Tacrolimus (Prograf™-FK506) has been commercially available since June, 1994, for use as an immunosuppressive agent. It has thus far been approved only for patients undergoing liver transplantation,1 although it has been used both clinically and experimentally in virtually all other organ or cell transplant settings.2–11 It has been used successfully in renal transplant recipients, both as a primary immunosuppressive agent12–25 and as a rescue agent,25–27 and several reports have suggested that it is more efficacious than cyclosporine-based therapy.17,18,23
In this report, we will present an update of our experience with tacrolimus as the primary immunosuppressive agent, and as a rescue agent, in renal transplant recipients. In addition, we will discuss the use of tacrolimus in kidney/pancreas transplantation, and in our program of bone marrow augmentation. With increasing follow-up, it is becoming clear that tacrolimus is a superior immunosuppressive agent.
PRIMARY THERAPY
Adults
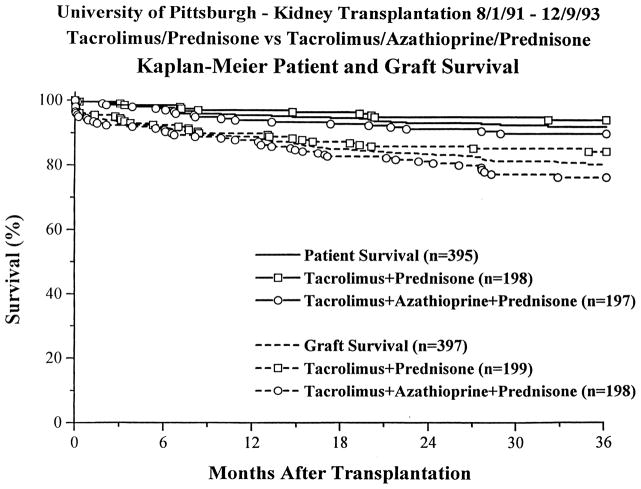
After an initial experience demonstrated comparable efficacy between tacrolimus and cyclosporine, but with an improved secondary profile in tacrolimus-treated patients (lower steroid and antihypertensive medication requirements, and lower cholesterol levels),12–14 a prospective, randomized trial was begun in August, 1991, comparing two tacrolimus-based regimens, with and without azathioprine.15–18 Induction antilymphocyte therapy was not used. 397 cases were entered into this trial, which ended in December, 1993. Analysis was by intention-to-treat, with no patients censored. With a mean follow-up of 33 ± 10 months, overall 1 and 3 actuarial patient survival was 95% and 92%, with no difference between the two groups (Figure 1). Overall 1 and 3 actuarial graft survival was 89% and 80% (Table 1), with a significantly worse 3 year outcome in the triple therapy than in the double therapy group, 76% versus 84% (p =.031). When first cadaver grafts were analyzed, overall 1 and 3 actuarial graft survival was 91% and 82%, with no difference between the two groups. Subgroup analysis revealed that patients with delayed graft function or steroid-resistant rejection were at increased risk for graft loss. In addition, recipients of kidneys from donors over 60 years of age had worse outcomes (Table 2). Black recipients, retransplant recipients, and sensitized patients did not have statistically worse outcomes, nor did recipients of female donor or pediatric enbloc kidneys.
Figure 1.
Renal Transplantation Under Tacrolimus – Patient &. Graft Survival in Adults.
Table 1.
Actuarial Survival N=397
| FK/Pred | FK/Aza /Pred | Overall | ||
|---|---|---|---|---|
| Patient | ||||
| 1 year | 97% | 94% | 95% | |
| 2 year | 95% | 91% | 93% | |
| 3 year | 94% | 90% | 92% | |
| p=NS | ||||
| Graft | ||||
| 1 year | 90% | 88% | 89% | |
| 2 year | 86% | 81% | 84% | |
| 3 year | 84% | 76% | 80% | |
| p=.031 | ||||
| Graft-First Cadaver | ||||
| 1 year | 90% | 91% | 91% | |
| 2 year | 85% | 83% | 84% | |
| 3 year | 84% | 80% | 82% | |
| p=NS | ||||
Table 2.
