Abstract
Background
Retinoblastoma is the most common primary malignant intraocular neoplasm of childhood. The poor outcomes of patients with metastatic retinoblastoma have encouraged the search for new therapies. In the current study, the efficacy of combination therapy with calcitriol and cisplatin in athymic mice with subcutaneous Y-79 human retinoblastoma tumors was assessed.
Methods
60 athymic mice were subcutaneously injected with human Y79 retinoblastoma cells. Animals were randomized into four groups: group 1 - 50 μg of cisplatin; group 2 - 0.05 μg of calcitriol; group 3 - 0.05μg of calcitriol and 50 μg of cisplatin; group 4 - control. The cisplatin was administered once a week, and the calcitriol was given 5 times a week.
Results
There was a significant inhibition of tumor growth in animals treated with the combination therapy of calcitriol and cisplatin as compared to controls and cisplatin alone (p=0.0001 and p=0.0041 respectively). In terms of toxicity, serum calcium levels were increased, but there was no mortality and minimal nephrotoxicity in any of the groups.
Conclusion
The present study shows that cisplatin given in combination with calcitriol may be a viable multidrug therapy option in the treatment of high risk retinoblastoma.
Keywords: Calcitriol, Cisplatin, Retinoblastoma, Xenograft model
Introduction
Vitamin D compounds may be of potential usefulness in the treatment of human retinoblastoma based on its antitumor activity in Y-79 athymic mouse xenograft model.[1] The antineoplastic mechanism of action of vitamin D compounds used in models of human retinoblastoma has been demonstrated to be apoptosis due to increased expression of p53 and p21 [2], as well as inhibition of angiogenesis.[3] Furthermore, vitamin D compounds are non-mutagenic [4] and therefore would be particularly useful in the treatment of hereditary retinoblastoma where second malignant neoplasm is the leading cause of death in the U.S.[5] A major obstacle to their use in human studies has been drug-induced hypercalcemia.[6] However, the hypercalcemia-related toxic effects can be minimized by controlling the dose given, increasing hydration, and, when necessary, skipping doses in response to elevated serum calcium levels.[7, 8]
Cisplatin (cis-diamminedichloroplatinum) is widely used in various chemotherapy regimens for the treatment of retinoblastoma.[9] It acts by formation of covalent, bifunctional DNA adducts.[10] The number of cisplatin-DNA adducts in human subjects undergoing cisplatin-based chemotherapy increases as a function of platinum dose.[11] The DNA damage and cell transformation caused by cisplatin makes it a mutagenic and clastogenic agent, thereby acting as an initiator of carcinogenesis in laboratory animals.[12] In humans, cisplatin produces long-lasting, detectable platinol-DNA adducts and chromosomal aberrations which put patients at risk for development of second primary neoplasms.[12] Furthermore, nephrotoxicity, bone marrow suppression, and ototoxicity are the major side-effects of cisplatin. The nephrotoxic effect is dose-dependent, cumulative, and primarily affects the proximal tubules.[13] The dose limiting adverse effects of cisplatin have encouraged the search for new combination therapies. In the present study, we attempted to minimize the adverse effects of cisplatin by using a low dose (50 μg) in combination with calcitriol in various treatment regimens in the Y-79 murine xenograft model.
Materials and Methods
Cell Culture
Y79 cell line was grown as non-adherent cells at 37°C under 5% CO2 in IMDM medium with 2mM L-glutamine (Cambrex Biosciences, Walkersville, MD) supplemented with 25% (v/v) fetal bovine serum and 1% penicillin-streptomycin-amphotericin B (Sigma-Aldrich, St. Louis, MO).[14]
Tumor inoculation
Y-79 human retinoblastoma cells were counted with a hemocytometer using an isotonic trypan blue dye solution (Sigma) to verify cell viability. A Y-79 cell suspension was then mixed in a 1:1 ratio with Matrigel basement membrane matrix suspension (BD Biosciences) to a concentration of 5 × 106 cells per 0.50 ml. The mice were injected with 0.50 ml of this suspension subcutaneously in the dorsal right flank.[7] All the mice developed a palpable subcutaneous tumor by day 3.
