Abstract
Purpose
The incidence of hypoxemia in patients undergoing surgery is largely unknown and may have a clinical impact. The objective of this study was to determine the incidence of intraoperative hypoxemia in a large surgical population.
Methods
We performed a retrospective study of electronically recorded pulse oximetry data obtained from two large academic medical centres. All adults (age ≥ 16 yr) undergoing non-cardiac surgery during a three-year period at the two hospitals were included in the analysis. Our main outcome measure was the percentage of patients with episodes of hypoxemia (SpO2 < 90) or severe hypoxemia (SpO2 ≤ 85) for two minutes or longer during the intraoperative period (induction of anesthesia, surgery, and emergence).
Results
We evaluated 95,407 electronic anesthesia records at the two hospitals. During the intraoperative period, 6.8% of patients had a hypoxemic event, and 3.5% of patients had a severely hypoxemic event of two consecutive minutes or longer. Seventy percent of the hypoxemic episodes occurred during either induction or emergence— time periods that represent 21% of the total intraoperative time. From induction to emergence, one episode of hypoxemia occurred every 28.9 hr, and one episode of severe hypoxemia occurred every 55.7 hr of intraoperative time.
Conclusion
Despite advances in monitoring technology, hypoxemia continues to occur commonly in the operating room and may be a serious safety concern because of its potential impact on end organ function and long-term outcomes. Further studies are needed to improve our understanding of the clinical impact of intraoperative hypoxemia and the strategies that will be most useful in minimizing its occurrence.
Hypoxemia is recognized as one of the most serious risks patients face during anesthesia and surgical care. Pulse oximetry has become an essential component of operating room technology to detect, treat, and reduce the degree of intraoperative hypoxemia in the developed world.1 Pulse oximetry has been endorsed by the Canadian Anesthesiologists' Society, American Society of Anesthesiologists (ASA), World Federation of Societies of Anaesthesiologists, and the World Health Organization as a minimal monitoring standard during surgery.2-5 Before the widespread adoption of pulse oximetry in the 1980s and the establishment of anesthesia monitoring standards in the 1990s, hypoxemia was the leading cause of anesthesia-related mortality.6,7 Since then, anesthesia-related mortality has dropped nearly 20-fold in developed settings.8-10 Much of this decline is attributable to improvements in the safety of anesthesia administration and monitoring, including the nearly universal use of pulse oximetry that has been associated with the earlier diagnosis and correction of hypoxemia.7,11-14
With the decline in anesthesia-related mortality, we have focused our attention on understanding the long-term effects of exposure to anesthesia.15 One area of great uncertainty is the clinical impact of transient hypoxemia during surgery. To date, no published studies using current-generation pulse oximetry technology have characterized the incidence or severity of hypoxemia during surgery. Additionally, the clinical impact of transient intraoperative hypoxemia on outcomes such as surgical site infections, postoperative cognitive dysfunction, and other end organ dysfunction, such as myocardial and renal insufficiency, has not been studied.
The recent advent of electronic anesthesia record keeping systems16-18 that automatically record intraoperative physiologic parameters has made it possible, for the first time, to examine pulse oximetry data from a large number of surgical patients during routine care. Therefore, the aim of this retrospective study was to utilize electronic intraoperative records to determine the incidence, severity, and duration of hypoxemia in a large population of surgical patients at two major academic medical centres.
Methods
Following approval of the study protocol by the Institutional Review Boards of two large academic tertiary hospitals— both with active transplant programs and surgical volumes in excess of 40,000 cases annually— electronically recorded pulse oximetry data were obtained from the anesthesia information management systems at both centres utilizing a structured query language. Data were collected over a three-year period (Hospital A: April 2006 to April 2009; Hospital B: November 2005 to January 2009) for each patient who underwent general anesthesia and for whom a completed electronic anesthesia record was available.
For each patient, the database query returned all pulse oximetry values, patient demographics (age, sex, American Society of Anesthesiologists' [ASA] physical status classification), surgical procedure, and case duration. Pediatric patients (age < 16 yr), patients undergoing cardiopulmonary bypass, and procedures performed outside of the operating room (e.g., magnetic resonance imaging, endos-copy, radiology) were excluded from the study. Case milestones (induction of anesthesia, start of surgery, end of surgery, patient departure from the operating room) were also obtained from the electronic anesthesia databases in order to separate pulse oximetry signals into discrete case phases.
Current generation pulse oximeters (Hospital A: Radical 7 - Masimo, Irvine, CA, USA; Hospital B: Nellcor - Boulder, CO, USA) utilizing conventional red and infrared photoplethysmography, digital signal processing, and adaptive filtration were used during the study period. The pulse oximeters were configured to provide either an eight-second (Hospital A) or 20-sec (Hospital B) average of oxygenation (SpO2) values. This information was polled by the information management systems either every 60 sec (Hospital A) or every 30 sec (Hospital B). All values recorded by the information management systems were included in the analysis.
