Summary
The motor cortices are active during both movement and movement preparation. A common assumption is that preparatory activity constitutes a sub-threshold form of movement activity: a neuron active during rightwards movements becomes modestly active during preparation of a rightwards movement. We asked whether this pattern of activity is in fact observed. We found that it was not: at the level of a single neuron, preparatory tuning was weakly correlated with movement-period tuning. Yet somewhat paradoxically, preparatory tuning could be captured by a preferred direction in an abstract ‘space’ that described the population-level pattern of movement activity. In fact, this relationship accounted for preparatory responses better than did traditional tuning models. These results are expected if preparatory activity provides the initial state of a dynamical system whose evolution produces movement activity. Our results thus suggest that preparatory activity may not represent specific factors, and may instead play a more mechanistic role.
Introduction
Voluntary movements are prepared before they are generated (Ghez et al., 1991; Rosenbaum, 1980). Similarly, changes in neural activity occur well before movement onset in both motor and premotor cortex (Tanji and Evarts, 1976; Weinrich et al., 1984). Such ‘preparatory’ activity likely plays a key role in movement generation: preparatory activity is predictive of reaction time and movement variability (Bastian et al., 2003; Churchland et al., 2006a; Churchland and Shenoy, 2007a; Churchland et al., 2006c; Riehle and Requin, 1993) and its disruption delays movement onset (Churchland and Shenoy, 2007a). An understanding of preparatory activity is also central to the study of the cognitive processes preceding movement. For example, an understanding of the preparatory activity preceding saccades has made approachable the cognitive processes that determine where and when to move the eyes (Schall and Thompson, 1999; Shadlen and Newsome, 2001). Yet it is still unclear how preparatory activity in motor and premotor cortex contributes to movement generation.
A common assumption is that preparatory activity constitutes a sub-threshold version of movement activity. If a neuron will become active during rightwards movement, it may be beneficial for that neuron to be weakly active during preparation of rightwards movement. Assuming a threshold for producing movement, preparatory activity could advance the system closer to that threshold. This sub-threshold view of preparatory activity dates to early studies (Tanji and Evarts, 1976), accords with our understanding of the saccadic system (e.g., Hanes and Schall, 1996) and is assumed by most models of reach generation (Bastian et al., 1998; Cisek, 2006a; Erlhagen and Schoner, 2002). A related hypothesis holds that preparatory and movement activity are tuned for different but concordant factors (e.g., rightwards target locations and rightwards hand velocity). An alternative proposal is that preparatory activity functions as the initial state of a dynamical system, and may not explicitly represent movement parameters (Churchland et al., 2006b; Churchland et al., 2006c; Cisek, 2006b; Fetz, 1992). Under this hypothesis, preparatory and movement activity are closely related (via those dynamics) yet that relationship needn't be transparent at the level of the individual cell.
Neural responses consistent with the sub-threshold view are often observed, especially in population averages (Bastian et al., 1998; Bastian et al., 2003; Cisek, 2006a; Erlhagen et al., 1999; Georgopoulos et al., 1989; Requin et al., 1988; Riehle and Requin, 1989). Yet other reports argue that, for individual neurons, tuning can differ during the two epochs (Crammond and Kalaska, 2000; Kaufman et al., 2010; Turner, 1991; Wise et al., 1986) and is in general inconstant with time (Churchland and Shenoy, 2007b; Fu et al., 1995; Hatsopoulos et al., 2007; Rickert et al., 2009).
If preparatory activity constitutes a sub-threshold precursor of movement activity, the two should share similar tuning. Yet under the dynamical systems view there is little reason why ‘tuning’ should be similar for the initial and subsequent states of the system. Muddying the waters further, preparatory activity appears tuned for a dizzying variety of factors, including reach direction and distance (Messier and Kalaska, 2000; Riehle and Requin, 1989), reach speed (Churchland et al., 2006b), visual location of the target (Shen and Alexander, 1997), target location relative to the eye and hand (Batista et al., 2007; Pesaran et al., 2006) and reach curvature (Hocherman and Wise, 1991). It is unclear which – if any – of those factors is primary. We are thus left with two open and fundamental questions: what is preparatory activity tuned for, and how does it relate to movement activity?
We addressed these questions using four datasets employing delayed-reach tasks. We found that the tuning of individual neurons was typically dissimilar during the preparatory and movement epochs. This finding is inconsistent with a sub-threshold role for preparatory activity. Nevertheless, preparatory tuning could be captured by a preferred direction in a ‘space’ describing the population-level movement-period responses. Remarkably, preferred directions in this unconventional space accounted for preparatory tuning better than did preferred directions in more traditional spaces (e.g., reach endpoint or velocity). This result has a simple mechanistic interpretation: it is expected under the hypothesis that preparatory activity acts as the initial state of a dynamical system.
Results
Behavior and recordings
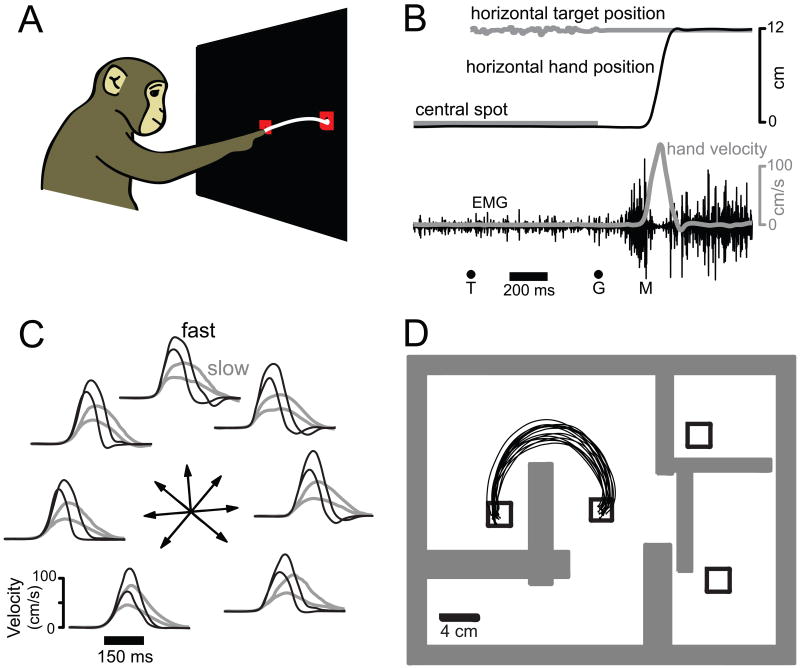
Three monkeys performed variants of a delayed-reach task. In the ‘speed task’ (monkeys A,B) target color instructed reach speed (28 conditions, Figure 1C). In the ‘maze task’ (monkey J) reaches were either straight or curved to avoid virtual barriers (Figure 1D). In the present study this complex cognitive-motor task simply provides a way to evoke many different reaching movements (27 and 108 conditions for ‘monkey J’ and ‘monkey J-array’ datasets).
Figure 1.
Illustration of behavior. A. Reaches were from a central spot to a target. An example trajectory is shown. B. Task timeline. Upon appearing (T) the target jittered slightly. Cessation of jitter provided the go cue (G). M indicates movement onset. C. Behavior: speed task. Velocity in the target direction for the 7 directions, 2 distances and 2 instructed speeds. D. Behavior: maze task. For this example condition the reach had to curve over a virtual barrier. In other conditions, reaches avoided different arrangements of barriers, or were straight with no barriers. Reaches lasted ∼200 to ∼600 ms (depending on distance/curvature).
Neural recordings employed single electrodes (monkey A, B and J datasets, 310 total single-unit isolations) and a pair of implanted 96-electrode arrays (J-array dataset, 146 single- and multi-unit isolations). Recordings were made from the caudal portion of dorsal premotor cortex (PMd) and from surface and sulcal primary motor cortex (M1). High trial counts (mean trials/neuron = 311, 492, 388 and 2166 for monkey A, B, J, J-array) allowed us to resolve peri-movement responses that were often complex and multiphasic (Churchland and Shenoy, 2007b, also see example PSTHs below).