Mice and treatment
Sixty 5- to 6-week-old athymic nude female mice were randomized three days after tumor inoculation into one of four treatment groups:
Group 1 - 50 μg of cisplatin in 0.25 ml of saline and 0.1 ml of vehicle (mineral oil)
Group 2 - 0.05 μg of calcitriol in 0.1 ml of vehicle and 0.25 ml saline
Group 3 - 0.05 μg of calcitriol in 0.1 ml of vehicle and 50 μg of cisplatin in 0.25 ml of saline
Group 4 - 0.1 ml vehicle and 0.25 ml saline (control)
This resulted in 4 groups of 15 animals each. To control for variance in initial tumor volumes, the mice were randomized according to estimated initial tumor volume. The mice were kept in conditions consistent with the University of Wisconsin-Madison Research Animals Resources Center (RARC) guidelines and were fed Purina Test Diet deficient in vitamin D and calcium (Purina Mills LLC, St. Louis, Missouri, USA).
Calcitriol was provided by Bone Care International, now a part of Genzyme Corp. (Cambridge, MA, USA), in a crystalline form, which was then dissolved with 100% ethanol for a stock solution of 2.98 mg/ml. This solution was diluted in mineral oil (vehicle) to concentrations of 0.05 μg of calcitriol in 0.1 ml of vehicle. All the dosing solutions were injected intraperitoneally. The calcitriol, vehicle, and saline were administered once daily from Monday to Friday (5 times) a week for 5 weeks, whereas the cisplatin was injected once a week for 5 weeks. Mice were weighed twice a week to monitor their health and dosing was withheld from mice that had a weight loss of more than 15% from baseline. During the treatment period, animals were euthanized under the following conditions: (1) the tumor became ulcerated; (2) the tumor exceeded the allowable size by the University of Wisconsin-Madison Research Animals Resources Center standards (greater than 20 mm in any dimension); (3) the animal did not regain lost body weight despite withholding treatment for more than 2 days; or (4) the animal appeared ill (e.g. dehydrated, cold, lethargic, or anorexic). All animals remaining at the end of 5 weeks were euthanized and underwent necropsy. The tumor, serum, and kidneys were collected at this time.
Tumor measurement/evaluation
Tumor sizes were quantified using methods employed in previous studies.[7] First, the tumor was measured in vivo with calipers, providing a means of monitoring both tumor volume and tumor growth during the study. Second, caliper measurement of the tumor was performed after it was removed from the mouse to provide a more accurate end-of-study tumor volume. Third, tumor weight was recorded after excision of the tumor. The tumor was then evaluated histologically with hematoxylin-eosin stain for differentiation, necrosis, and calcification.
Toxicity
Toxicity was evaluated as previously described.[7] The parameters examined included mortality, body weight change, serum calcium level, and kidney calcification and necrosis. At necropsy, serum was drawn and sent for quantitation of total calcium. Both kidneys of 4 mice randomly selected from each group were evaluated histologically with hematoxylin-eosin and von Kossa stains for evidence of calcification and necrosis.
Statistical analysis
Serum calcium level, change in body weight, and final tumor volume were compared by t tests. The effect of treatment dose on tumor volume was assessed using analysis of variance. Tumor volume was transformed to the cube root scale to obtain approximately constant variance. All significant tests for effect of treatment dose were followed by pair-wise analyses to assess differences between specific treatment groups.
Results
Tumor measurements/evaluation
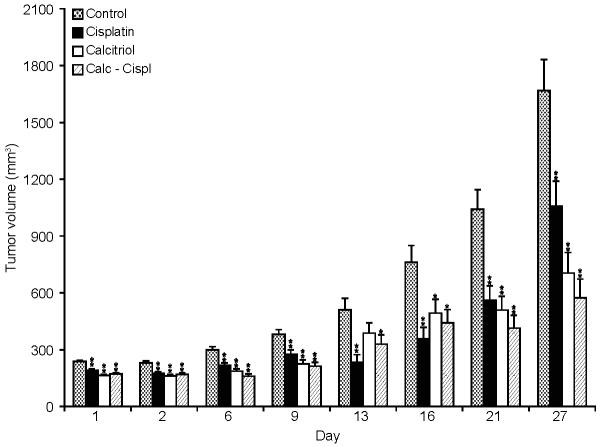
All the animals in each group developed palpable tumors. The effect of calcitriol and cisplatin on tumor growth in various groups was measured by end tumor volume (Table 1). After 5 weeks of treatment there was a significant inhibition of tumor growth in animals treated with the combination therapy of calcitriol and cisplatin (group 3) as compared to controls (p<0.0001). Tumor volume was approximately 65% less as compared to the control group. Further subset analysis revealed that the mean end tumor volume was significantly less after combination therapy with calcitriol and cisplatin (group 3) as compared to cisplatin alone (group 1; p=0.0017). No statistical significance was found between the combination therapy with calcitriol and cisplatin (group 3) and calcitriol alone (group 2; p=0.4165). Growth kinetic data show that significant differences between the treated and untreated tumors arose soon after initiation of drug treatment (Figure 1). However, no regression was observed. Histopathologic evaluation of these tumors did not show any differences in differentiation, necrosis, or calcification among the groups. A quantitative analysis of the differentiation, necrosis, or calcification in the tumors in various groups was not performed.