We calculated the number and duration of episodes of both hypoxemia (SpO2 < 90) and severe hypoxemia (SpO2 ≤ 85) for each patient. We chose these definitions on the basis of the accepted definition of hypoxemia (SpO2 < 90 that correlates with a PaO2 of < 60 mmHg) and previous studies that have defined severe hypoxemia as SpO2 ≤ 85.12,19,20 Episodes of hypoxemia and severe hypoxemia were then categorized according to maximum duration per patient and according to the phase of intraoperative care during which they occurred: induction of anesthesia to start of surgery (induction), start of surgery to end of surgery (surgery), or end of surgery to departure from the operating room (emergence).
When calculating episode durations, we categorized hypoxemic events as lasting one, two, three, four, five, or ≥ six minutes. We treated each individual value recorded in the anesthesia information management systems as persisting until replaced by a new value. Thus, at Hospital A, two consecutive abnormal values sampled every 60 sec represented a two-minute hypoxemic episode. Three consecutive abnormal readings represented a three-minute hypoxemic event. At Hospital B, four consecutive abnormal values sampled every 30 sec represented a two-minute hypoxemic episode, while six consecutive abnormal values represented a three-minute hypoxemic event. In order to allow comparison of data between Hospital A and Hospital B, episodes at Hospital B that consisted of an odd number of values were divided evenly into adjacent bins (e.g., the group of patients with three consecutive abnormal values representing 1.5 min was split evenly into either the one-minute bin or the two-minute bin). In order to understand the effect of our treatment of the odd number of consecutive readings at Hospital B, a sensitivity analysis was performed by modelling the effects of downscaling data recorded at 30-sec intervals into data recorded at one-minute intervals using a Weibull distribution.
For each episode of hypoxemia and severe hypoxemia that lasted two minutes or longer, we then calculated the average number of hours between instances. Our goal was to report how often providers encounter hypoxemia in routine practice. We performed this calculation only on episodes that lasted two minutes or longer, because we wished to increase our specificity for detecting episodes that were truly reflective of hypoxemia by including episodes where multiple abnormal SpO2 recordings occurred sequentially—and excluding non-consecutive readings that might be more reflective of artefact.
Mean age, sex, ASA physical status classification, number of emergency cases, and average surgical times for the entire sample were also calculated. To validate the accuracy of the electronic data query, a random sample of print-outs of anesthesia information management system records from 100 patients at each hospital was manually compared with the information returned from the data query.
Results
Our search returned data from 245,458 completed electronic anesthesia records, and 95,407 of those met our inclusion criteria (Hospital A: 55,775; Hospital B: 39,632). The majority of cases excluded from our sample (52%) failed to meet our age criteria (≥ 16 yr), with another large sample (33%) representing cases performed under regional anesthesia or monitored anesthesia care. The case demographics are shown in Table 1. The average age and sex distributions were similar between Hospital A and Hospital B. The ASA physical status distributions revealed a larger percentage of patients with no medical co-morbidities (i.e., ASA I) at Hospital B compared with Hospital A (36.4% vs 15.7%, respectively), but the number of patients rated ASA I or II was very similar (71.5% vs 71.6%, respectively). Finally, the mean surgical times at Hospital A were moderately longer than those at Hospital B (107 min vs 73 min, respectively).
Table 1. Patient demographics.
| Hospital A (n = 55,775) |
Hospital B (n = 39,632) |
|
|---|---|---|
| Mean age (yr) | ||
| (standard error) | 53.4 (0.07) | 48.3 (0.10) |
| Sex (%) | ||
| Male | 46% | 45% |
| Female | 54% | 55% |
| ASA physical status (%) | ||
| 1 | 15.7% | 36.4% |
| 2 | 55.8% | 35.0% |
| 3 | 24.4% | 22.6% |
| 4 | 2.6% | 4.1% |
| 5 & 6 | 0.1% | 0.1% |
| Unknown | 1.5% | 1.8% |
| Emergency cases (%) | 4.7% | – |
| Surgical time (min). | ||
| Mean (standard error) | 107 (0.4) | 73 (0.4) |
ASA = American Society of Anesthesiologists
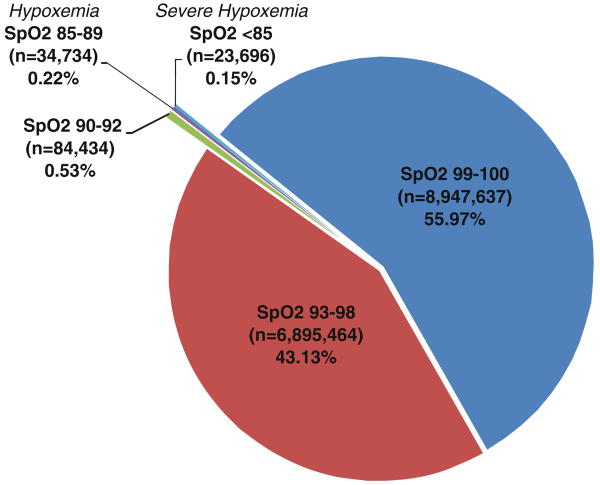
The total distribution of pulse oximetry values returned by the data query for both hospitals is shown in Fig. 1. Of the 15,985,965 SpO2 readings, 99.63% were within the normal range (≥ 90%) and 0.37% of all readings represented values < 90%. Additionally, as shown in Fig. 1, the values 99% and 100% represent the majority (56%) of all readings. The occurrence of hypoxemic events was associated with higher ASA physical status.
