCI could be used in future longitudinal studies of natural and postoperative changes in AL to give a better understanding of eye growth and refractive outcomes in pediatric patients. It is recognized that a subset of patients too young or otherwise unable to cooperate with preoperative biometric measurements of any kind will continue to require immersion US under general anesthesia.
The purpose of the present study was to determine whether AL measurements in children obtained by PCI correlate well with those obtained using immersion US. Although several previous studies5–8 compared the biometric data obtained with PCI and with various forms of US in adults, the only study comparing PCI and A-scan US measurements of ocular AL in children relied on contact A-scan ultrasonography,9 which can reduce AL measurement by 0.20 to 0.33 mm as a result of corneal compression.6 In 1986, Holladay et al.10 stated that obtaining accurate biometry is more important than choosing the appropriate IOL power calculation formula in achieving good refractive outcomes after surgery. Obtaining accurate AL measurements in children before cataract surgery is particularly important because biometric errors can be of greater significance in shorter eyes.
PATIENTS AND METHODS
The charts of pediatric patients who had cataract surgery from August 2008 to September 2009 at Emory Eye Center Pediatric Ophthalmology Clinic were reviewed. The study was approved by the Emory University Institutional Review Board and was in compliance with the U.S. Health Insurance Portability and Accountability Act.
The patients were evaluated for their ability to cooperate with in-clinic PCI testing. Patients who were sufficiently cooperative were evaluated during the preoperative clinic visit using the IOLMaster PCI biometer (Carl Zeiss Meditec). This involved placing the chin in a slitlamp-like apparatus and forehead against a band to stabilize the head for a period of seconds while measurements of the distance from the corneal vertex to the retinal pigment epithelium (RPE) were taken. The default settings of the biometer were used, and the biometer was calibrated daily in the clinic. The biometer takes at least 5 and up to 20 AL measurements and determines a composite AL.A A signal curve of the AL measurement and a signal-to-noise ratio (SNR) were also generated, both reflecting the validity of the AL measurement.
Subsequently, on the day of cataract surgery after general anesthesia was induced, all patients had AL measurements by immersion US (Eye Cubed, Ellex). This involved placing a Kohn immersion shell containing the ultrasonic probe tip over the operative eye and filling the shell with a balanced salt solution to obtain an AL measurement. The US was operated in manual mode during acquisition. The measurement was repeated several times, and the reading with the best alignment pattern and gate placement was chosen for IOL calculations. The measurements were performed by 1 of 2 experienced pediatric ophthalmologists (A.K.H., S.R.L.).
Although fellow eyes were measured, this paper reports results in the cataractous eyes only. The mean AL was compared between the PCI and immersion US measuring techniques using a paired t test.
RESULTS
Twenty-seven cataractous eyes of 18 patients cooperative enough to have PCI testing were included in this study (Table 1). The PCI AL measurements could not be obtained in 6 eyes (22%) of 3 patients. Of the remaining 21 eyes, 11 were right eyes and 10 left eyes.
Table 1.
Patient characteristics and ALs obtained by PCI and immersion US.
| Pt | Age (Y) |
Eye | AL (mm) PCI |
SNR (PCI) | AL (mm) Immersion US |
|---|---|---|---|---|---|
| 1 | 6 | R | NA | 1.2 | 23.88 |
| L | 21.08 | 147 | 21.24 | ||
| 2 | 5 | R | 20.28 | 71.4 | 20.54 |
| L | 20.08 | 96.6 | 20.39 | ||
| 3 | 10 | R | 22.45 | 9.8 | 22.45 |
| 4 | 7 | R | 24.37 | 42.7 | 24.26 |
| L | 24.58 | 63.2 | 24.43 | ||
| 5 | 8 | L | 22.59 | 56.9 | 23.04 |
| 6 | 13 | R | 21.91 | 85.7 | 21.99 |
| L | NA | NA | 21.78 | ||
| 7 | 10 | R | 23.77 | 181 | 23.8 |
| L | 23.94 | 105.6 | 23.9 | ||
| 8 | 10 | R | 24.17 | 253.6 | 24.23 |
| 9 | 8 | R | 21.82 | 181.4 | 21.9 |
| 10 | 16 | R | NA | 1.8 | 23.23 |
| L | NA | 1.7 | 21.5 | ||
| 11 | 5 | R | NA | 2 | 24.23 |
| 12 | 4 | L | 21.91 | 40.8 | 22.15 |
| 13 | 4 | R | 20.44 | 297.3 | 20.73 |
| L | 20.5 | 107 | 20.87 | ||
| 14 | 4 | R | 21.84 | 154.7 | 22.21 |
| L | 21.69 | 245.4 | 21.88 | ||
| 15 | 5 | R | 21.41 | 19.2 | 21.39 |
| L | 21.39 | 67.5 | 21.4 | ||
| 16 | 9 | R | 22.41 | 200 | 22.45 |
| 17 | 5 | R | NA | NA | 21.35 |
| 18 | 4 | L | 22.27 | 22.2 | 22.41 |
AL = axial length; NA = attempted but not obtainable; PCI = partial coherence interferometry; Pt = patient; SNR = signal-to-noise ratio; US = ultrasound
The mean patient age for the remaining 15 patients was 7.1 years. Six of the 15 patients (40%) were 5 years or younger; 4 (27%) were girls.