Neural responses during preparation and movement
Figure 2A illustrates an idealized response pattern consistent with preparatory activity serving as a sub-threshold form of movement activity. Preparatory activity shows a preference for one condition (red, perhaps the preferred direction) over another (green, perhaps the opposite direction). This preference is maintained as the plateau of preparatory activity gives rise to a burst of peri-movement activity (defined as activity immediately before, during, and just after the movement).
Figure 2.
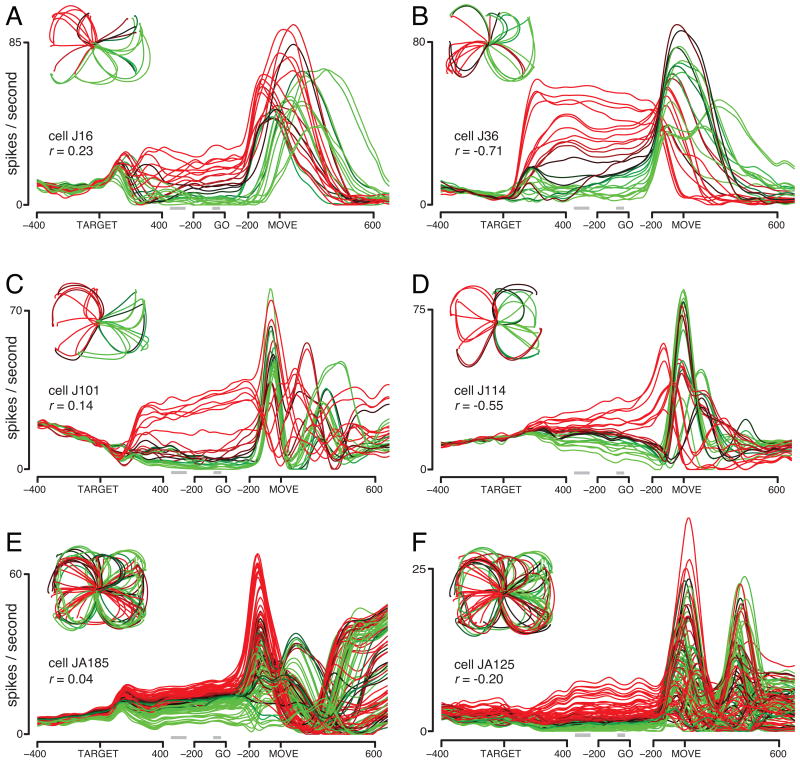
An idealized schematic of neural activity (A) and responses of example neurons (B-E, instructed speed task). A. Traces are colored red (preferred) and green (non-preferred). In this conception, preparatory activity is present during the delay period, rises to a threshold following the go cue, and produces a burst of peri-movement activity. B. Firing rate versus time for an example neuron whose responses resemble the schematic in A. Responses are shown for all 7 reach directions for the fast instructed speed and shorter distance. Traces are shaded from red to green based on the firing rate 50 ms before the go cue. Data were averaged separately locked to target onset, the go cue, and movement onset. To aid viewing, data have been interpolated across the gaps between these three epochs. See Supplemental Figure 4 for a description of the smoothing used to produce these PSTHs. Inset plots mean hand trajectory using the same color-coding. C-E. Similar plots for three more example neurons. Data have been down-selected to a single distance and instructed speed (far/fast for these panels). These three neurons were selected to illustrate the fact that preparatory and peri-movement tuning were typically different, even in the simplest paradigm: straight reaches at one distance / instructed-speed.
Figure 2B plots the response of a neuron that approximates the schematic in A. Data are for one distance/instructed speed. Traces (one per condition) are colored based on the level of preparatory activity, to allow visual comparison with the subsequent pattern of peri-movement activity. This neuron ‘prefers’ reaches ending up and leftwards. This preference is shared between preparatory and peri-movement epochs. This agreement is not perfect (two green traces surpass the black trace) but the overall correlation is quite high (r=0.72, see section on correlations below for details).
Figure 2C-E plot responses of three additional neurons recorded using the speed task. Despite clear preparatory tuning, preferences are not maintained between the preparatory and peri-movement epochs (color ordering differs between epochs). Furthermore, peri-movement activity was often complex and multiphasic (Churchland and Shenoy, 2007b; Sergio et al., 2005). These neurons show negative correlations between preparatory and peri-movement tuning.
Figure 3 plots responses of six neurons recorded using the maze task. Panels A-D plot single-electrode recordings (27 conditions). Panels E-F plot array-based recordings (108 conditions). Some neurons maintained similar tuning between preparatory and peri-movement epochs (panel A) but most did not. Also, while most neurons showed a rough directional preference during the preparatory period (insets), it was not uncommon for a few (panel B) or even many (panels E,F) conditions to evoke activity not easily explained by a pure directional preference.
Figure 3.
Responses of six example neurons (maze task, similar format to Fig. 2). Responses are shown for all conditions, including straight and curved reaches. Different conditions, involving different maze configurations, sometimes evoked similar reach trajectories (although not necessarily with similar velocity profiles). The same 108 conditions were used for the J and J-array datasets. For the former a given neuron was recorded using one of four 27-condition subsets. The insets in A-D thus show different reach patterns, corresponding to different subsets. In addition to temporal filtering, additional de-noising was accomplished via the method in Supplemental Figure 4. This method removes uncorrelated noise, and thus cannot eliminate noise in the firing rate that is accidentally similar across conditions (e.g., during the delay period in F).
Peri-movement response complexity is not due to noise, but is a real feature of most neural responses (Churchland and Shenoy, 2007b). Still, in Figure 3 response complexity is partly due to the variety of reach paths. Given this, it is worth stressing two things. First, any model regarding how preparatory activity leads to peri-movement activity should work for curved as well as straight reaches. Second, both peri-movement response complexity and the failure of preparatory tuning to predict peri-movement tuning are quite apparent even for simple center-out reaches (Figure 2).
Correlations between preparatory and peri-movement tuning
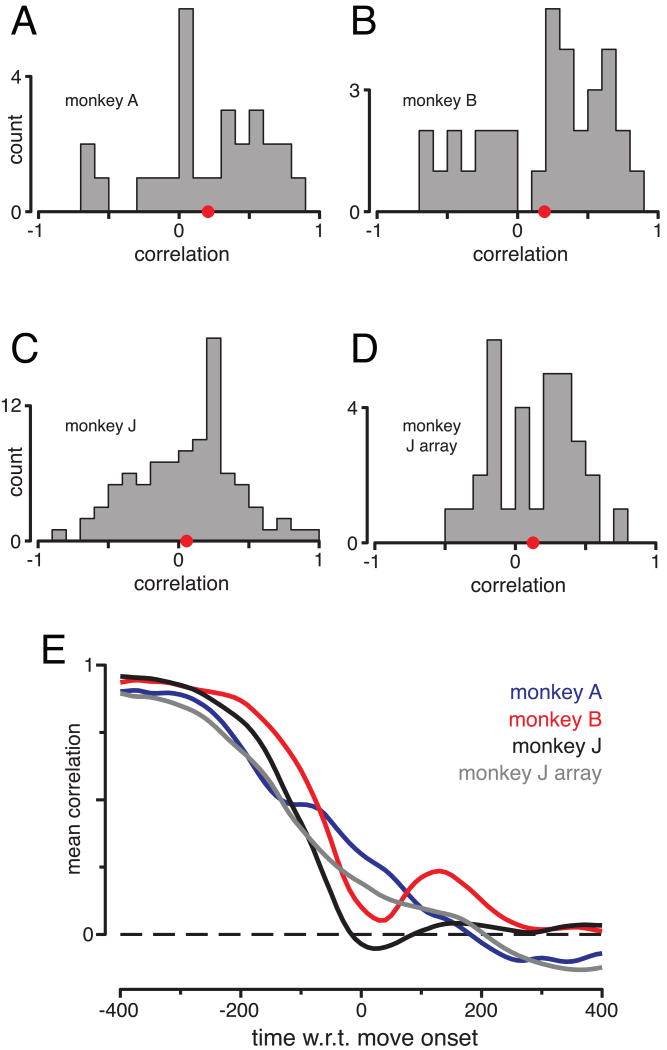
Similar levels of preparatory activity can lead to opposing patterns of peri-movement activity (e.g., middlemost traces in Figure 2D and traces near top of Figure 3C). Thus, it is not simply that tuning preferences can reverse; tuning is often weakly correlated between the two epochs (population analysis in Figure 4A-D). To compute correlations, for each condition we took the average firing rate during the preparatory and peri-movement epochs (Methods). A neuron's tuning in one epoch is then described by a vector of firing rates, with one entry per condition. We intentionally chose to not fit a specific tuning model, but simply computed the correlation between epochs. This correlation will be high if a neuron's preference remains similar. Across the four datasets the mean correlation was positive (p=0.01, p=0.01, p=0.12, p=0.03) but only very modestly so (mean r = 0.21, 0.19, 0.06 and 0.13). Non-parametric correlations were similarly modest (mean Spearman's r = 0.20, 0.20, 0.06 and 0.11). Thus, preparatory and peri-movement tuning agree weakly on average, and individual neurons span a wide spectrum.