Actuarial Graft Survival - Subgroup Analysis
| 1 year | 2 year | 3 year | p | |||||
|---|---|---|---|---|---|---|---|---|
| Immediate Function | 95% | 90% | 86% | |||||
| Delayed Graft Function | 78% | 72% | 69% | 0.00001 | ||||
| No Rejection | 91% | 87% | 87% | NS | 0.00001 | |||
| Rejection – Steroids | 92% | 86% | 80% | |||||
| Rejection – 0KT3/ATG | 71% | 57% | 53% | |||||
| Donor | ≤ 60 years | 91% | 86% | 83% | 0.0001 | |||
| >60 years | 74% | 65% | 62% | |||||
| En Bloc | 84% | 84% | 84% | NS | ||||
| Other Cadaver | 89% | 83% | 79% | |||||
| Female Donor | 90% | 83% | 80% | NS | ||||
| Male Donor | 88% | 84% | 81% | NS | ||||
| Black Recipients | 88% | 76% | 70% | NS | ||||
| Non-Black | 89% | 85% | 82% | |||||
| First Transplant | 91% | 85% | 82% | NS | ||||
| Retransplant | 83% | 80% | 75% | NS | ||||
| PRA <40% | 89% | 84% | 81% | NS | ||||
| PRA ≥40% | 87% | 83% | 78% | NS | ||||
At most recent follow-up, the mean serum creatinine was 1.9 ± 1.5 mg/dl, and 69% of successfully transplanted patients had been taken off steroids; 38% were off antihypertensive medications (Table 3).
Table 3.
| FK/Pred | FK/Aza/Pred | Overall | |
|---|---|---|---|
| S. Creatinine (mg/dl) | 1.9±1.0 | 1.9±1.8 | 1.9 ± 1.5 |
| Off Steroids | 70% | 68% | 69% |
| Off Antihypertensive Medications | 39% | 36% | 38% |
A half-life analysis was performed, and the projected half-life for all cadaver recipients was 11.4 ± 2.0 years; for first cadaver recipients, it was 11.9 ± 2.5 years.
These data have continued to demonstrate the efficacy and superiority of tacrolimus in adult renal transplant recipients, but have called into question the utility of azathioprine as a third agent. A subsequent randomized trial assessing the efficacy of one week of low dose cyclophosphamide is currently being analyzed, and a new randomized trial of tacrolimus and steroids with and without mycophenolate mofetil28 is currently in progress.
Pediatrics
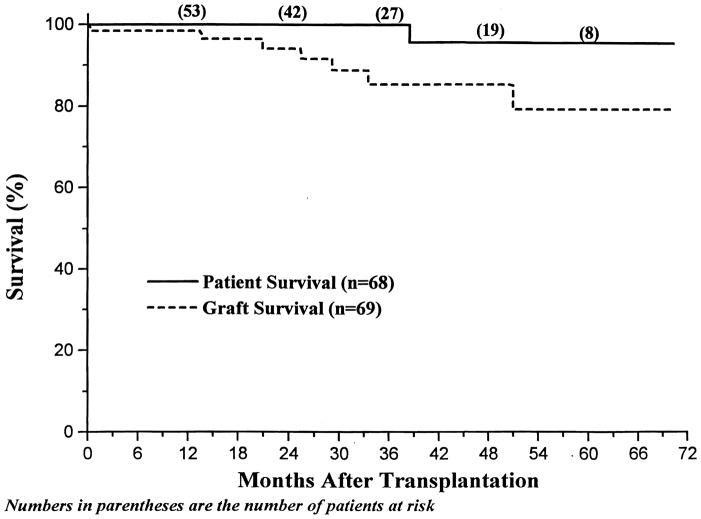
Between December 17, 1989, and June 30, 1995, sixty-eight pediatric patients underwent 69 renal transplantations and received tacrolimus-based therapy, again without induction antilymphocyte therapy (this analysis excludes children undergoing concomitant or previous liver transplantation). The mean age was 10.3 ± 5.0 years (range 0.7 – 17.5); 17 (24.6%) children were undergoing retransplantation, and 6 (8.7%) had a PRA of 40% or higher. 39 (57%) transplants were with cadaveric kidneys, and 30 (43%) were with living donors. With a mean follow-up of 32 ± 20 months, overall 1 and 4 year actuarial patient survival was 100% and 96% (Figure 2). The one patient who died lost her kidney to noncompliance and died on dialysis 17 months after allograft nephrectomy. Overall 1 and 4 year actuarial graft survival was 99% and 85%.