Fig. 1.
Distribution of All SpO2 Readings. The distribution of SpO2 readings from the 95,407 anesthesia cases. Of the 15,985,965 SpO2 readings, 99.63% were within the normal range (≥ 90%) and 0.37% of all readings represented values < 90%
Table 2 shows the relative timing of hypoxemic episodes of all durations, lists the percentage of patients experiencing hypoxemia and severe hypoxemia during the three phases of intraoperative care, and shows the mean duration of each of the three time intervals. During each of the three time intervals, i.e., induction, surgery, and emergence, a similar percentage of patients experienced hypoxemia (7.4%, 7.2%, and 9.3%, respectively) and severe hypoxemia (4.1%, 3.8%, and 5.4%, respectively). Patients spent almost eight times longer in the surgical phase (93 min mean duration) as they did in either the induction or emergence time periods (mean duration 12 min each).
Table 2. Incidence and timing of hypoxemia.
| Hospital A and B (n = 95,407) | Induction of anesthesia to start of surgery | Start of surgery to end of surgery | End of surgery to exit OR |
|---|---|---|---|
| Mean duration of each interval | 12 min | 93 min | 12 min |
| Patients with hypoxemia (SpO2 < 90) n (%) | 7,101 (7.4%) | 6,908 (7.2%) | 8,875 (9.3%) |
| Patients with severe hypoxemia (SpO2 ≤ 85) n (%) | 3,954 (4.1%) | 3,582 (3.8%) | 5,157 (5.4%) |
OR = operating room
The incidence and duration of intraoperative hypoxemic episodes are shown in Fig. 2, with details by hospital shown in the Appendix. The episodes are grouped by maximum duration per patient for both hypoxemic (SpO2 < 90) and severely hypoxemic (SpO2 ≤ 85) episodes. The percentage of patients experiencing hypoxemia or severe hypoxemia for two minutes or longer was 6.8% and 3.5%, respectively, and the percentage of patients who were hypoxemic or severely hypoxemic for five consecutive minutes or longer was 1.6% and 0.8%, respectively. In our sample, the average intraoperative time between each two-minute or longer episode of hypoxemia and severe hypoxemia was 28.9 hr and 55.7 hr, respectively. The overall rate of hypoxemia was stable over the three-year period examined at both centres. The manual comparison of print-outs of the records yielded 100% concordance with the electronic data queries performed at each hospital, confirming our confidence in data extraction methodology. Finally, the sensitivity analysis of the impact of splitting data from Hospital B into adjacent 60-sec intervals revealed a small degree of error, suggesting that our approach was a reliable method for joining the two data sets.
Fig. 2.
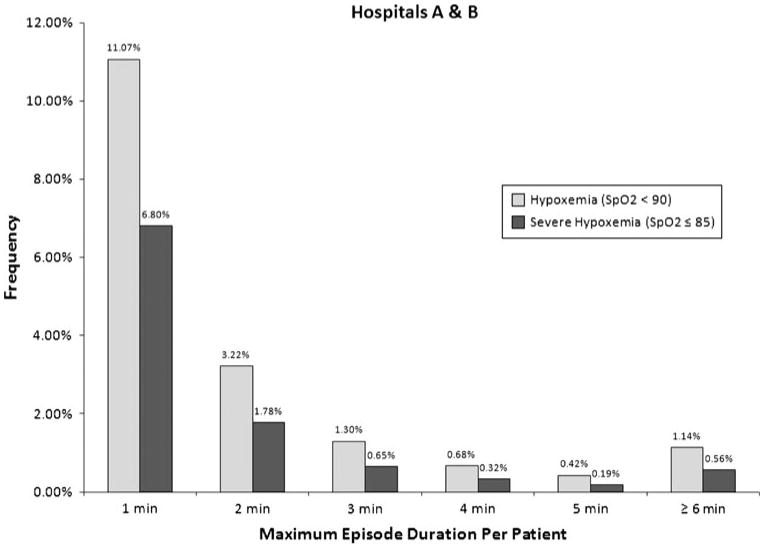
Duration of Hypoxemic Episodes at Hospitals A & B. The incidence and maximum duration of intraoperative hypoxemic episodes. Episodes are grouped by maximum duration per patient for both hypoxemic (SpO2 < 90) and severely hypoxemic episodes (SpO2 ≤ 85). The percentage of patients experiencing two consecutive minutes or longer of hypoxemia and severe hypoxemia was 6.8% and 3.5%, respectively
Discussion
Summary of findings
Our data revealed that 6.8% of patients were hypoxemic and 3.5% were severely hypoxemic for two consecutive minutes or longer during the intraoperative period. Among the SpO2 readings reflective of hypoxemia, episodes appeared to be relatively evenly divided among the three phases of intraoperative care, even though the induction phase (average time 12 min) and emergence phase (average time 12 min) were significantly shorter than the surgical phase (average time 93 min). From induction to emergence, an anesthesia provider in a tertiary care centre can expect to manage a period of hypoxemia of two-minute duration or longer at least once every 29 hr of intraoperative time.