Figure 4.
Correlation between preparatory and peri-movement tuning. A-D. Distribution of correlations (measured once per neuron) for the four datasets. Analysis was restricted to neurons robustly tuned during both epochs (Methods). Red dot indicates the distribution mean. E. Average correlation as a function of when peri-movement activity was assessed. Peri-movement activity was measured at a single time-point, after smoothing with a 20 ms Gaussian kernel. Correlations are initially high, as preparatory tuning is being correlated with itself.
The example neurons with correlations closest to the population mean are shown in Figure 3A,C, E. These neurons illustrate a further point: preparatory and peri-movement tuning often agreed when the later was assessed ∼150 ms before movement onset, but not when assessed close to movement onset. To examine this time-course, we measured peri-movement activity at individual time-points. The correlation with preparatory activity began to decline ∼200 ms prior to movement onset, and reached values near zero by movement onset (Figure 4E).
The above results agree with some prior observations at the single neuron level (Churchland and Shenoy, 2007b; Crammond and Kalaska, 2000; Kaufman et al., 2010; Wise et al., 1986) but appear to disagree with findings made at the population level (Bastian et al., 1998; Bastian et al., 2003; Cisek, 2006a; Georgopoulos et al., 1989). This discrepancy can be resolved by noting that preparatory and peri-movement tuning are weakly but positively correlated on average, especially early in the peri-movement epoch (Figure 4). In population averages (Supp. Fig. 1) the lack of correlation averages out and the means are dominated by the weak agreement between preparatory and peri-movement tuning. However, such effects grow dramatically smaller with time. By movement onset even population averages show little relationship with preparatory tuning (Supp. Fig. 1).
Our results argue against a common assumption: that preparatory activity constitutes a sub-threshold version of peri-movement activity. The cartoon in Figure 2A bears little resemblance to the responses of most neurons. Even more disconcertingly, different neurons show markedly different relationships between preparatory and peri-movement activity, with a broad range of correlations centered near zero. It has been suggested that neurons with concordant preparatory and peri-movement tuning might prime the desired movement, while neurons with opposing tuning might suppress movement until execution (Wise et al., 1986). Yet there is little evidence in Figure 4 (except perhaps weakly in panel B) for one population of concordant tuning (r ≈ 1) and another of opposing tuning (r ≈ -1). Instead, most neurons have weakly correlated tuning.
There are at least four possible explanations for the above findings. First, perhaps preparatory activity is a largely idiosyncratic phenomenon with no lawful relationship with peri-movement activity. Second, perhaps preparatory and peri-movement activity code the same thing (e.g., reach direction) with unrelated preferences (e.g., different PDs). Third, preparatory and peri-movement activity may code fundamentally different things and do so with unrelated preferences. The motor system might decode reach endpoint from preparatory activity and then produce peri-movement activity representing the velocity trajectory necessary to reach that endpoint. Finally, under the dynamical systems view, preparatory activity may be directly and mechanistically linked with peri-movement activity, but in a manner not obvious at the single-neuron level (Churchland et al., 2006b; Cisek, 2006b). These possibilities are addressed below.
Testing preparatory tuning in different spaces / reference frames
Of the above hypotheses, the first supposes that preparatory activity is tuned for nothing reliable. The next two suppose that preparatory activity is tuned for task or movement parameters (e.g., reach endpoint, trajectory or speed). The last supposes that preparatory activity should somehow relate to the population-level pattern of peri-movement activity. What is preparatory activity in fact tuned for?
It is worth expanding upon the usual caveat that most experimentally-quantifiable variables (reach endpoint, reach velocity, muscle activity) correlate with one another (Mussa-Ivaldi, 1988; Sanger, 1994; Scott, 2000, 2008). This has important consequences. Suppose preparatory activity truly represents reach endpoint (i.e., is some straightforward function of reach endpoint and can be readily decoded to infer endpoint). Suppose peri-movement activity represents reach velocity. Preparatory activity will then appear tuned not only for reach endpoint, but also for reach velocity and peri-movement activity (all these factors are correlated). Yet reach endpoint does not correlate perfectly with reach velocity, particularly for the tasks used here. Preparatory activity (which in this example is tuned for endpoint) would therefore have its strongest relationship with endpoint, and would have somewhat weaker relationships with the other variables. The central question is thus: what is preparatory activity best tuned for?
To investigate this question, we measured how well a preferred direction (PD) accounts for preparatory tuning. We asked in what ‘space’ that PD best captures tuning. If preparatory activity is tuned for endpoint, then the PD will capture tuning most effectively when expressed in a ‘reach-endpoint space’, where the axes capture horizontal and vertical endpoint. If preparatory activity is tuned for initial reach velocity, the PD will be most effective in an ‘initial-velocity space’. A wide variety of such spaces is possible. Indeed, a central goal of this field has been to determine in which space, or ‘reference frame’, the PD best captures tuning.
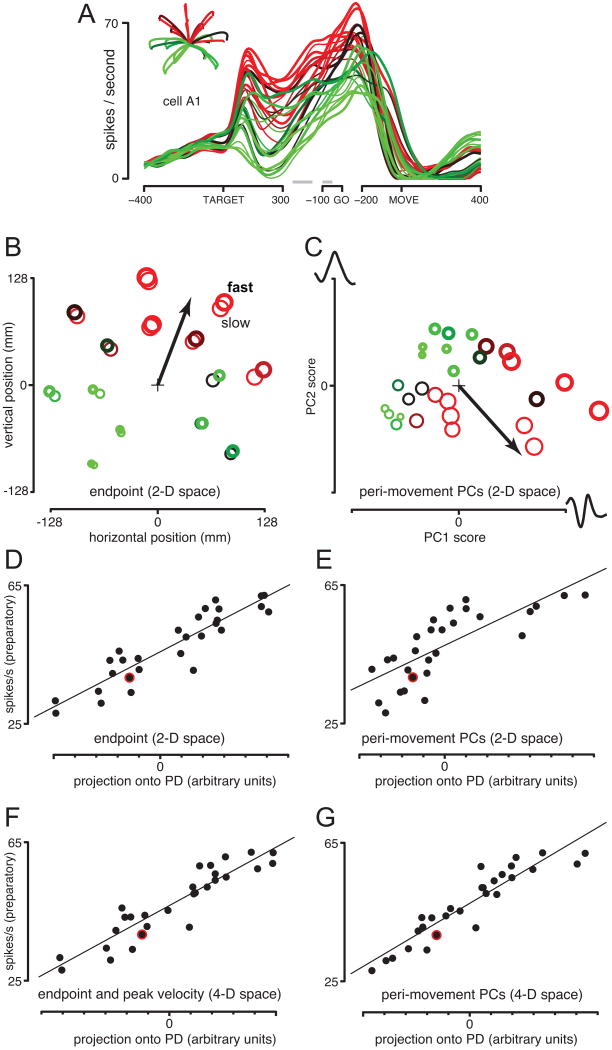
Figure 5 illustrates, for one example neuron, how the PD can capture tuning in different spaces. Preparatory activity showed a clear preference for some conditions (shaded red) over others (shaded green). Figure 5B illustrates a traditional method for accounting for such preferences. The 28 conditions (each corresponding to a target location and instructed speed) are located in a space defined by the horizontal and vertical reach endpoints. Preparatory firing rate is indicated by symbol size/color. The PD points towards the most active responses, and attempts to provide a fit according to:
Figure 5.