Figure 2.
Pediatric Renal Transplantation Under Tacrolimus - Patient and Graft Survival
The mean serum creatinine was 1.2 ± 0.8 mg/dl (Table 4). 73% of successfully transplanted children have been weaned off prednisone. Growth in the pre-adolescent children off steroids has been particularly gratifying.
Table 4.
Pediatric Recipients - Actuarial Survival n=69
| 1 year | 4 year | |
|---|---|---|
| Patient | 100% | 96% |
| Graft | 99% | 85% |
| S. Creatinine (mg/dl) | 1.2 ± 0.8 | |
| Off Steroids | 73% |
The incidence of rejection was 49%, and antilymphocyte therapy was required in 6% of children. There is a suggestion that the incidence of rejection has been less over the past 18 months, as more experience with tacrolimus has been acquired.
An early concern with our pediatric patients receiving tacrolimus was the incidence of Epstein-Barr virus associated post-transplant lymphoproliferative disorder (PTLD) 24,25,29 Between December 17, 1989, and December 31, 1992, five (17%) cases were seen in the first 29 recipients, and although no child died or lost his/her kidney, temporary sensation of immunosuppression and a prolonged course of antiviral therapy with gancyclovir were required. Beginning in 1993, a conscious change in immunosuppressive management was implemented, with aggressive tapering of both tacrolimus and steroids beginning 6–8 weeks after transplantation. The incidence of PTLD in the 40 patients transplanted since January 1, 1993, has decreased to 5% (2 cases); in both cases, the PTLD resolved with medical therapy as outlined above. There has also been one case of a late PTLD showing a Burkitt’s lymphoma-like histology 46 months after transplantation, after a 50% increase in the tacrolimus dosage was instituted. This patient ressponded to aggressive chemotherapy.
RESCUE THERAPY
Prior to FDA approval, a significant experience was acquired in Pittsburgh with tacrolimus as a rescue agent for renal transplant recipients who had failed conventional therapy.25–27 Most of these patients were transplanted at other centers. Over 200 patients with refractory acute rejection were eventually converted, and while a detailed analysis of this group is still in progress, the overall success rate was comparable to the 74% originally reported in the first 77 patients. The previous analysis had indicated that, even in cases of patients who arrived on dialysis, successful rescue was possible in 50% of cases.27
KIDNEY/PANCREAS TRANSPLANTATION
Between July 4, 1994, and September 30, 1995, 43 simultaneous kidney/pancreas transplantations were performed under tacrolimus-based immunosuppression. As in the kidney alone patients, induction antilymphocyte therapy was not given. With a median follow-up of 6 months, all patients are alive, with 95% renal allograft survival and 79% pancreas allograft survival (Table 5). Steroid tapering has been possible, and 8 (19%) patients have had steroids completely withdrawn.
Table 5.