Table 2 shows the mean AL by method and the difference between the measurements. The PCI values were, on average, 0.1 mm less than the immersion US values (95% confidence interval, −0.2 to −0.1; P = .002).
Table 2.
Comparison of AL measurements between PCI and immersion US.
| Method | Eyes | Mean ± SD | Axial Length (mm) Range |
95% CI for Mean |
|---|---|---|---|---|
| PCI | 21 | 22.14 ± 1.37 | 20.08 to 24.58 | 21.52 to 22.76 |
| Immersion US | 21 | 22.27 ± 1.26 | 20.39 to 24.43 | 21.69 to 22.85 |
| Difference* | 21 | −0.13 ± 0.17 | −0.45 to 0.15 | −0.21 to −0.06 |
CI = confidence interval; PCI = partial coherence interferometry, US = ultrasound
The difference was calculated as PCI – Immersion US. The P value for comparison of means between PCI and US was 0.002 (paired t test)
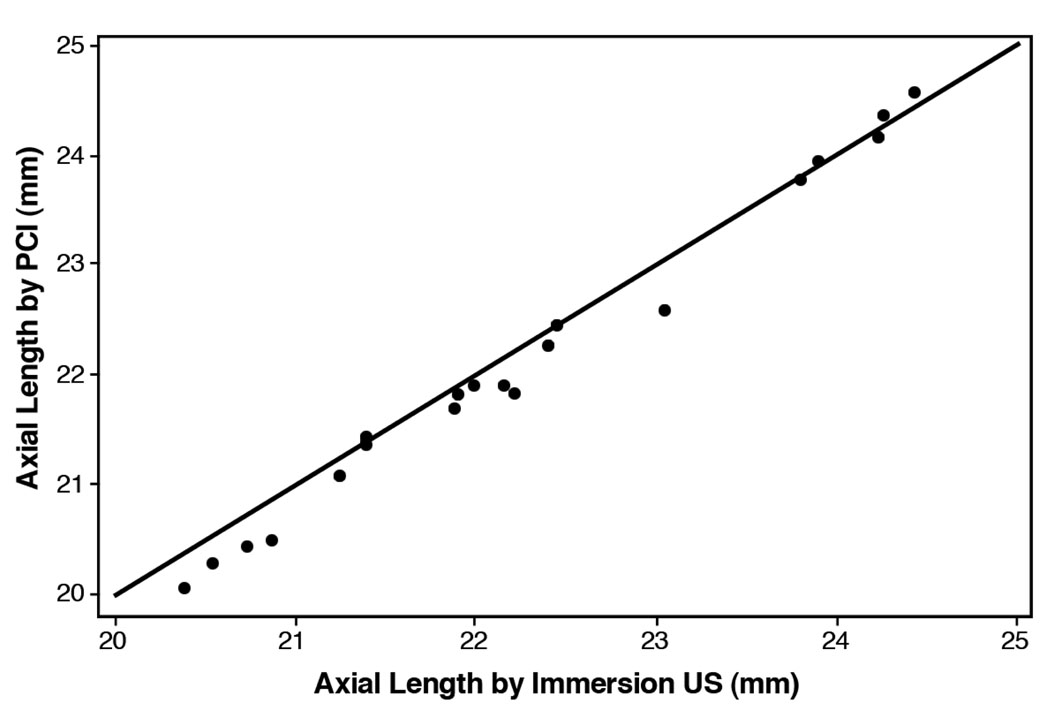
In all eyes with an AL of 23.5 mm or less, the PCI values that were less than or equal to the immersion ultrasonography values (Figure 1). In eyes with an AL greater than 23.5 mm, however, there was no systematic pattern of 1 measurement being greater or less than the other.