Example PDs in a space based on movement endpoints (left column) and the ‘peri-movement space’ (right column). A. Firing rate as a function of time for an example neuron. All 28 conditions are shown. B. Mean reach endpoint for each condition. Red-to-green shading and symbol size indicate preparatory firing rate for that condition. Symbol thickness indicates instructed speed. The PD (arrow) points towards conditions with the greatest preparatory activity. C. The same 28 conditions located in the peri-movement space. Each axis corresponds to a population-level pattern of peri-movement activity (a PC). The subsection of this pattern coming from a single neuron (A34, chosen arbitrarily) is plotted at the end of each axis. Thus, the rightmost red symbol corresponds to a condition where the ‘PC1 pattern’ was strongly present. D. Firing rate versus each condition's projection onto the PD, using the endpoint space from B. E. Similar to D, but for the peri-movement space. F. Same as D but for a 4-D space defined by reach endpoint and peak velocity. G. Same as E but for a 4-D peri-movement space.
| eqn. 1 |
where xn is a c×1 vector containing the neuron's preparatory response for the c conditions, bo and g are the firing rate offset and gain, S is a c×k matrix containing the location of each condition in a k dimensional space, and the PD is a k×1 direction in that space. The performance of the PD can be gauged by plotting the true responses (xn) versus the fit provided by the PD (right hand side of eqn. 1). Doing so (Figure 5D) shows that the PD in endpoint space captures the pattern of preparatory activity reasonably well.
Might the PD might perform better still if expressed in a different space? Many spaces are possible. For example, to construct an ‘endpoint-and-peak-velocity’ space, one needs simply to measure average endpoint and peak velocity for each condition. Each condition is then described by four numbers (horizontal and vertical endpoint and peak velocity) and ‘lives’ in a four-dimensional space. We can then ask how well a PD in this four-dimensional space captures the pattern of preparatory activity (Figure 5F). We refer to spaces constructed in this manner as ‘task’ spaces. Task spaces are constructed by the experimenter to capture salient aspects of the task (e.g., target location) or of performance (e.g., peak velocity). The use of such task spaces is common in systems neuroscience. The many studies that ask which reference frame best captures neural responses (e.g., Kakei et al., 1999) are asking which task space is preferable. More generally, whenever neural responses are posited to depend linearly on some set of k factors, that relationship can be formalized via a PD in k-dimensional space. How well the PD performs is a test of whether activity truly depends on those k factors. Traditional ‘cosine tuning’ models fall into the above category: cosine tuning for velocity is equivalent to a linear dependence on horizontal and vertical velocity (Georgopoulos et al., 1982).
Testing preparatory tuning in PCA-based spaces
A presumed purpose of preparatory tuning is for changes in a given neuron's activity to contribute, in a small but specific way, to changes in some future aspect of movement. A neuron tuned for reach endpoint would, through its firing, contribute some small change to the eventual reach endpoint. Of course, preparatory activity might be tuned not for static factors (e.g., endpoint) but for time-varying parameters (e.g., velocity). A change in preparatory firing rate would then presumably impact the future temporal profile of that parameter (perhaps scaling velocity overall). This situation should still be experimentally tractable. Provided one can describe, using a small number of parameters, how velocity varies with condition, then preparatory activity should faithfully co-vary with those parameters (if it is truly tuned for future velocity). Such parameterizations can be provided by dimensionality reduction techniques such as principal component analysis (PCA).
This approach can also address the hypothesis that preparatory activity constitutes an initial state that largely determines (via local and feedback dynamics) the population-level pattern of peri-movement activity. In this view, changes in preparatory activity produce specific changes in the pattern of peri-movement activity. For a large class of dynamical systems this relationship is straightforward: the greater a given neuron's preparatory rate, the stronger some subsequent peri-movement pattern becomes. PCA can be used to reduce peri-movement activity to a simple set of component patterns. Under the dynamical systems view, preparatory activity should be ‘tuned’ for these component patterns. If a particular pattern is prominent for a given condition, then the preparatory rate of a neuron contributing to that pattern should be high.
The PCA-based approach (Figure 5C,E,G, Supp. Fig. 2) constructs a low-dimensional space that captures how time-varying factors (velocity, peri-movement activity, etc.) differ across conditions. To illustrate the necessity of dimensionality reduction, consider the full-dimensional space describing the peri-movement response. The relevant matrix (call this T) is large: c×nt, where c is the number of conditions, n is the number of neurons, and t is the number of time-points. The c conditions thus reside in a space with thousands of dimensions. PCA can reduce this dimensionality from c×nt to c×k, with k in the range of 3-14. The resulting c×k matrix, Tred, then conveniently yields the location of each condition in a k-dimensional space (Supp. Fig. 2).
To illustrate, Figure 5C plots the 28 conditions in a two-dimensional (k=2) peri-movement space. The location of each symbol indicates, for that condition, the strength of the two most prominent peri-movement patterns (the first 2 PCs). The PD captures preparatory tuning reasonably well (Figure 5C,E). In fact, the PD generally performed adequately regardless of the space in which it was expressed. As discussed above, this is expected given correlations between movement parameters. One thus wishes to ask in which space the PD best captures the pattern of preparatory responses. We tested a variety of spaces, falling into three categories. First, we tested ‘task’ spaces, constructed by hand-selecting likely important factors (as in Figure 5B). Second, we tested the peri-movement space (Figure 5C). Finally, PCA was used to produce a kinematics-based space and an EMG-based space (substituting time-varying kinematic variables or EMG records for peri-movement activity).
Most tested spaces were greater than 2-dimensional. Figure 5F,G plots PD performance in a 4-dimensional task space and a 4-dimensional peri-movement space. The improvement in fit (relative to D,E) is expected The use of a PD is equivalent to linear regression; we are now regressing against four explanatory factors rather than two. To combat potential over-fitting, we computed the PD based on all but one condition (red-circled points in Figure 5D-G), and assessed performance for the left-out condition. This cross-validation procedure was repeated, leaving out each condition in turn. We define performance as one minus the squared error, averaged across all left-out conditions and normalized appropriately (Methods).
PD performance in a variety of spaces
We quantified the ability of the PD, in each space, to account for preparatory tuning (Figure 6). Points above zero indicate performance considerably better than chance. Points below zero indicate that the PD provided a largely spurious fit (or suffered from overfitting) resulting in poor generalization. For task spaces (green) performance is plotted against the dimensionality of the space. Given the lack of agreement regarding the key task variables, we tried many candidate spaces. A number of task spaces performed reasonably well, including ‘targs + inst. spd.’: a 3-dimensional space based on the horizontal and vertical target location and the instructed speed (speed task only); ‘endpoints + max. spd.’: a similar space based on the empirical reach endpoints and peak speeds; ‘segment dirs.’: a 6-dimensional space built by dividing the reach trajectory into three vectors; and ‘initial vel.’: the average horizontal and vertical velocity during the first 150 ms of the reach. That the PD can perform well in different task spaces highlights the historical difficulty of determining which factors activity truly depends upon.
Figure 6.
Ability of the PD to account for preparatory tuning. A-D. Performance of the PD for the four datasets. For PCA-based spaces (peri-movement, kinematic, EMG) performance is plotted over a range of tested dimensionalities. ‘Task’ spaces (green) are plotted versus their respective dimensionalities. Gray line is an estimate of the upper-limit on performance given measurement error (Methods). E. Summary of performance, spanning datasets from monkeys A, B and J. We combined only across spaces defined for all datasets (e.g., ‘targs + inst. spd.’ was not included, but ‘endpoints + max. spd.’ was included). Subpanels plot performance for spaces of the indicated dimensionality or less. Bars plot SEs. Asterisks indicate performance significantly worse (p<0.001) than the best space.
Performance of the PCA-based spaces is plotted for a range of dimensionalities (values of k). Performance typically improved with dimensionality. This increase is nontrivial, as generalization performance is being assessed. Still, because the dimensionality of preparatory activity itself is moderately high (at least 7-10 dimensions, methods), performance should increase with dimensionality assuming the PD is in approximately the ‘right’ space. Performance typically declined at dimensionalities >10, an indication of over-fitting.