Kidney - Pancreas Transplantation n=43
| Patient Survival | 100% |
| Renal Allograft Survival | 95% |
| Pancreas Allograft Survival | 79% |
KIDNEY/BONE MARROW
Based on the observation that extremely long-term graft survival was associated with systemic microchimerism, 30–33 a program of combined, simultaneous kidney/bone marrow transplantation was begun in December, 1992, with the goal of augmenting chimerism.34–37 Thirty-six cases were transplanted by October 31, 1994.38 Patients received 3–5 × 108 unmodified bone marrow cells/kg at the conclusion of the kidney transplant. Immunosuppression was with tacrolimus and steroids. Seven patients receiving a kidney/pancreas transplant were also given azathioprine. Radiation, cytoreduction, or induction antilymphocyte therapy were not given. With a mean follow-up of 11.1 ± 5.8 months, all patients were alive and 33 (92%) patients had functioning renal allografts (Table 6). A group of 20 patients who did not received bone marrow were studied as controls. Patient and graft survival, quality of allograft function, and the incidence of rejection, delayed function, or cytomegalovirus were not different between the two groups. Graft versus host disease was not seen in any patient. Chimerism was detected in 97% of the kidney/bone marrow group, and 64% of the control group (p=.02). These early results suggest that bone marrow augmentation is safe and is associated with reasonable patient and graft survival and routine augmentation of chimerism. The long-term consequences of the increased chimerism await further follow-up.
Table 6.
Kidney/Bone Marrow Transplantation
| n=3 6 K/BM | n=20 Control | p | |
|---|---|---|---|
| Patient Survival | 100% | 90% | NS |
| Graft Survival | 92% | 85% | NS |
| Chimerism | 97% | 64% | 0.02 |
DISCUSSION
As more experience has been acquired with tacrolimus in renal transplant recipients, it is becoming increasingly clear that better outcomes are being seen, both in the form of higher short-term graft survival rates, longer projected half-lives, and steroid withdrawal in a majority of recipients. These outcomes have lead to the routine use of tacrolimus in our renal and pancreas transplant recipients. Current strategies call for a pre-operative oral dose of 0.15 mg/kg and a continuous intravenous infusion of 0.05–0.075 mg/kg/24 hours, beginning in the recovery room. Oral tacrolimus is begun at a dose of 0.15 mg/kg twice daily, after which the intravenous tacrolimus is quickly tapered. The target levels vary over time; we aim for levels of 20-25 ng/ml (whole blood IMX) for the first 2 weeks after transplantation, tapering down to 15–20 ng/ml by 1 month, 10–15 ng/ml by 3 months, and 5–12 ng/ml chronically, although many long-term patients do well with chronic levels between 3–5 ng/ml. In the ideal circumstance, steroids are decreased from 20 to 15 mg/d 3 weeks after transplantation, and then by 2.5 mg decrements to 10 mg/d by 2 months. Tapering by 2.5 mg/month continues until steroids are discontinued altogether. The doses are proportionally lower for pediatric recipients.
The toxicities of tacrolimus are well-known and include nephrotoxicity, neurotoxicity, and diabetogenicity.39–45 They are similar to those seen with cyclosporine46–51 and are largely reversible with dosage reduction and steroid tapering. The infectious complications associated with tacrolimus also appear to be qualitatively and quantitatively similar to those seen with cyclosporine.14,17
A continuing problem with tacrolimus is a moderately high incidence of rejection of approximately 50%. While most rejections are steroid-responsive, those requiring antilymphocyte therapy are associated with relatively poor outcomes. It is hoped that the addition of mycophenolate mofetil as a third agent will be associated with less rejection and equally good or perhaps even better graft survival. For now, however, tacrolimus appears to represent an advance in immunosuppression for renal transplant recipients, and should be considered to be the agent of choice.
SUMMARY
Tacrolimus is a superior immunosuppressive agent in patients undergoing renal transplantation. In adults, the 1 and 3 year actuarial patient survival was 95% and 92%, and the 1 and 3 year actuarial graft survival was 89% and 80%. For first cadaver kidneys, the 1 and 3 year actuarial graft survival was 91% and 82%, with a projected half-life of 11.9 years. 69% of successfully transplanted patients were able to be weaned off steroids.