Figure 1.
Scatterplot comparing AL obtained by PCI with AL obtained by immersion US (PCI = partial coherence interferometry; US = ultrasound).
Partial coherence interferometry and immersion ultrasound were used to obtain axial length measurements in children. Axial lengths by PCI were 0.1 mm less than immersion US values (P<.002).
DISCUSSION
How well AL measurements in children obtained using PCI and immersion US correlate has not been well researched. However, studies comparing biometric measurements by PCI and by US in adult patient populations found a good correlation.5–8 In 2002, Packer et al.11 compared AL measurements obtained by PCI and immersion US in 50 cataractous eyes and found that the measurements correlated in “a highly positive manner.” In 2003, Németh et al.7 reported that AL values were “significantly larger with the IOLMaster than with the Ultrascan Digital 2000” (a contact scan device); however, they still found a high correlation between US and optical (PCI) AL measurements (P<.001).
Other recent studies have compared refractive outcomes in adults based on US biometry and PCI biometry. In a 2007 study calculating IOL power in 467 consecutive cataract operations using both PCI and applanation A-scan US, Olsen8 concluded that the use of calibrated AL reading obtained with PCI contributed to significant improvement in the accuracy of IOL power calculation. In 2009, Landers and Goggin12 found that PCI biometry produced a more predictable refractive outcome than immersion US. Although Narváez et al.6 found no difference in measurements or postoperative refractive outcomes in eyes measured by both PCI and immersion US, the study emphasized the limitations of interferometry in eyes with dense media opacity.
Few studies have evaluated the use of PCI in children. Some simply evaluated the feasibility of testing children with the IOLMaster PCI biometer.3,4 In a 2003 study, Quinn et al.4 used PCI to measure AL in children ages 3.4 to 12.9 years of age and found that, even in young children, PCI provided reproducible and precise AL measurements. The authors indicated that the data provided by PCI might be instrumental in studying eye growth and refractive development. In 2008, Borchert et al.3 evaluated the testability of PCI ocular biometry in children aged 30 to 72 months as part of the Multi-Ethnic Pediatric Eye Disease Study. The study found that 91% of children were testable with the PCI device and that testability rose sharply with age. The authors conclude that the knowledge that even young children can be reliably tested for ocular biometry with the PCI device might have an impact on management strategies for cataracts and refractive error in preschool children.
Two studies9,13 compared AL measurements obtained by IOLMaster PCI and US in children. In 2004, Carkeet et al.13 compared the repeatability of PCI axial dimension measurements with that of conventional contact or applanation US (Echoscan US 800, Nidek) in 179 Chinese children. The PCI AL measurements were, on average, slightly longer (by 0.14 mm) than US AL measurements. Carkeet et al. conclude that PCI techniques should be considered standard technique for AL measurement in children because of the noninvasive, highly precise, and user-friendly nature of the modality. In 2006, Hussin et al.9 compared PCI and contact A-scan US ocular AL measurements in children and found the PCI measurements to be more accurate and reproducible than those of contact US. Hussin et al. also noted the potential role of the PCI biometry in studying ocular growth and refractive development in children.
The results in our study of children indicate there is a systematic difference between PCI and immersion US in that PCI tends to give lower values in eyes with an AL of 23.5 mm or less. Although the magnitude of the difference (mean 0.13 mm) may seem small, it may be clinically important because errors in AL measurement have greater significance in the relatively shorter eyes of most children.
There are several limitations to our study. First is the small number of eyes. Second, our patient data do not represent all cataractous eyes of all children at the center who required cataract extraction with or without IOL placement during the study period. Only children who were physically and behaviorally able to cooperate with positioning in the slitlamp-like apparatus of the PCI device were selected to participate. Therefore, the number of untestable patients, such as infants, in a given pediatric ophthalmology population would be much higher than in our series. Third, there we were unable to obtain more than 5 AL measurements in many cases. Although obtaining at least 10 AL measurements in each eye would have been desirable, this was not possible in some cases because of limited patient cooperation.