The data in Figure 6A-D lend themselves to many comparisons, but one central finding stands out: performance was nearly always best for the space derived from the population-level peri-movement response. For every dataset, the highest level of performance was achieved – usually for a middle range of dimensionalities – when PDs were expressed in the peri-movement space. Even if we consider each dimensionality individually, performance was almost always higher for the peri-movement space. An exception was that, among 3-dimensional spaces, the ‘targs+instr. spd.’ and ‘endpoints + max. spd.’ performed very well for the datasets collected using the speed task. The former is undefined for the maze task, but the latter did not perform well.
We did not test all possible task spaces. Nor is it clear that the ‘right’ space should stay constant across tasks. Still, if we restrict ourselves to the tested spaces, and assume that the correct tuning model should account for both straight and curved reaches, then a clear rank order of spaces is present. Figure 6E summarizes performance across the datasets (excluding J-array, for which EMG was not recorded). For the first sub-panel, performance was computed for spaces of 3 dimensions or less. The other subpanels allow spaces up to 6 and 12 dimensions. For PCA-based spaces, if the best dimensionality was less than the maximum dimensionality allowed, then the best was used. Performance (averaged across neurons) was highest when the PD employed the space based on peri-movement neural activity. This performance differential increased at higher dimensionalities, indicating that the peri-movement space better captured higher-dimensional aspects of the data. Although a few task spaces performed well at lower dimensionalities, no single task space performed consistently well across datasets. As a result, the task spaces were largely outcompeted by the PCA-based kinematic and EMG spaces, which were in turn outperformed by the peri-movement space. Notably, the peri-movement space performed well despite the low correlation between preparatory and peri-movement activity at the single-neuron level (Figure 4).
Controls
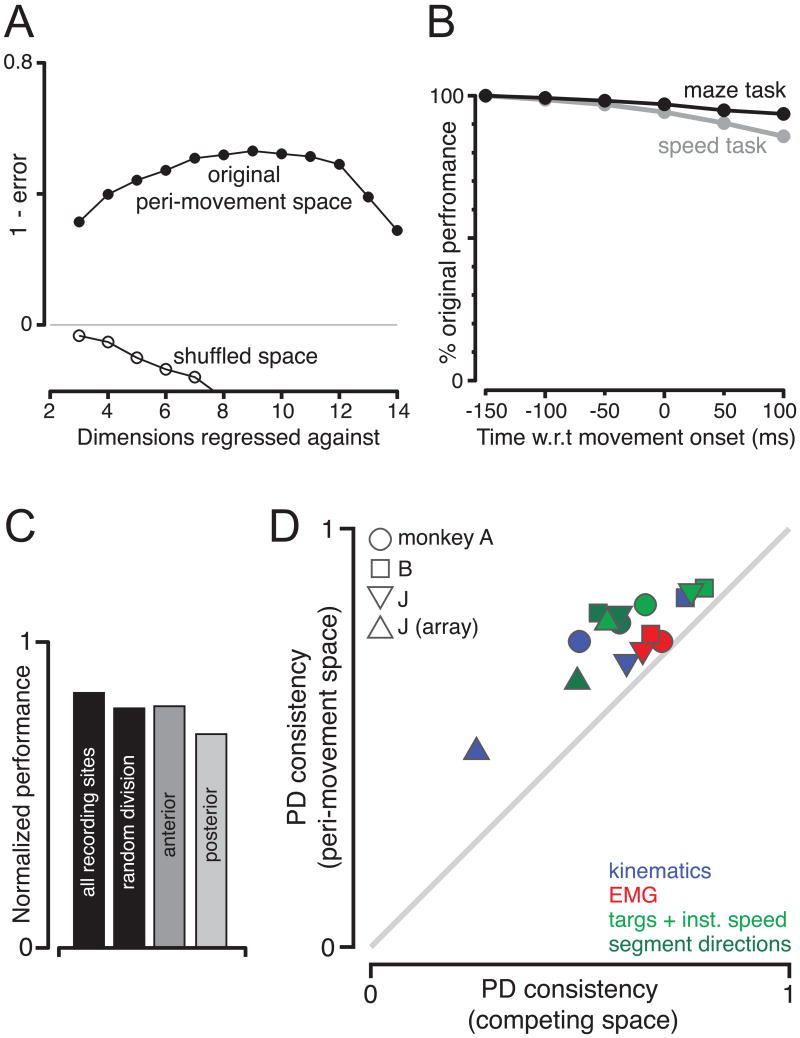
Might the peri-movement space perform well because it is based in part on activity (the beginning of the peri-movement epoch) that correlates with preparatory activity? This is unlikely for three reasons. First, when using a PD to capture a given neuron's preparatory activity, the peri-movement space was based on the responses of all the other neurons (Methods). Second, because peri-movement activity is typically stronger than preparatory activity, the peri-movement space is unlikely to be dominated by the trailing end of preparatory activity. To illustrate this, we employed a shuffled control where each condition's peri-movement response was randomly inverted on half the conditions (legend). The PCA-based ‘shuffled-space’ performed poorly (Figure 7A); any contribution from the trailing end of preparatory activity (preserved despite shuffling) is insufficient to allow good performance. Finally, the start of the peri-movement window was 150 ms before movement onset but could be slid later in time with little decline in performance (Figure 7B). Even when the window began at movement onset (at which point the correlation between preparatory and peri-movement tuning is almost zero, Figure 4E), performance was still at 97% (monkey J), and 94% (monkeys A, B) of its original value.
Figure 7.
Further controls and analyses. A. Performance of the ‘shuffled’ peri-movement spaces, averaged over all the datasets. During shuffling, the firing rate for each neuron/condition, was either left intact (50% probability) or the peri-movement pattern was inverted. This was done by preserving activity up 150 ms before movement onset, and reflecting (vertically) all subsequent activity around the firing rate at that time. B. Performance (averaged across neurons) versus the start of the peri-movement interval. Performance was measured using the best dimensionality for that dataset/start-time. C. Performance of the peri-movement space for subsets of the original data. Performance was computed for a six-dimensional space, normalized, and averaged across datasets. Left-most bar: performance for all data. Gray bars: performance for sites with AP locations >= the median (anterior), <= to the median (posterior). Second black bar: performance when data were randomly subdivided. D. PD consistency for the peri-movement space versus that for a variety of other spaces. Kinematic and EMG spaces were chosen because they had performed the best overall (after the peri-movement space) in the analysis in Fig. 6. ‘Targs. + inst. speed’ and ‘segment directions’ spaces were chosen because they performed well for the speed and maze tasks respectively. ‘Targs. + inst. speed’ was undefined for the maze task; the similar ‘endpoints + max. spd.’ space was analyzed instead.
Might the peri-movement space perform well simply because it is high dimensional? This is unlikely. Regressing against a high-D space will improve fit but not generalization performance (unless those higher dimensions really are relevant). For example, the shuffled space is high-dimensional but performed poorly. Also, the high-dimensional task spaces (including the gratuitously high-dimensional ‘kitchen-sink’ space) always underperformed the peri-movement space. Finally, the peri-movement space performed well even at the lower dimensionalities.
A final possible concern is that the peri-movement space is somehow at an advantage because it involves relating neural activity with other neural activity. This is unlikely for a number of reasons. First, the analysis in Figure 4 similarly related neural activity during the two epochs, yet found little consistent relationship. Second, the measurement of neural activity is noisier than the measurement of kinematic parameters or of EMG. Based on a finite number of neurons, the peri-movement space will, if anything, be at a disadvantage. Third, a given neuron's preparatory activity was explained using a space derived from the peri-movement activity of all the other neurons. Finally, one expects preparatory activity that is tuned for one thing (e.g., reach endpoint) to relate to peri-movement activity tuned for another (e.g., reach velocity). Yet any such secondary relationship should be weaker than the direct relationship between preparatory activity and the factor it is truly tuned for. Yet we found that preparatory activity was best explained in the peri-movement space.
Comparison of anterior versus posterior sites
A number of response properties – including the prevalence of preparatory activity – vary with anterior/posterior location within and between PMd/M1 (Kalaska et al., 1997; Kalaska et al., 1998; Weinrich et al., 1984). We similarly found preparatory activity to be more prevalent at anterior sites (data not shown). However, with respect to our central results, anterior and posterior sites were similar. The poor correlation between preparatory and peri-movement tuning (at the single-cell level) was equally prevalent at anterior and posterior sites. Regressing tuning correlation (as in Figure 4) against AP location yielded little or no effect: r=-0.05, p=0.82; r=-0.03, p=0.85; r=-0.07, p=0.52; and r=0.20, p=0.27 for the four datasets.