Table 1.
End tumor volumes after 5 weeks of treatment with cisplatin, calcitriol, combination therapy and control in Y-79 retinoblastoma xenograft mice
| Tumor size (mm3) | Standard error | p-value* | |
|---|---|---|---|
| Cisplatin/calcitriol | 577.44 | 102.52 | <0.0001 |
| 0.05 μg calcitriol | 696.89 | 112.22 | <0.0001 |
| 50 μg cisplatin | 1125.8 | 141.49 | <0.0227 |
| Control | 1626.87 | 169.24 |
p value when compared to the control group
Figure 1.
In vivo tumor volumes (mm3) at various time points (day) during 5 weeks of treatment with cisplatin, calcitriol, combination therapy and control in Y-79 retinoblastoma xenograft mice. There was a significant inhibition of tumor growth in animals treated with the combination therapy of calcitriol and cisplatin as compared to controls (p=0.0001). ** refer to a p value of <0.005 and * refer to a p value of 0.005-0.05.
Toxicity
The toxicity was evaluated by its effects on mortality, body weight change, serum calcium and kidney calcification (Table 2, and 3). There was no mortality in any of the groups. There was net weight gain in the cisplatin and control group, whereas net weight loss in the calcitriol and combination therapy group (Table 2). There was no evidence of weight loss greater than 15% from baseline in any of the groups, and hence no doses were held. The weight loss in the calcitriol and combination therapy group was statistically significant as compared to other groups. Mean serum calcium levels in the various groups are listed in Table 2. Differences in serum calcium levels were highly significant between the cisplatin and combination therapy group (p=0.0001), but minimally significant between the calcitriol and combination therapy group (p=0.0218). There was no significant difference in serum calcium levels between the cisplatin and control group (p=0.5803). The degree of kidney calcification was similar in the calcitriol and combination therapy group and ranged from no calcification to low-grade calcification (Table 3). There was absence of kidney calcification in the cisplatin and control group.
Table 2.
Mortality, body weight change, and serum calcium levels in Y-79 retinoblastoma xenograft mice after 5 weeks of treatment
| Group | Mortality | Number with weight loss |
Mean weight change (%) |
Mean serum calcium (mg/dl) |
|---|---|---|---|---|
| Cisplatin | 0 | 0 | +12.6 | 9.18±0.15 |
| Calcitriol | 0 | 10 | −10.4 | 11.14±0.15 (p<0.0001)* |
| Combination therapy | 0 | 15 | −11.5 | 11.68±0.15 (p<0.0001)* |
| Control | 0 | 1 | +9.5 | 9.30±0.15 |
Significant different from cisplatin group as well as control group. There is also a significant difference between the calcitriol and combination therapy groups (p=0.0218)
Table 3.
Kidney calcification as measure of toxicity in Y-79 retinoblastoma xenograft mice after 5 weeks of treatment
| Group | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Cisplatin | 15 | 0 | 0 | 0 |
| Calcitriol | 5 | 10 | 0 | 0 |
| Combination therapy | 2 | 13 | 0 | 0 |
| Control | 15 | 0 | 0 | 0 |
Discussion
When detected early, retinoblastoma confined to the eye can be successfully managed with various treatment modalities such as enucleation, chemotherapy, external beam radiation therapy, plaque brachytherapy, cryotherapy, and laser photocoagulation.[15] However, extraocular and metastatic disease can be lethal and is treated with high dose chemotherapy with autologous stem cell rescue (ASCR) and radiation therapy.[16] The various chemotherapeutic agents being used include vincristine, doxorubicin, cyclophosphamide, cisplatin, carboplatin, and etoposide.[17] Despite evidence of long-term event free survival, primary second tumors are the greatest cause of death in these patients in the U.S. particularly in bilateral heritable form of retinoblastoma.[18] The Rb1 mutation present in all cells of a patient with hereditary retinoblastoma predisposes them to developing other non-ocular malignant tumors.[19] The cumulative incidence of these additional tumors is around 1% per year and radiation therapy greatly increases the risk, particularly if administered before the age of 1 year.[20] Chemotherapy can be mutagenic and is also linked to an increased risk of second primary cancers in treated patients.