Interestingly, patient cooperation in our pediatric population (aged 4 to 16 years) was not the limiting factor in most cases in which we were unable to obtain valid PCI readings. Axial length measurements recorded by the IOLMaster device must be interpreted on the basis of a valid signal curve and an acceptable SNR (<10). In the most eyes in which measurements were attempted but not obtainable, the signal curve or SNR accompanying the results of the AL measurements was unacceptable. The SNR may be low for many reasons, including dense media opacity along the visual axis, poor fixation in a restless patient, poor alignment of the device to the patient’s eye, very high ametropia (>6.0 diopters), corneal scars, and pathological changes in the retina.A All eyes for which we were unable to obtain PCI measurements had dense cataract; AL measurements were possible in the noncataractous eye in the same patients in all cases.
Our percentage of untestable children compares well with the percentage of untestable children documented in previous studies of IOLMaster measurements in adults. In 2003, Németh et al.7 reported a success rate of 82% for AL measurement with the PCI device in adults. Reasons they listed for why reliable measurements could not be obtained included young or old age; poor cooperation; dense or posterior central cortical–capsular cataract; high myopia; corneal dystrophy or degeneration; vitreous opacity or hemorrhage; fibrin accumulation in the anterior chamber or on the lens surface; and recent retinal detachment surgery, vitrectomy, keratoplasty, excimer laser keratectomy, or applanation tonometry.7 In the study by Narváez et al.,6 that 22% of adult eyes having surgery could not be measured using interferometry. Poor laser penetration in eyes with dense media opacities, particularly with posterior subcapsular cataract, and poor fixation in cataractous eyes have been the most significant limitations to interferometry.6,8
The strength of the present study lies in the comparison of PCI and immersion US in a pediatric population. In previous studies of adults and children comparing PCI and applanation A-scan US, the potential for contact-induced distortion of AL measurements may have limited direct comparison of values obtained by the 2 devices. One would expect that AL measurement using the PCI device would more closely approximate immersion US values because the system’s software is calibrated using a regression model; thus, the optically measured AL value (tear film to RPE) is can be directly compared with that obtained by acoustic immersion US (cornea to internal limiting membrane).11 The PCI device automatically adjusts for the distance disparity between the internal limiting membrane and the RPE. Because the optical method (PCI) measures along the optical axis of the eye and the ultrasound technique more likely measures on the anatomic axis,7 it is conceivable that the difference between PCI and immersion US in our study could have been due to poor alignment of the scans along the anatomic axis of the eye or poor gate positioning with US.
Our study supports previous assertions that when testing is possible, PCI is a quick, noncontact method of obtaining accurate preoperative biometric measurements in children. In general, the PCI measurements in the clinic were less time consuming than immersion US measurements in the operating room. Furthermore, knowing that reliable measurements have been obtained using PCI in a preoperative clinic may permit a surgeon to minimize the child’s time under general anesthesia. Finally, noncontact PCI measurements eliminate the potential risk for corneal abrasion or infection from immersion US.
Although our study points to the advantages of PCI, the method has limitations. A percentage of patients with dense cataract were untestable using the PCI device. In our group of patients between the ages of 4 years and 16 years, it was the density of the media opacity rather than the age of the patient that limited the ability to obtain PCI measurements. Clearly, a subset of patients (adults or children) unable to cooperate with preoperative biometric measurements of any kind will continue to require immersion US under general anesthesia. Finally, the high cost of the PCI device may be prohibitive for some practitioners.
Further studies are needed to determine the use of PCI biometry in honing postoperative refractive outcome in children and to evaluate the PCI as a tool for the longitudinal follow-up of AL measurement in children.
Acknowledgments
Supported by National Institutes of Health (NIH) grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY06360, Bethesda, Maryland, and Research to Prevent Blindness, Inc., New York, New York, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery, Boston, Massachusetts, USA, April 2010.