The ability of the peri-movement space to capture tuning also held for both anterior and posterior sites (Figure 7C). Some caution is required: any division of the data will likely yield poorer performance, as the peri-movement space is impacted more by sampling noise. To combat this complication, we normalized performance by its estimated upper limit (e.g., the gray line in Figure 6A-D). Normalized performance was only slightly different than if we down-selected the data randomly. Performance was slightly better at anterior sites, though this may occur simply because stronger tuning can be more accurately measured and thus more accurately captured by the PD.
Stability of the PD
A common experimental design involves measuring the PD before and after an imposed manipulation, such as a change in arm posture or the addition of a load (Caminiti et al., 1990; e.g., Kakei et al., 1999; Kalaska et al., 1989; Scott et al., 1997). Often the manipulation is carefully chosen to be maximally revealing with respect to the candidate spaces being considered. However, the central logic holds more generally: if the PD is expressed in the ‘right’ space, it ought not change when re-measured in another context. Our datasets employed many (27-108) conditions, affording the opportunity to perform a less creative, but not necessarily less effective, version of this class of experiment. For each neuron we randomly chose 25 conditions (with replacement), measured the PD, then randomly chose another 25 conditions and re-measured the PD. (This analysis is possible because the PD is computed via regression, which does not require a uniform arrangement of conditions). ‘PD consistency’ was the dot product of the two PDs.
Figure 7D plots average PD consistency in the peri-movement space versus that in a variety of other spaces. The peri-movement space always employed the same dimensionality as the space with which it was compared. We chose two ‘task’ spaces that had performed well for at least one of the two tasks in the analysis in Figure 6. For PCA-based kinematic and EMG spaces, we employed the dimensionality that yielded the highest performance for that dataset in Figure 6. Thus, the task, kinematic, and EMG spaces were given the best possible chance to compete favorably with the peri-movement space. Yet for every comparison, and for all four datasets, the PD was most stable when measured in the peri-movement space.
‘Tuning’ from a dynamical systems perspective
The finding that the PD performs best in a peri-movement space initially appears paradoxical, given the generally weak correlation between preparatory and peri-movement tuning (Figure 4). More broadly, what kind of a representation does this finding imply? How might that representation be decoded? These issues can be addressed under the interpretation that preparatory activity serves as the initial state of a dynamical system whose subsequent evolution produces peri-movement activity (Churchland et al., 2006b; Churchland et al., 2010; Cisek, 2006b; Fetz, 1992; Schaffer et al., 2006; Sussillo and Abbott, 2009; Yu et al., 2009). This suggestion is compatible with both of our basic findings. The critical observation is that the trajectory of a linear dynamical system is a weighted sum of component patterns. Changes in the initial state lead to linear changes in the magnitudes of those component patterns (Figure 8A). If one knows how strongly each pattern is present, one can infer the initial state. This will be approximately true for a nonlinear system, to the degree that it can be approximated by a time-varying linear system (see Supp. Materials for a derivation and further illustration).
Figure 8.
Illustration of the behavior of a simple linear dynamical system: a harmonic oscillator. A. Changes in the initial state produce a linear scaling of the subsequent activity pattern. In this case, the larger the initial state of unit 1, the greater the amplitude of the oscillation. B. State space containing 25 randomly chosen initial states (25 ‘conditions’). Symbol size/color indicates the initial firing rate of unit 1. For the simulations below, activity evolved counterclockwise from the initial state: x(t+1) = Wx(t), where x is the 2-dimensional vector of unit activities, and the matrix W = [1 - 2π/360; 2π/360 1]. Thus, the state rotates one degree with each time-step. C. Activity of unit 1 versus time for the initial states shown in B. The initial state was at first held constant (as if W were the identity matrix) to emulate preparatory activity. The dynamics were then released to produce sinusoidal ‘peri-movement’ activity. Asterisks indicate the same two conditions as in B. D. The pattern of initial states (the ‘preparatory tuning’) from C is captured by a PD in peri-movement space. Analysis/format are the same as for figure 5C. Traces on each axis plot the time-evolving pattern corresponding to that PC (black and blue traces for units one and two). The pattern at the end of the arrow is the weighted sum of the two PCs, with weights corresponding to the PD.
To illustrate the above, Figure 8 shows simple simulated dynamics: circular trajectories through state-space. We employed 25 initial states (25 ‘conditions’, Fig. 8B). These determine the phase and amplitude of the subsequent activity. The system was held at its initial state (a rough analogy with preparatory activity) and then released. At the single-unit level (Figure 8C) preparatory activity seems to bear little relationship with peri-movement activity. The correlation of preparatory activity with peri-movement activity (averaged over the gray interval) was 0.08. Furthermore, a given preparatory firing rate could lead to opposing patterns of peri-movement activity (asterisks, Figure 8C) much as was the case for the data (e.g., Figure 3C). This occurs because a given firing rate for unit 1 can correspond to different initial states (asterisks, Figure 8B).
Yet hidden in Figure 8C is a straightforward relationship. When the initial rate of unit one is high, the subsequent pattern contains a strong cosine-shaped modulation for unit one, and a strong sine-shaped modulation for unit two. The orthogonal activity pattern – a negative sine for unit one and a cosine for unit two – is completely uncorrelated with the initial state of unit one (it relates to the initial state of unit two). Thus, unit one can be said to have a ‘preferred direction’ that points towards the first of these population-level patterns and is orthogonal to the second pattern.
Figure 8D illustrates such a PD (same format as for Figure 5C). PCA yields a space that captures all activity patterns that are possible for this system. Traces on each axis plot the pattern captured by that principal component (black and blue for units one and two). Each symbol's location is given by the weights required so that those patterns sum to the actual peri-movement pattern seen for that condition. Symbol size/color indicates the preparatory rate of unit one for that condition. The PD points towards the pattern recruited by the activity of unit one (panel A), and perfectly captures preparatory ‘tuning’. This occurs because the space in D is a linear transformation (in this case a nearly pure rotation) of the space in B. Given that there was a direction in that original space (the horizontal axis) that captured the initial activity of unit 1, there will also be a direction (the PD) in the transformed space that captures the initial activity of unit 1.
Discussion
Preparatory activity – along with related forms of delay/memory-period activity – has ranked among the most heavily studied varieties of neural activity. This is not accidental: preparatory activity potentially provides a critical link between mechanisms related to cognition and those related to movement. Yet in motor and premotor cortex, the nature of the link between preparatory and movement activity has remained unclear.
We found that preparatory and peri-movement tuning are typically dissimilar. This finding argues against models in which preparatory activity constitutes a sub-threshold version of peri-movement activity. This finding might initially appear to rule out any straightforward relationship between preparatory and peri-movement activity. However, even simple mechanistic models (Fig. 8) can reproduce two key features of the data. First, such models reproduce the poor correlation between preparatory and peri-movement tuning at the single-neuron level. Second, such models reproduce a deeper relationship: preparatory tuning is captured by a PD in the space describing peri-movement activity.
Thus, one interpretation of our data is that preparatory activity exists not to represent specific movement features but to initialize a dynamical system whose evolution will produce peri-movement activity (Churchland et al., 2006b; Cisek, 2006b; Fetz, 1992; Schaffer et al., 2006; Sussillo and Abbott, 2009). Consistent with this interpretation, recent studies (Churchland and Shenoy, 2007a; Churchland et al., 2006c; Rickert et al., 2009; Yu et al., 2009) suggest that preparatory activity is optimized to a state appropriate to drive the movement. In this perspective delay-period activity is truly ‘preparatory’, in the causal sense of setting up the conditions that will permit the generation of appropriate peri-movement activity. Preparatory activity is ‘tuned’ for parameters such as direction and distance only in an indirect way: via its relationship with peri-movement activity. In this view the two central questions – ‘what is preparatory activity tuned for’ and ‘how do preparatory and movement activity relate’ – are in fact the same question. This presents a possible escape (Cisek, 2006b) from the unresolved debate regarding what preparatory activity represents. It has been similarly suggested (Churchland and Shenoy, 2007b; Fetz, 1992; Scott et al., 2001; Todorov, 2000) that peri-movement activity is best understood via its mechanistic role in producing movement.