In pediatric patients, the 1 and 4 year actuarial patient survival was 100% and 96%, and the 1 and 4 year actuarial graft survival was 99% and 85%. 73% of successfully transplanted children were weaned off steroids.
Tacrolimus was also useful as a rescue agent, with an initial success rate of 74%.
Tacrolimus has been used successfully in kidney/pancreas transplantation, with 100% patient, 95% kidney, and 79% pancreas graft survival.
Tacrolimus should be considered to be the immunosuppressive agent of choice in renal transplantation.
References
- 1.Physicians’ Desk Reference®. Montvale: Medical Economics Data Production Company, Prograf™ (tacrolimus); 1995. p. 1050. [Google Scholar]
- 2.Starzl TE, Todo S, Fung J, et al. FK506 for human liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Starzl TE. Liver, kidney and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung JJ, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK506 vs. cyclosporine. Trans Proc. 1991;23(6):2977. [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Fung JJ, Starzl TE, et al. Single-center experience with primary orthotopic liver transplantation under FK506 immunosuppression. Ann Surg. 1994;220:297. doi: 10.1097/00000658-199409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage JM, Kormos RL, Griffith BP, et al. The clinical trial of FK506 as primary and rescue immunosuppression in adult cardiac transplantation. Trans Proc. 1991;23(6):3054. [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith BP, Brando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57(6 ):848. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without the colon. Transplantation. 1994;57(6):840. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzakis A, Abu-Elmagd K, Fung JJ, et al. FK506 rescue in chronic graft versus host disease after bone marrow transplantation. Trans Proc. 1991;23(6):3225. [PMC free article] [PubMed] [Google Scholar]
- 10.Masaoka T, Shibata H, Kakishita E, et al. Phase II study of FK506 for allogeneic bone marrow transplantation. Transplant Proc. 1991;23(6 ):3228. [PubMed] [Google Scholar]
- 11.Tzakis AG, Ricordi C, Alejandro R, et al. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336:402. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Fung JJ, Jordan M, et al. Kidney transplantation under FK506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro R, Jordan M, Fung J, McCauley J, Johnston J, Iwaki Y, Tzakis A, Hakala T, Todo S, Starzl TE. Kidney transplantation under FK506 immunosuppression. Trans Proc. 1991;23:920. [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro R, Jordan ML, Scantlebury V, et al. FK506 in clinical kidney transplantation. Trans Proc. 1991;23:3065. [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro R, Jordan M, Scantlebury V, et al. Randomized trial of FK506/prednisone vs FK506/azathioprine/prednisone after renal transplantation: preliminary report. Trans Proc. 1993;25:669. [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro R, Jordan M, Scantlebury V, et al. A prospective, randomized trial of FK506 in renal transplantation–a comparison between double and triple drug therapy. Clin Trans. 1994;8:508. [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Fung JJ, McCauley J, Randhawa P, Demetris AJ, Irish W, Mitchell S, Hakala TR, Simmons RL, Starzl TE. A prospective, randomized trial of FK506-based immunosuppression after renal transplantation. Transplantation. 1995;59:485–490. doi: 10.1097/00007890-199559040-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro R, Jordan ML, Scantlebury VP, Vivas C, Fung JJ, McCauley J, Randhawa P, Demetris AJ, Irish W, Jain A, Mitchell S, Hakala TR, Simmons RL, Starzl TE. A prospective, randomized trial of FK506/prednisone vs FK506/azathioprine/prednisone in renal transplant patients. Trans Proc. 1995;27 (1 ):814–817. [PMC free article] [PubMed] [Google Scholar]