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
Other Cited Material
A. Carl Zeiss Meditec AG. IOLMaster with Advanced Technology Software Version 5.4. User Manual, 2008. Available at: http://doctor-hill.com/zeiss_iolmaster/iolmaster_5-4.pdf. Accessed August 26, 2010
REFERENCES
- 1.Gordon RA, Donzis PB. Refractive development of the human eye. [Accessed August 26, 2010];Arch Ophthalmol. 1985 103:785–789. doi: 10.1001/archopht.1985.01050060045020. Available at: http://archopht.ama-assn.org/cgi/reprint/103/6/785?ck=nck. [DOI] [PubMed] [Google Scholar]
- 2.Griener ED, Dahan E, Lambert SR. Effect of age at time of cataract surgery on subsequent axial length growth in infant eyes. J Cataract Refract Surg. 1999;25:1209–1213. doi: 10.1016/s0886-3350(99)00158-3. [DOI] [PubMed] [Google Scholar]
- 3.Borchert M, Wang Y, Tarczy-Hornoch K, Cotter S, Deneen J, Azen S, Varma R. Testability of the Retinomax autorefractor and IOLMaster in preschool children; the Multi-ethnic Pediatric Eye Disease Study; the MEPEDS Study Group. Ophthalmology. 2008;115:1422–1425. doi: 10.1016/j.ophtha.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn GE, Francis EL, Nipper KS, Flitcroft DI, Ying G-S, Rees RC, Schmid GF, Maguire MG, Stone RA. Highly precise eye length measurements in children aged 3 through 12 years. Arch Ophthalmol. 2003;121:985–990. doi: 10.1001/archopht.121.7.985. [DOI] [PubMed] [Google Scholar]
- 5.Lara F, Fernández-Sánchez V, López-Gil N, Cerviño A, Montés-Micó R. Comparison of partial coherence interferometry and ultrasound for anterior segment biometry. J Cataract Refract Surg. 2009;35:324–329. doi: 10.1016/j.jcrs.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Narváez J, Cherwek DH, Stulting RD, Waldron R, Zimmerman GJ, Wessels IE, Waring GO., III Comparing immersion ultrasound with partial coherence interferometry for intraocular lens power calculation. Ophthalmic Surg Lasers Imaging. 2008;39:30–34. doi: 10.3928/15428877-20080101-08. [DOI] [PubMed] [Google Scholar]
- 7.Németh J, Fekete O, Pesztenlehrer N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg. 2003;29:85–88. doi: 10.1016/s0886-3350(02)01500-6. [DOI] [PubMed] [Google Scholar]
- 8.Olsen T. Improved accuracy of intraocular lens power calculation with the Zeiss IOLMaster. [Accessed August 26, 2010];Acta Ophthalmol Scand. 2007 85:84–87. doi: 10.1111/j.1600-0420.2006.00774.x. Available at: http://www3.interscience.wiley.com/cgi-bin/fulltext/118515533/PDFSTART. [DOI] [PubMed] [Google Scholar]
- 9.Hussin HM, Spry PGD, Majid MA, Gouws P. Reliability and validity of the partial coherence interferometry for measurement of ocular axial length in children. Eye. 2006;20:1021–1024. doi: 10.1038/sj.eye.6702069. [DOI] [PubMed] [Google Scholar]
- 10.Holladay JT, Prager TC, Ruiz RS, Lewis JW, Rosenthal H. Improving the predictability of intraocular lens power calculations. [Accessed August 26, 2010];Arch Ophthalmol. 1986 104:539–541. doi: 10.1001/archopht.1986.01050160095020. Available at: http://archopht.ama-assn.org/cgi/reprint/104/4/539. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Fine IH, Hoffman RS, Coffman PG, Brown LK. Immersion A-scan compared with partial coherence interferometry; outcomes analysis. J Cataract Refract Surg. 2002;28:239–242. doi: 10.1016/s0886-3350(01)01259-7. [DOI] [PubMed] [Google Scholar]
- 12.Landers J, Goggin M. Comparison of refractive outcomes using immersion ultrasound biometry and IOLMaster biometry. Clin Exp Ophthalmol. 2009;37:566–569. doi: 10.1111/j.1442-9071.2009.02091.x. [DOI] [PubMed] [Google Scholar]
- 13.Carkeet A, Saw S-M, Gazzard G, Tang W, Tan DTH. Repeatability of IOLMaster biometry in children. Optom Vis Sci. 2004;81:829–834. doi: 10.1097/01.opx.0000145020.33250.c0. [DOI] [PubMed] [Google Scholar]