Comparison with previous reports
Our findings are consistent with previous studies12,21-23 that have suggested that intraoperative hypoxemia occurs at a significant rate. The largest randomized evaluation of pulse oximetry23 revealed a combined incidence of hypoxemia in the operating room and postanesthesia care unit of 7.9% (n = 10,312), although it did not define the duration of hypoxemic episodes and used first generation pulse oximetry technology that is subject to additional signal artefact (Nellcor N-200, Ohmeda 3700 & Radiometer OXI). It appears that our results are generalizable, given the similarity between our results and this previous work, the large number of patients included in our study, and the similarity of results between our two centres (see Appendix).
The finding that 70% of hypoxemia occurs during only one-fifth of the time spent in the operating room is consistent with our clinical experience, which suggests that the time close to induction of anesthesia and emergence are particularly high-risk periods for hypoxemia. This intuition is supported by data from the ASA Closed Claims database, which has shown that difficult airway claims continue to comprise the highest percentage of claims.24 The majority of these claims are related to incidents that occurred during induction (67%), with similar numbers occurring during surgery (15%) and extubation (12%).25
Implications
Our data suggest that a surprisingly high percentage of patients experience sustained hypoxemia during surgery. Even in highly advanced surgical settings, approximately one in 15 patients experienced hypoxemia for at least two consecutive minutes, and one in 64 patients experienced hypoxemia for at least five consecutive minutes. These frequencies are likely higher in resource-limited settings throughout the world where pulse oximetry is often unavailable, and hypoxemia may not be recognized by the perioperative team. Given that approximately 234 million surgical procedures are performed annually worldwide,26 our report suggests that at least three million patients each year experience prolonged (≥ five minutes) and potentially preventable hypoxemia during surgery.
The thresholds for the duration and severity of hypoxemia that are likely to affect clinical outcomes are unknown, but the levels of hypoxemia reported in this study may have a clinical impact. Although the effect of oxygen saturation levels on surgical patient morbidity has been studied in several clinical trials, little has been published on the impact of transient hypoxemic events on surgical outcomes. Reduced cerebral oxygen saturation levels have been correlated with higher postoperative complication rates in thoracic surgery.27 Perioperative administration of supplemental oxygen has been shown to reduce the incidence of surgical-wound infections,28,29 to improve immune function,30 and to decrease the incidence of postoperative nausea and vomiting.31 Furthermore, hypoxemia has been demonstrated in a variety of animal models to have detrimental effects on almost every end organ.32 At the cellular level, hypoxia has been shown to lead to acute heart failure,33 pulmonary hypertension,34 and acute renal failure.35 Hypoxia-induced changes in neural tissue have been associated with decreased cognitive function.36 Additionally, numerous studies have demonstrated that even modest fluctuations in oxygen delivery may lead to cognitive dysfunction.37 One study has linked intraoperative desaturation (measured by cerebral oxygen monitoring) with a decline in postoperative cognitive function (POCD).38 In that study, patients who experienced a drop in cerebral oxygen levels of ≥ 25% were found to have a statistically significant decline in their Mini Mental State Examination scores measured seven days after surgery. Although the predictors of POCD are still not well understood, further examination of the impact of transient hypoxemia on POCD is warranted, because 5-12% of patients demonstrate POCD at three-month follow-up visits.39
The evidence base for the use of pulse oximetry has been questioned in previous studies.40 However, even the largest prospective randomized controlled trial of pulse oximetry23,41 was insufficiently powered to reveal a mortality benefit from pulse oximetry (nearly two million patients would have been required). Furthermore, a post-hoc analysis of this trial41 suggests that the use of pulse oximetry was associated with several key benefits, including a reduction in myocardial ischemia (from 0.2 to 0.1%; P = 0.03) and cardiac arrest (from 0.1 to 0.04%; P = 0.06). Endobronchial intubation and hypoventilation were also recognized more frequently.42
These clinical data and animal model studies suggest only that the hypoxemia we observed during surgery may have been clinically relevant. They do not establish a direct link between perioperative hypoxemia and poor clinical outcomes. However, as the oxygen-hemoglobin dissociation curve indicates, there is little reserve when saturations fall to < 90%, let alone 85%; undoubtedly, there is a threshold below which hypoxemia causes survivable end organ damage. The potential public health impact of our findings is difficult to quantify, but given the very large numbers of patients undergoing surgery with anesthesia globally, it may well be substantial.