Still, it remains possible that preparatory and peri-movement activity represent unknown parameters, and do so with tuning that is largely unrelated between preparation and movement. Preparatory activity may represent complex biomechanical features of the upcoming reach (features not currently measured or guessed at) and peri-movement activity may represent similar features in a different way. If so, a space based on peri-movement activity would likely outperform all other tested spaces, if none involved an accurate guess regarding the right features. In this view, the success of the peri-movement space underscores our ignorance regarding the true factors being represented.
The ‘representational’ and ‘dynamical systems’ views are not necessarily at odds. Even if we accept the representational view, the dynamical systems perspective in some sense has to be true. Suppose preparatory activity represents reach endpoint and peri-movement activity represents muscle activity. There must exist lawful and quantifiable dynamics that convert the former representation into the latter. However, such dynamics would need to be fairly complex and non-linear. Preparatory activity would probably not be readily explained by a PD in peri-movement space. Such a PD would certainly underperform the PD in the correct (endpoint) space. Under the representational perspective, if one can identify the correct factors, the PD should perform best in that space. Under the dynamical systems perspective, so long as the dynamics are even approximately linear, the peri-movement space should outperform all traditional spaces.
A caveat under either interpretation is that, while the peri-movement space performed better on average, there were individual neurons whose activity was better captured in one of the other spaces. It seems plausible that some neurons do truly ‘represent’ known task parameters (e.g., visual target location) even if others play a different role. In particular, responses in rostral premotor cortex (from which we did not record) seem more closely tied to visual aspects of the task (Pesaran et al., 2006; Shen and Alexander, 1997).
In summary, we found that the preparatory tuning of an M1/PMd neuron was typically weakly related to its subsequent peri-movement tuning. We then asked which set of variables (which ‘reference frame’) best explained preparatory tuning. The most successful reference frame was a space built to capture the population-level patterns of movement-related activity. These findings are consistent with the view that preparatory tuning serves not to represent specific factors, but to initialize a dynamical system whose future evolution will produce movement.
Methods
Task design and behavior
Animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee. Our basic methods have been described previously (Churchland et al., 2006c). Briefly, monkeys performed delayed reaches on a fronto-parallel screen. Delays ranged from 0-1000 ms (the exact range varied by monkey). To allow sufficient preparatory activity, only trials with delays >400 ms were analyzed. Fixation was enforced (at the central spot) during the delay for monkey J only.
For single-electrode recordings using the maze task, we employed 4 sets of 27 mazes each. Each neuron was recorded for one or more of these, but rarely for all. The monkey J dataset thus consists of four smaller datasets, of ∼53 neurons each. Population-level analyses were performed at the level of these smaller datasets and then averaged. For the monkey J-array dataset, all 108 conditions were interleaved in the standard way.
Neural recordings and datasets
Penetrations were guided by stereotaxic critera, the known response properties of M1 and PMd, and the effects of microstimulation. Recordings were medial to the arcuate spur and lateral to – or in a few instances within the lateral bank of – the precentral dimple. Few if any recordings were made within rostral PMd, near the arcuate sulcus. Analysis of preparatory responses was restricted to neurons with at least 10 spikes/s of preparatory tuning.
Electrode arrays were implanted in PMd and surface M1 (Supp. Fig. 3). The resulting dataset involved simultaneous recordings and much larger trial counts per neuron (2155). However, isolations were only occasionally of the same high isolation quality as for the single-electrode recordings. Many array recordings were of contaminated single-unit isolations or isolations of two or more neurons. Still, some array-based isolations were of high quality, including those in Figure 3E,F. Firing rate modulation was typically lower for the array recordings, presumably due to the lack of selection bias. We thus used a lower inclusion criterion: >5 spikes/s of preparatory tuning, which was acceptable given the higher signal-to-noise ratio provided by the higher trial count.
Correlations between preparatory and peri-movement tuning
For the preparatory epoch, firing rate was averaged from 200 ms before the go cue until 100 ms after. For the peri-movement epoch, activity was averaged from 100 ms before movement onset until 350 ms after. The vector of preparatory rates (one per condition) was correlated with the vector of peri-movement rates. Analysis was restricted to neurons with robust peri-movement tuning (at least 1.5 times as strong as preparatory tuning). For all other analyses (e.g., those in figures 5-7) neurons were analyzed regardless of the strength of peri-movement activity.
Using a PD to capture preparatory tuning
Activity during the preparatory epoch was fit using the model, xn ≈ bo + Sb, where xn is a c×1 vector of preparatory firing rates (one for each of the c conditions) for neuron n, S is a c×k matrix containing the location of every condition in a k dimensional space, bo is a scalar firing rate offset, and b is a k×1 vector of coefficients. b and bo were found using linear regression (Matlab, Mathworks). E.g., if S describes the location of each condition in the space of horizontal and vertical target locations, b might be [1;0] for a neuron with a rightwards preference. The matrix S is the same for every neuron, but all other variables differ. The vector b can be represented as g*PD: a gain times a preferred direction of unit length.
Task spaces
The ‘targets + instructed speed’ space was 3 dimensional: horizontal and vertical target location plus instructed speed (1 for slow, 2 for fast). A similar space, ‘endpoints + maximum speed’, was constructed based on the actual reach endpoints and peak speed. Other task spaces were ‘initial velocity’ (2-D), the average horizontal and vertical velocity during the first 150 ms of the reach; ‘endpoints + orthogonal excursion’ (3-D), the horizontal and vertical endpoints and the maximum excursion from a straight reach (positive for counterclockwise); ‘endpoints + viapoint’ (4-D), the horizontal and vertical endpoints and the horizontal and vertical co-ordinates of the maximum departure from a straight reach; ‘endpoints + maximum velocity’ (4-D), the horizontal and vertical endpoints and peak horizontal and vertical velocities; ‘endpoints + halfpoint’ (4-D), the horizontal and vertical endpoints and horizontal and vertical co-ordinates of the point halfway along the reach trajectory; ‘segment directions’ (6-D), the individual horizontal and vertical displacements after dividing the reach trajectory into three segments; and ‘kitchen sink’ (9-D and 11-D for the speed and maze tasks). This last space was designed primarily as a control for whether high-dimensionality alone is sufficient to yield good performance. It included the segment directions, the maximum counter-clockwise excursion from a straight line, and the horizontal and vertical location of that maximum excursion. For monkey J it also included whether any barriers were present (0 or 1) and whether distractor targets were present (0 or 1).
PCA-based spaces
To illustrate this approach, consider that reaches for different conditions differ in velocity at most times. To fully characterize such differences, we can create a c×2t matrix T, in which each row corresponds to a condition and contains horizontal and vertical reach velocities for all t times. We can then PCA to reduce dimensionality from c×2t to c×k. The resulting matrix ‘Tred’ then captures the differences between conditions in a k-dimensional space. Note that PCA is more commonly applied to a matrix where each row corresponds to a time and each column to a neuron. Dimensionality reduction is then used to denoise and visualize responses (for review see Churchland et al., 2007). Here PCA is used to parameterize key differences between conditions, rather as one could have done by hand-picking k features such as peak velocity. See Supp. Fig. 2 for further illustration.
The peri-movement T-matrix contained data from 150 ms before movement onset until 400 ms (speed task) or 800 ms (maze task) after onset. Each row contained data from all neurons except the neuron whose preparatory activity we were trying to capture. Responses were not normalized; neurons with weak peri-movement activity were included but had little impact on Tred.
Each row of the kinematic T-matrix included horizontal and vertical position, velocity and acceleration (150 ms before movement onset until after the reach), normalized to have unity range across times/conditions. Each neuron contributed one kinematic variable (so that the T-matrix was of similar size to that for peri-movement activity).
Each row of the EMG T-matrix contained activity for multiple muscles (same time-window as for the neural data). Each muscle's activity was normalized to have a unity range across times/conditions (a necessity, given the arbitrary units of EMG).
PD performance
Performance was assessed using leave-one-out cross validation. One condition was left out and we found the PD that best captured tuning for the remaining conditions. We then computed the error between the left-out preparatory firing rate and that predicted by the PD. Performance was quantified as one minus the mean squared error (averaged across all conditions, each left out in turn) normalized by the variance of the data. Thus, performance equal to one indicates that the PD generalized perfectly. Performance equal to zero indicates that one could have done similarly well by ignoring the PD, and predicting every condition's firing rate to be the mean firing rate. Performance below zero typically indicates overfitting.