Our prior studies with calcitriol and other vitamin D analogs revealed consistent inhibition of tumor growth by more than 50 % compared with controls.[1, 6, 7] In the case of transgenic mice, animals were observed in the treatment groups with total regression of tumors.[8] The currently utilized chemoreduction protocols, which include carboplatin, etopiside, and vincristine has not been evaluated by us in comparison with calcitriol in experimental models. However, the mechanism of action of calcitriol, which differs from that of existing retinoblastoma chemotherapeutic agents [21], and the fact that they are non-mutagenic agents [4], makes these compounds a suitable choice in multi-drug therapy. The results of the present study are consistent with our prior results and demonstrate potent anti-tumor activity of calcitriol in the Y-79 xenograft model of human retinoblastoma. Furthermore, the combination of calcitriol with cisplatin results in a greater inhibition of tumor growth as compared to controls and cisplatin alone. At the doses studied, the antitumor activity of calcitriol alone is not statistically different from the combination of calcitriol with cisplatin. This suggests that in clinical situations it may be possible to lower the dose of cisplatin with combined treatment with calcitriol, while maintaining a similar level of tumor inhibition. Cisplatin has been shown to enhance calcitriol-induced apoptotic signaling and this may be responsible for increased cytotoxicity of the combination therapy.[22]
The toxicity of the combination of calcitriol with cisplatin was relatively low with no deaths in this group. There was statistically significant weight loss and elevation in serum calcium levels in the combination therapy and calcitriol group. However, kidney calcification was low grade in both combination therapy and calcitriol groups. Thus, combination therapy may be a viable option in human clinical trials to decrease the dose of cisplatin. In view of the concerns of nephrotoxicity with calcitriol, serum creatinine levels must be evaluated at regular intervals during human clinical trials. It was not possible to obtain regular serum creatinine levels in the experimental model.
In conclusion, we believe these laboratory findings have important clinical implications. The addition of calcitriol may allow for a decreased dosage of cisplatin which will potentially reduce acute and long-term toxic effects while maintaining a strong anti-tumor effect.
Acknowledgements
The research reported in this paper was funded by NIH Grant R01-EY001917 and a Core Grant for Vision Research EY016665 with supplemental funding from Research to Prevent Blindness. The authors thank Brittany Radke, Elaina Gates, Janice Lokken, and Joshua Harder for technical assistance.
Footnotes
Presentations
Presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting in Fort Lauderdale, FL in 2007
Competing Interest: None
Licence for Publication: The Corresponding Author grants on behalf of all authors an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in BJO and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in the licence.