- 19.Ochiai T, Ishibashi M, Fukao K, Takahashi K, Endo T, Yokoyama I, Uchida K, Ohshima S, Takahara S, Morozumi K, Yamaguchi Y, Dyo M, Sonoda T, Takagi H, Ota K, Iwasaki Y the Japanese FK506 Study Group. Japanese Multicenter Studies of FK506 in Renal Transplantation. Trans Proc. 1995;22(1):50. [PubMed] [Google Scholar]
- 20.Japanese FK506 Study Group. Yokoyama I, Uchida K, Fukao K, Ochiai K, Takahara S, Iwasaki Y, Ota K, Takagi H, Sonoda T. FK506: long-term study in kidney transplantation. Trans Proc. 1995;22(1):818. [PubMed] [Google Scholar]
- 21.Japanese FK506 Study Group. Ochia K, Fukao K, Takahashi K, Endo T, Oshima S, Uchida K, Yokoyama I, Ishibashi M, Takahara S, Iwasake Y, Ota K, Takai H, Sonoda T. Phase II Study of FK506 in Kidney Transplantation. Trans Proc. 1995;22(1):829. [PubMed] [Google Scholar]
- 22.Laskow DA, Vincenti F, Neylan J, Mendez R, Matas A. Phase II FK506 Multicenter Concentration Control Study. One-year follow-up. Trans Proc. 1995;22(1):809. [PubMed] [Google Scholar]
- 23.Gjertson DW, Cecka JM, Terasaki PI. The relative effects of FK506 and cyclosporine on short-and long-term kidney graft survival. Transplantation. 1995 doi: 10.1097/00007890-199560120-00002. (In Press) [DOI] [PubMed] [Google Scholar]
- 24.Scantlebury V, Shapiro R, Tzakis A, et al. Pediatric kidney transplantation at the University of Pittsburgh. Trans Proc. 1994;26(1):46. [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro R, Scantlebury VP, Jordan ML, Vivas C, Tzakis AG, Ellis D, Gilboa N, Hopp L, McCauley J, Irish W, Mitchell S, Hakala TR, Simmons RL, Starzl TE. FK506 in pediatric kidney transplantation-primary and rescue experience. Pediatric Nephrology. 1995;9:S43–S48. doi: 10.1007/BF00867683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan ML, Shapiro R, Jensen C, et al. FK506 conversion of renal allografts failing cyclosporine immunosuppression. Trans Proc. 1991;23:3078. [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan M, Shapiro R, Vivas C, et al. FK506 rescue for resistant rejection of renalallografts under primary cyclosporine immunosuppression. Transplantation. 1994;57(6):860. doi: 10.1097/00007890-199403270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sollinger HW for the U.S. Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995;60(3):225–232. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro R, Tzakis A, Scantlebury V, Jordan M, Vivas C, Ellis D, Gilboa N, Irish W, Hopp L, Reyes J, Hakala T, Simmons RL, Starzl TE. Improving results of pediatric kidney transplantation. J Amer Col of Surg. 1994;179(4):1424–432. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donorspecific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Cell chimerism permitted by immunosuppressive drugs is the basis of organ transplant acceptance and tolerance. Immunol Today. 1993;14:326–332. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontes P, Abdul R, Demetris AJ, Zeevi A, Massimo T, Carroll P, Rybka W, Ricordi C, Dodson F, Shapiro R, Tzakis A, Todo S, Abu-Elmagd K, Jordan M, Fung J, Starzl TE. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao AS, Fontes P, Zeevi A, Trucco M, Shapiro R, Demetris AJ, Tzakis AG, Carroll PB, Rudert WA, Dodson FS, Rybka WB, Scantlebury V, Rohal S, Ricordi C, Fung JJ, Starzl TE. Combined bone marrow and whole organ transplantation from the same donor. Trans Proc. 1994;26 (6 ):3377–3378. [PMC free article] [PubMed] [Google Scholar]