Limitations
Our data were derived objectively from routine clinical practice at two geographically distinct centres and demonstrate real-world validity by eliminating the biases often associated with study observation. However, the retrospective nature of our study does limit the conclusions we can draw. Specifically, our data do not provide information on the etiology of the episodes of hypoxemia, and we also lack data on patient outcomes.
Pulse oximeters have well-known technical limitations and various sources of artefact that may have influenced the quality of our data.43 Sensor malposition can lead to apparent hypoxemia; the incidence of this is unknown, but such artefacts are thought to be rare.44 Poor peripheral perfusion, hypothermia, hypotension, and advanced age have been shown to yield low-quality pulse oximetry readings.45
Since our study was a retrospective analysis of current practice, we were unable to compare our electronically recorded data with actual plethysmographic signals or to estimate the specific performance characteristics of the devices used in the study. However, pulse oximeters have been validated previously in multiple clinical trials,46-49 including several within the perioperative environment.50-52 These studies have shown that current generation pulse oximeters provide reliable readings when compared with arterial blood gas sampling – even in the circumstances with poor peripheral perfusion, hypothermia, and motion.50,51,53 All of the pulse oximeters used in the study adhere to the International Organization for Standardization (ISO) standards for pulse oximetry accuracy.54
We attempted to minimize the impact of spurious readings on our results in two ways. First, we validated our hypoxemia data on 200 randomly selected patients. The concordance between the data returned by the electronic query and the patients' anesthesia records was 100%, providing considerable reassurance in relation to our algorithms. Second, we focused on the percentage of patients who experienced episodes of hypoxemia that lasted two minutes or longer. We chose this interval because it ensured that each hypoxemic episode represented at least two consecutive abnormal readings - as opposed to single isolated values. While there is no standardized definition of what constitutes a hypoxemic episode under anesthesia, we believe that the selection of this interval strikes an appropriate balance between capturing events that are likely to be reflective of true hypoxemia and eliminating isolated spurious values. Additionally, in our practice, we consider any episode of hypoxemia that lasts at least two minutes to be clinically significant.
Since our data were recorded every 30 or 60 sec rather than continuously, they could have been subject to an undersampling artefact because a single value was used to summarize a series of values over a discrete time interval.55,56 Thus, undersampling may have underestimated the true frequency of hypoxemic episodes. However, since our sampling intervals were either 30 or 60 sec, such episodes would be sub-minute episodes of uncertain clinical significance. On the other hand, we treated each single value as being representative of the entire time interval over which it was recorded. This may have either over- or underestimated the duration of any given hypoxemic episode. Despite this possibility, we believe our methodology represents a logical compromise between these two competing concepts and aligns with the interpretation of these data points in clinical practice.
Finally, although pulse oximetry data were collected automatically by electronic systems without user intervention, the case milestones (i.e., induction of anesthesia, start of surgery, end of surgery, and departure from operating room) were manually entered events and, as such, may be subject to errors in data entry, for example, event latency.57 Furthermore, there is no universal definition for these events, although attempts have been made at standardization of anesthesia procedural times.58 While a discrepancy in case times might lead to the misclassification of the case phase for a particular hypoxemic episode, it would not affect the overall SpO2 data.
Conclusions
Anesthesia providers strive to avoid hypoxemia because of the risk of irreversible damage to the myocardium, brain, and other end organs. Despite these efforts, hypoxemia continues to occur in the operating room at a surprisingly high rate. This may represent a serious safety concern because of its potential impact on end organ function and long-term outcomes. Our findings suggest that a typical anesthesia provider is likely to encounter at least one episode of sustained (≥ two minutes) hypoxemia every 29 hr of intraoperative time. In the developing world where pulse oximetry is not universally available, hypoxemic episodes may well be more frequent and longer. Future studies that focus on whether these episodes are preventable and have a clinical impact are warranted.
Acknowledgments
Financial support for the preparation of this manuscript was provided from department funds of the Department of Anesthesia, Critical Care & Pain Medicine, Massachusetts General Hospital and from 5T32GM007592 from the National Institute of Health.
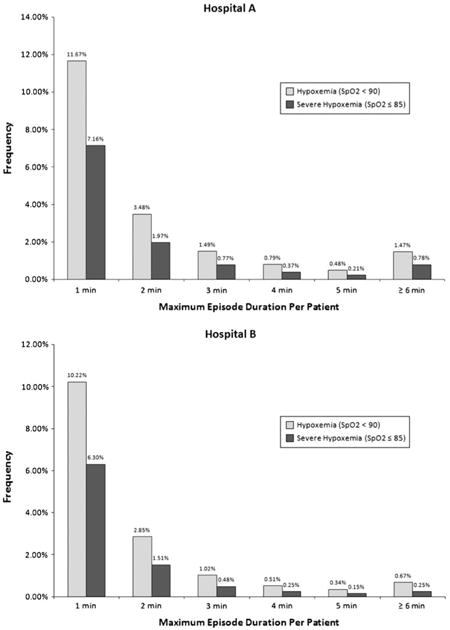
Appendix
The figures below show the incidence and duration of intraoperative hypoxemic episodes by hospital. Episodes are grouped by maximum duration per patient for both hypoxemic (SpO2 < 90) and severely hypoxemic episodes (SpO2 ≤ 85).