References
- 1.Albert DM, Nickells RW, Gamm DM, et al. Vitamin D analogs, a new treatment for retinoblastoma: The first Ellsworth Lecture. Ophthalmic Genet. 2002;23(3):137–56. doi: 10.1076/opge.23.3.137.7883. [DOI] [PubMed] [Google Scholar]
- 2.Audo I, Darjatmoko SR, Schlamp CL, et al. Vitamin D analogues increase p53, p21, and apoptosis in a xenograft model of human retinoblastoma. Invest Ophthalmol Vis Sci. 2003;44(10):4192–9. doi: 10.1167/iovs.02-1198. [DOI] [PubMed] [Google Scholar]
- 3.Shokravi MT, Marcus DM, Alroy J, et al. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci. 1995;36(1):83–7. [PubMed] [Google Scholar]
- 4.Sarkar A, Saha K, Basak R, et al. Anticlastogenic potential of 1alpha,25-dihydroxyvitamin D3 in murine lymphoma. Cancer Lett. 2000;150(1):1–13. doi: 10.1016/s0304-3835(99)00327-4. [DOI] [PubMed] [Google Scholar]
- 5.Acquaviva A, Ciccolallo L, Rondelli R, et al. Mortality from second tumour among long-term survivors of retinoblastoma: a retrospective analysis of the Italian retinoblastoma registry. Oncogene. 2006;25(38):5350–7. doi: 10.1038/sj.onc.1209786. [DOI] [PubMed] [Google Scholar]
- 6.Albert DM, Kumar A, Strugnell SA, et al. Effectiveness of vitamin D analogues in treating large tumors and during prolonged use in murine retinoblastoma models. Arch Ophthalmol. 2004;122(9):1357–62. doi: 10.1001/archopht.122.9.1357. [DOI] [PubMed] [Google Scholar]
- 7.Grostern RJ, Bryar PJ, Zimbrick ML, et al. Toxicity and dose-response studies of 1alpha-hydroxyvitamin D2 in a retinoblastoma xenograft model. Arch Ophthalmol. 2002;120(5):607–12. doi: 10.1001/archopht.120.5.607. [DOI] [PubMed] [Google Scholar]
- 8.Dawson DJ, Gleiser J, Zimbric ML, et al. Toxicity and dose-response studies of 1-alpha hydroxyvitamin D2 in LH-beta-tag transgenic mice. Ophthalmology. 2003;110(4):835–9. doi: 10.1016/S0161-6420(02)01934-6. [DOI] [PubMed] [Google Scholar]
- 9.Makimoto A. Results of treatment of retinoblastoma that has infiltrated the optic nerve, is recurrent, or has metastasized outside the eyeball. Int J Clin Oncol. 2004;9(1):7–12. doi: 10.1007/s10147-003-0364-2. [DOI] [PubMed] [Google Scholar]
- 10.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 11.Silva MJ, Costa P, Dias A, et al. Comparative analysis of the mutagenic activity of oxaliplatin and cisplatin in the Hprt gene of CHO cells. Environ Mol Mutagen. 2005;46(2):104–15. doi: 10.1002/em.20138. [DOI] [PubMed] [Google Scholar]
- 12.Greene MH. Is cisplatin a human carcinogen? J Natl Cancer Inst. 1992;84(5):306–12. doi: 10.1093/jnci/84.5.306. [DOI] [PubMed] [Google Scholar]
- 13.Yao X, Panichpisal K, Kurtzman N, et al. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334(2):115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 14.Reid TW, Albert DM, Rabson AS, et al. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974 Aug;53(2):347–60. doi: 10.1093/jnci/53.2.347. [DOI] [PubMed] [Google Scholar]
- 15.Gombos DS, Chevez-Barrios AP. Current treatment and management of retinoblastoma. Curr Oncol Rep. 2007;9(6):453–8. doi: 10.1007/s11912-007-0063-7. [DOI] [PubMed] [Google Scholar]
- 16.Gunduz K, Müftüoglu O, Günalp I, et al. Metastatic retinoblastoma clinical features, treatment, and prognosis. Ophthalmology. 2006;113(9):1558–66. doi: 10.1016/j.ophtha.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Chintagumpala M, Chevez-Barrios P, Paysse EA, et al. Retinoblastoma: review of current management. Oncologist. 2007;12(10):1237–46. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 18.Mohney BG, Robertson DM, Schomberg PJ, et al. Second nonocular tumors in survivors of heritable retinoblastoma and prior radiation therapy. Am J Ophthalmol. 1998;126(2):269–77. doi: 10.1016/s0002-9394(98)00146-9. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher O, Easton D, Anderson K, et al. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96(5):357–63. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 20.Schlienger P, Campana F, Vilcoq JR, et al. Nonocular second primary tumors after retinoblastoma: retrospective study of 111 patients treated by electron beam radiotherapy with or without TEM. Am J Clin Oncol. 2004;27(4):411–9. doi: 10.1097/01.coc.0000128861.46357.ee. [DOI] [PubMed] [Google Scholar]
- 21.Albert DM, Plum LA, Yang W, et al. Responsiveness of human retinoblastoma and neuroblastoma models to a non-calcemic 19-nor Vitamin D analog. J Steroid Biochem Mol Biol. 2005;97(1-2):165–72. doi: 10.1016/j.jsbmb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Hershberger PA, McGuire TF, Yu WD, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1(10):821–9. [PubMed] [Google Scholar]