- 36.Rao AS, Fontes P, Zeevi A, Trucco M, Dodson FS, Rybka WB, Shapiro R, Jordan M, Phan SM, Rilo HL, Seskey T, Todo S, Scantlebury V, Vivas C, Demetris AJ, Fung JJ, Starzl TE. Augmentation of chimerism in whole organ recipients by simultaneous infusion of donor bone marrow cells. Trans Proc. 1995;27(1):210–212. [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro R, Rao AS, Fontes P, Jordan ML, Scantlebury VP, Vivas C, Demetris AJ, Zeevi A, Rybka W, Carroll P, Trucco M, Starzl TE. Combined kidney/bone marrow transplantation – Evidence for augmentation of chimerism. Transplantation. 1995;59(2):306–309. doi: 10.1097/00007890-199501000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro R, Rao AS, Fontes P, Zeevi A, Jordan M, Scantlebury V, Vivas C, Gritsch HA, Corry RJ, Egidi MF, Rugeles MT, Rilo H, Abdelouahab A, Demetris AJ, Rosner G, Trucco M, Rybka W, Irish W, Fung JJ, Starzl TE. Combined simultaneous kidney/bone marrow transplantation. Transplantation. 1995 (In Press) [PMC free article] [PubMed] [Google Scholar]
- 39.McCauley J, Takaya S, Fung J, et al. The question of FK506 nephrotoxicity after liver transplantation. Trans Proc. 1991;23(1):1444. [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Selected topics on FK506, with special references to rescue of extrahepatic whole organ grafts, transplantation of “Forbidden Organs,” side effects, mechanisms, and practical pharmacokinetics. Trans Proc. 1991;23(1):914. [PubMed] [Google Scholar]
- 41.Starzl TE. FK506 versus cyclosporine. Trans Proc. 1993;25(1):511. [PMC free article] [PubMed] [Google Scholar]
- 42.Demetris AJ, Banner B, Fung JJ, et al. Histopathology of human renal allograft function under FK506: A comparison with cyclosporine. Trans Proc. 1991;23:944. [PMC free article] [PubMed] [Google Scholar]
- 43.Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506: Clinical significance and comparison with cyclosporine. Am J Surg Pathol. 1993;17(1 ):60. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro R, Fung JJ, Jain AB, et al. The side effects of FK506 in humans. Trans Proc. 1990;22(1 Suppl 1):35. [PMC free article] [PubMed] [Google Scholar]
- 45.Scantlebury V, Shapiro R, Fung JJ, et al. New onset of diabetes in FK506 vs. cyclosporine-treated kidney transplant recipients. Trans Proc. 1991;23(6):3169. [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimura N, Nakai I, Ohmori Y, et al. Effect of cyclosporine on the endocrine and exocrine pancreas in kidney transplant recipients. Am J Kidney Dis. 1988;12:11. doi: 10.1016/s0272-6386(88)80065-9. [DOI] [PubMed] [Google Scholar]
- 47.Boudreaux J, McHugh L, Canafax D, et al. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation. 1987;44:376. doi: 10.1097/00007890-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Roth D, Milgrom M, Esquenazi V, et al. Posttransplant hyperglycemia. Transplantation. 1989;47:278. [PubMed] [Google Scholar]
- 49.Krentz AJ, Doussett B, Mayer D, et al. Metabolic effects of cyclosporine A and FK506 in liver transplant recipients. Diabetes. 1993;42:1753. doi: 10.2337/diab.42.12.1753. [DOI] [PubMed] [Google Scholar]
- 50.Jindal RM, Emre S, Menesses P, et al. Diabetogenicity of FK506 versus CyA in liver transplant recipients. Hepatology. 1993;18:745. [Google Scholar]
- 51.Jindal RM, Popsecu I, Schwartz ME, et al. Diabetogenicity of FK506 versus cyclosporine in liver transplant recipients. Transplantation. 1994;58:370. [PubMed] [Google Scholar]