Footnotes
Author contributions: Dr. Ehrenfeld had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ehrenfeld, Gawande, Funk.
Acquisition of data: Ehrenfeld, van Schalkwyk.
Analysis and interpretation of data: Ehrenfeld, Funk, Gawande, Sandberg, Merry, van Schalkwyk.
Drafting of the manuscript: Ehrenfeld, Funk.
Critical revision of the manuscript for important intellectual content: Sandberg, Merry, Gawande, van Schalkwyk.
Conflicts of interest Dr. Merry has a financial interest in Safer Sleep LLC, a manufacturer of anesthesia information management systems.
Contributor Information
Jesse M. Ehrenfeld, Email: jehrenfe@post.harvard.edu, Department of Anesthesia, Critical Care, & Pain Medicine, Massachusetts General Hospital, 55 Fruit St, Jackson 458, Boston, MA 02114, USA.
Luke M. Funk, Department of Surgery, Brigham and Women's Hospital, Boston, MA, USA; Department of Health Policy and Management, Harvard School of Public Health, Boston, MA, USA.
Johan Van Schalkwyk, Department of Anaesthesia, Auckland City Hospital, Auckland, New Zealand.
Alan F. Merry, Department of Anaesthesia, Auckland City Hospital, Auckland, New Zealand; Department of Anaesthesiology, University of Auckland, Auckland, New Zealand.
Warren S. Sandberg, Department of Anesthesia, Critical Care, & Pain Medicine, Massachusetts General Hospital, 55 Fruit St, Jackson 458, Boston, MA 02114, USA.
Atul Gawande, Department of Surgery, Brigham and Women's Hospital, Boston, MA, USA; Department of Health Policy and Management, Harvard School of Public Health, Boston, MA, USA.
References
- 1.Severinghaus JW, Kelleher JF. Recent developments in pulse oximetry. Anesthesiology. 1992;76:1018–38. doi: 10.1097/00000542-199206000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Merchant R, Bosenberg C, Brown K, et al. Guidelines to the practice of anesthesia - Revised edition 2010. Can J Anesth. 2010;57:58–87. doi: 10.1007/s12630-009-9209-4. [DOI] [PubMed] [Google Scholar]
- 3.Anesthesiologists ASA. Standards of the American Society of Anesthesiologists: Standards for Basic Anesthetic Monitoring. [April 2010]; Available from URL: http://www.asahq.org/publicationsAndServices/standards/02.pdf.
- 4.Eichhorn JH, Cooper JB, Cullen DJ, Maier WR, Philip JH, Seeman RG. Standards for patient monitoring during anesthesia at Harvard Medical School. JAMA. 1986;256:1017–20. [PubMed] [Google Scholar]
- 5.World Alliance for Patient Safety. WHO guidelines for safe surgery. Geneve: World Health Organization; 2008. [April 2010]. Available from URL: http://www.who.int/patientsafety/safesurgery/knowledge_base/SSSL_Brochure_finalJun08.pdf. [Google Scholar]
- 6.Cooper JB, Newbower RS, Long CD, McPeek B. Preventable anesthesia mishaps: a study of human factors. Anesthesiology. 1978;49:399–406. doi: 10.1097/00000542-197812000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JB, Newbower RS, Kitz RJ. An analysis of major errors and equipment failures in anesthesia management: considerations for prevention and detection. Anesthesiology. 1984;60:34–42. doi: 10.1097/00000542-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Keenan RL, Boyan CP. Cardiac arrest due to anesthesia. A study of incidence and causes. JAMA. 1985;253:2373–7. [PubMed] [Google Scholar]
- 9.Keenan RL, Boyan CP. Decreasing frequency of anesthetic cardiac arrests. J Clin Anesth. 1991;3:354–7. doi: 10.1016/0952-8180(91)90174-l. [DOI] [PubMed] [Google Scholar]
- 10.Arbous MS, Meursing AE, van Kleef JW, et al. Impact of anesthesia management characteristics on severe morbidity and mortality. Anesthesiology. 2005;102:257–68. doi: 10.1097/00000542-200502000-00005. quiz 491-2. [DOI] [PubMed] [Google Scholar]
- 11.Moller JT, Jensen PF, Johannessen NW, Espersen K. Hypoxaemia is reduced by pulse oximetry monitoring in the operating theatre and in the recovery room. Br J Anaesth. 1992;68:146–50. doi: 10.1093/bja/68.2.146. [DOI] [PubMed] [Google Scholar]
- 12.Cote CJ, Rolf N, Liu LM, et al. A single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74:980–7. doi: 10.1097/00000542-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Canet J, Ricos M, Vidal F. Postanesthetic hypoxemia and oxygen administration. Anesthesiology. 1991;74:1161–2. doi: 10.1097/00000542-199106000-00039. [DOI] [PubMed] [Google Scholar]