Supplementary Material
Acknowledgments
This work was supported by a Helen Hay Whitney postdoctoral fellowship and NIH postdoctoral training fellowship (MMC), the Burroughs Welcome Fund Career Awards in the Biomedical Sciences (MMC, KVS),, the Michael Flynn Stanford Graduate Fellowship and NIH-NINDS-CRCNS-R01 (JPC), a National Science Foundation graduate research fellowship (MTK), and these awards to KVS: NIH Director's Pioneer Award 1DP1OD006409, NIH NINDS R01-NS054283, Stanford Center for Integrated Systems, NSF Center for Neuromorphic Systems Engineering at Caltech, Office of Naval Research, and the Whitaker Foundation. We thank M. Risch for surgical and veterinary assistance and S. Eisensee for administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastian A, Riehle A, Erlhagen W, Schoner G. Prior information preshapes the population representation of movement direction in motor cortex. Neuroreport. 1998;9:315–319. doi: 10.1097/00001756-199801260-00025. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schoner G, Riehle A. Preshaping and continuous evolution of motor cortical representations during movement preparation. Eur J Neurosci. 2003;18:2047–2058. doi: 10.1046/j.1460-9568.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- Batista AP, Santhanam G, Yu BM, Ryu SI, Afshar A, Shenoy KV. Reference frames for reach planning in macaque dorsal premotor cortex. J Neurophysiol. 2007;98:966–983. doi: 10.1152/jn.00421.2006. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Johnson PB, Urbano A. Making arm movements within different parts of space: dynamic aspects in the primate motor cortex. J Neurosci. 1990;10:2039–2058. doi: 10.1523/JNEUROSCI.10-07-02039.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron. 2006a;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol. 2006b;96:3130–3146. doi: 10.1152/jn.00307.2006. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol. 2007a;97:348–359. doi: 10.1152/jn.00808.2006. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Temporal complexity and heterogeneity of single-neuron activity in premotor and motor cortex. J Neurophysiol. 2007b;97:4235–4257. doi: 10.1152/jn.00095.2007. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J Neurosci. 2006c;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Sahani M, Shenoy KV. Techniques for extracting single-trial activity patterns from large-scale neural recordings. Curr Opin Neurobiol. 2007;17:609–618. doi: 10.1016/j.conb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: a computational model. J Neurosci. 2006a;26:9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Preparing for speed. Focus on: “Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach”. J Neurophysiol. 2006b doi: 10.1152/jn.00857.2006. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- Erlhagen W, Bastian A, Jancke D, Riehle A, Schoner G. The distribution of neuronal population activation (DPA) as a tool to study interaction and integration in cortical representations. J Neurosci Methods. 1999;94:53–66. doi: 10.1016/s0165-0270(99)00125-9. [DOI] [PubMed] [Google Scholar]
- Erlhagen W, Schoner G. Dynamic field theory of movement preparation. Psychol Rev. 2002;109:545–572. doi: 10.1037/0033-295x.109.3.545. [DOI] [PubMed] [Google Scholar]
- Fetz EE. Are movement parameters recognizably coded in the activity of single neurons? Behavioral and Brain Sciences. 1992;15:679–690. [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol. 1995;73:836–854. doi: 10.1152/jn.1995.73.2.836. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Crutcher MD, Schwartz AB. Cognitive spatial-motor processes. 3. Motor cortical prediction of movement direction during an instructed delay period. Exp Brain Res. 1989;75:183–194. doi: 10.1007/BF00248541. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Hening W, Gordon J. Organization of voluntary movement. Curr Opin Neurobiol. 1991;1:664–671. doi: 10.1016/s0959-4388(05)80046-7. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Xu Q, Amit Y. Encoding of movement fragments in the motor cortex. J Neurosci. 2007;27:5105–5114. doi: 10.1523/JNEUROSCI.3570-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocherman S, Wise SP. Effects of hand movement path on motor cortical activity in awake, behaving rhesus monkeys. Exp Brain Res. 1991;83:285–302. doi: 10.1007/BF00231153. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud'homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci. 1989;9:2080–2102. doi: 10.1523/JNEUROSCI.09-06-02080.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol. 1997;7:849–859. doi: 10.1016/s0959-4388(97)80146-8. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Sergio LE, Cisek P. Cortical control of whole-arm motor tasks. Novartis Foundation symposium. 1998:176–190. doi: 10.1002/9780470515563.ch10. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Santhanam G, Yu BM, Afshar A, Ryu SI, Shenoy KV. The roles of monkey premotor neuron classes in movement preparation and execution. J Neurophysiol. 2010 doi: 10.1152/jn.00231.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. J Neurophysiol. 2000;84:152–165. doi: 10.1152/jn.2000.84.1.152. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA. Do neurons in the motor cortex encode movement direction? An alternative hypothesis. Neurosci Lett. 1988;91:106–111. doi: 10.1016/0304-3940(88)90257-1. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Dorsal premotor neurons encode the relative position of the hand, eye, and goal during reach planning. Neuron. 2006;51:125–134. doi: 10.1016/j.neuron.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin J, Riehle A, Seal J. Neuronal activity and information processing in motor control: from stages to continuous flow. Biol Psychol. 1988;26:179–198. doi: 10.1016/0301-0511(88)90019-1. [DOI] [PubMed] [Google Scholar]
- Rickert J, Riehle A, Aertsen A, Rotter S, Nawrot MP. Dynamic encoding of movement direction in motor cortical neurons. J Neurosci. 2009;29:13870–13882. doi: 10.1523/JNEUROSCI.5441-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res. 1993;53:35–49. doi: 10.1016/s0166-4328(05)80264-5. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA. Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen. 1980;109:444–474. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Theoretical considerations for the analysis of population coding in motor cortex. Neural Computation. 1994;6:29–37. [Google Scholar]
- Schaffer ES, Rajan K, Churchland MM, Shenoy KV, Abbott LF. Society for Neuroscience Annueal Meeting (Atlanta, GA) 2006. Generating complex repeatable patterns of activity by gain modulating network neurons. [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Scott SH. Role of motor cortex in coordinating multi-joint movements: is it time for a new paradigm? Can J Physiol Pharmacol. 2000;78:923–933. [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586:1217–1224. doi: 10.1113/jphysiol.2007.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH, Gribble PL, Graham KM, Cabel DW. Dissociation between hand motion and population vectors from neural activity in motor cortex. Nature. 2001;413:161–165. doi: 10.1038/35093102. [DOI] [PubMed] [Google Scholar]
- Scott SH, Sergio LE, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. II. Activity of individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol. 1997;78:2413–2426. doi: 10.1152/jn.1997.78.5.2413. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Paquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol. 2005;94:2353–2378. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Shen L, Alexander GE. Preferential representation of instructed target location versus limb trajectory in dorsal premotor area. J Neurophysiol. 1997;77:1195–1212. doi: 10.1152/jn.1997.77.3.1195. [DOI] [PubMed] [Google Scholar]
- Sussillo D, Abbott LF. Generating coherent patterns of activity from chaotic neural networks. Neuron. 2009;63:544–557. doi: 10.1016/j.neuron.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol. 1976;39:1062–1068. doi: 10.1152/jn.1976.39.5.1062. [DOI] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Turner RS. Movement- and instruction-related activity in the globus pallidus of the monkey. University of Washington; 1991. [Google Scholar]
- Weinrich M, Wise SP, Mauritz KH. A neurophysiological study of the premotor cortex in the rhesus monkey. Brain. 1984;107(Pt 2):385–414. doi: 10.1093/brain/107.2.385. [DOI] [PubMed] [Google Scholar]
- Wise SP, Weinrich M, Mauritz KH. Movement-related activity in the premotor cortex of rhesus macaques. Prog Brain Res. 1986;64:117–131. doi: 10.1016/S0079-6123(08)63407-X. [DOI] [PubMed] [Google Scholar]
- Yu BM, Cunningham JP, Santhanam G, Ryu SI, Shenoy KV, Sahani M. Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. J Neurophysiol. 2009;102:614–635. doi: 10.1152/jn.90941.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.