- 14.Eichhorn JH. Effect of monitoring standards on anesthesia outcome. Int Anesthesiol Clin. 1993;31:181–96. doi: 10.1097/00004311-199331030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Sessler DI. Long-term consequences of anesthetic management. Anesthesiology. 2009;111:1–4. doi: 10.1097/ALN.0b013e3181a913e1. [DOI] [PubMed] [Google Scholar]
- 16.Merry AF, Webster CS, Mathew DJ. A new, safety-oriented, integrated drug administration and automated anesthesia record system. Anesth Analg. 2001;93:385–90. doi: 10.1097/00000539-200108000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Egger Halbeis CB, Epstein RH, Macario A, Pearl RG, Grunwald Z. Adoption of anesthesia information management systems by academic departments in the United States. Anesth Analg. 2008;107:1323–9. doi: 10.1213/ane.0b013e31818322d2. [DOI] [PubMed] [Google Scholar]
- 18.Epstein RH, Vigoda MM, Feinstein DM. Anesthesia information management systems: a survey of current implementation policies and practices. Anesth Analg. 2007;105:405–11. doi: 10.1213/01.ane.0000270214.58811.c4. [DOI] [PubMed] [Google Scholar]
- 19.Xue FS, Huang YG, Tong SY, et al. A comparative study of early postoperative hypoxemia in infants, children, and adults undergoing elective plastic surgery. Anesth Analg. 1996;83:709–15. doi: 10.1097/00000539-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Xue FS, An G, Tong SY, Liao X, Liu JH, Luo LK. Influence of surgical technique on early postoperative hypoxaemia in children undergoing elective palatoplasty. Br J Anaesth. 1998;80:447–51. doi: 10.1093/bja/80.4.447. [DOI] [PubMed] [Google Scholar]
- 21.Moller JT, Johannessen NW, Berg H, Espersen K, Larsen LE. Hypoxaemia during anaesthesia–an observer study. Br J Anaesth. 1991;66:437–44. doi: 10.1093/bja/66.4.437. [DOI] [PubMed] [Google Scholar]
- 22.Cote CJ, Goldstein EA, Cote MA, Hoaglin DC, Ryan JF. A single-blind study of pulse oximetry in children. Anesthesiology. 1988;68:184–8. doi: 10.1097/00000542-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Moller JT, Johannessen NW, Espersen K, et al. Randomized evaluation of pulse oximetry in 20, 802 patients: II. Perioperative events and postoperative complications. Anesthesiology. 1993;78:445–53. doi: 10.1097/00000542-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB. Trends in anesthesia-related death and brain damage: a closed claims analysis. Anesthesiology. 2006;105:1081–6. doi: 10.1097/00000542-200612000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW. Management of the difficult airway: a closed claims analysis. Anesthesiology. 2005;103:33–9. doi: 10.1097/00000542-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 27.Kazan R, Bracco D, Hemmerling TM. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br J Anaesth. 2009;103:811–6. doi: 10.1093/bja/aep309. [DOI] [PubMed] [Google Scholar]
- 28.Greif R, Akca O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342:161–7. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 29.Belda FJ, Aguilera L, Garcia de la Asuncion J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294:2035–42. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 30.Kotani N, Hashimoto H, Sessler DI, et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93:15–25. doi: 10.1097/00000542-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Greif R, Laciny S, Rapf B, Hickle RS, Sessler DI. Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology. 1999;91:1246–52. doi: 10.1097/00000542-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Korner PI. Circulatory adaptations in hypoxia. Physiol Rev. 1959;39:687–730. doi: 10.1152/physrev.1959.39.4.687. [DOI] [PubMed] [Google Scholar]
- 33.Cross CE, Rieben PA, Barron CI, Salisbury PF. Effects of arterial hypoxia on the heart and circulation: an integrative study. Am J Physiol. 1963;205:963–70. doi: 10.1152/ajplegacy.1963.205.5.963. [DOI] [PubMed] [Google Scholar]
- 34.Zielinski J. Effects of intermittent hypoxia on pulmonary haemodynamics: animal models versus studies in humans. Eur Respir J. 2005;25:173–80. doi: 10.1183/09031936.04.00037204. [DOI] [PubMed] [Google Scholar]
- 35.Brezis M, Rosen S. Hypoxia of the renal medulla–its implications for disease. N Engl J Med. 1995;332:647–55. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 36.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 38.Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101:740–7. doi: 10.1213/01.ane.0000166974.96219.cd. [DOI] [PubMed] [Google Scholar]
- 39.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen T, Moller AM, Pedersen BD. Pulse oximetry for perioperative monitoring: systematic review of randomized, controlled trials. Anesth Analg. 2003;96:426–31. doi: 10.1097/00000539-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Moller JT, Pedersen T, Rasmussen LS, et al. Randomized evaluation of pulse oximetry in 20, 802 patients: I. Design, demography, pulse oximetry failure rate, and overall complication rate. Anesthesiology. 1993;78:436–44. doi: 10.1097/00000542-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Merry AF, Eichhorn JH, Wilson IH. Extending the WHO ‘Safe Surgery Saves Lives’ project through Global Oximetry. Anaesthesia. 2009;64:1045–8. doi: 10.1111/j.1365-2044.2009.06104.x. [DOI] [PubMed] [Google Scholar]
- 43.Reich DL, Timcenko A, Bodian CA, et al. Predictors of pulse oximetry data failure. Anesthesiology. 1996;84:859–64. doi: 10.1097/00000542-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Guan Z, Baker K, Sandberg WS. Misalignment of disposable pulse oximeter probes results in false saturation readings that influence anesthetic management. Anesth Analg. 2009;109:1530–3. doi: 10.1213/ANE.0b013e3181b9a814. [DOI] [PubMed] [Google Scholar]
- 45.Freund PR, Overand PT, Cooper J, et al. A prospective study of intraoperative pulse oximetry failure. J Clin Monit. 1991;7:253–8. doi: 10.1007/BF01619270. [DOI] [PubMed] [Google Scholar]
- 46.Bohnhorst B, Peter CS, Poets CF. Pulse oximeters' reliability in detecting hypoxemia and bradycardia: comparison between a conventional and two new generation oximeters. Crit Care Med. 2000;28:1565–8. doi: 10.1097/00003246-200005000-00050. [DOI] [PubMed] [Google Scholar]
- 47.Alexander CM, Teller LE, Gross JB. Principles of pulse oximetry: theoretical and practical considerations. Anesth Analg. 1989;68:368–76. [PubMed] [Google Scholar]
- 48.Hanning CD, Alexander-Williams JM. Pulse oximetry: a practical review. BMJ. 1995;311:367–70. doi: 10.1136/bmj.311.7001.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee WW, Mayberry K, Crapo R, Jensen RL. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med. 2000;18:427–31. doi: 10.1053/ajem.2000.7330. [DOI] [PubMed] [Google Scholar]
- 50.Wax DB, Rubin P, Neustein S. A comparison of transmittance and reflectance pulse oximetry during vascular surgery. Anesth Analg. 2009;109:1847–9. doi: 10.1213/ANE.0b013e3181bbc446. [DOI] [PubMed] [Google Scholar]
- 51.Durbin CG, Jr, Rostow SK. Advantages of new technology pulse oximetry with adults in extremis. Anesth Analg. 2002;94:S81–3. [PubMed] [Google Scholar]
- 52.Golparvar M, Naddafnia H, Saghaei M. Evaluating the relationship between arterial blood pressure changes and indices of pulse oximetric plethysmography. Anesth Analg. 2002;95:1686–90. doi: 10.1097/00000539-200212000-00040. [DOI] [PubMed] [Google Scholar]
- 53.Barker SJ. “Motion-resistant” pulse oximetry: a comparison of new and old models. Anesth Analg. 2002;95:967–72. doi: 10.1097/00000539-200210000-00033. [DOI] [PubMed] [Google Scholar]
- 54.ISO 9919. Medical electrical equipment – Particular requirements for the basic safety and essential performance of pulse oximeter equipment for medical use. International Organization for Standardization; Geneva, Switzerland: 2005. [April 2010]. Available from URL: http://webstore.iec.ch/preview/info_iso9919%7Bed2.0%7Den.pdf. [Google Scholar]
- 55.Gregorini P. Comparison of four methods of automated recording of physiologic data at one minute intervals. J Clin Monit. 1996;12:299–303. doi: 10.1007/BF02221750. [DOI] [PubMed] [Google Scholar]
- 56.Derrick JL, Bassin DJ. Sampling intervals to record severe hypotensive and hypoxic episodes in anesthetised patients. J Clin Monit Comput. 1998;14:347–51. doi: 10.1023/a:1009978414365. [DOI] [PubMed] [Google Scholar]
- 57.Epstein RH, Dexter F, Ehrenfeld JM, Sandberg WS. Implications of event entry latency on anesthesia information management decision support systems. Anesth Analg. 2009;108:941–7. doi: 10.1213/ane.0b013e3181949ae6. [DOI] [PubMed] [Google Scholar]
- 58.Donham RT. Defining measurable OR-PR scheduling, efficiency, and utilization data elements: the Association of Anesthesia Clinical Directors procedural times glossary. Int Anesthesiol Clin. 1998;36:15–29. doi: 10.1097/00004311-199803610-00005. [DOI] [PubMed] [Google Scholar]