Summary
Oncogene addiction is thought to occur cell autonomously. Immune effectors are implicated in the induction and restraint of tumorigenesis, but their role in oncogene inactivation mediated tumor regression is unclear. Here, we show that an intact immune system, specifically CD4+ T-cells, is required for the induction of cellular senescence, shut down of angiogenesis and chemokine expression resulting in sustained tumor regression upon inactivation of the MYC or BCR-ABL oncogenes in mouse models of T-cell acute lymphoblastic lymphoma and pro-B-cell leukemia, respectively. Moreover, immune effectors knocked out for thrombospondins failed to induce sustained tumor regression. Hence, CD4+ T-cells are required for the remodeling of the tumor microenvironment through the expression of chemokines, such as thrombospondins, in order to elicit oncogene addiction.
Introduction
The inactivation of a single oncogene is sufficient to induce sustained tumor regression in vivo through the phenomenon of oncogene addiction, as has been demonstrated experimentally in many conditional transgenic mouse model systems (Felsher, 2008; Sharma and Settleman, 2007; Weinstein and Joe, 2008) and through the development of targeted therapeutics such as Gleevec (Weinstein and Joe, 2006). Oncogene addiction is associated with proliferative arrest, apoptosis, differentiation, and cellular senescence as well as the shut down of host programs such as angiogenesis (Felsher, 2003; Felsher and Bishop, 1999; Giuriato et al., 2006; Jain et al., 2002; Shachaf et al., 2004; Wu et al., 2007). To date, it has been presumed that oncogene inactivation induces tumor regression through cell autonomous mechanisms, independent of host effector cells.
The host immune system plays an important role in tumorigenesis. Both antigen-dependent and -independent mechanisms are implicated through multiple cellular effectors and effects on inflammation and the tumor microenvironment (Crowe et al., 2002; Shankaran et al., 2001). Indeed, it is well documented that CD8+ T-cells contribute to antigen-dependent and NK cell-mediated tumor elimination (Shanker et al., 2007; van der Bruggen et al., 1991). Additionally, CD4+ T-cells may also contribute to tumor regression (Corthay et al., 2005; Qin and Blankenstein, 2000). Chemokines produced by the immune system have been shown to play an important role during tumor evolution and therapeutic response (Rossi and Zlotnik, 2000; Smyth et al., 2004).
In general, tumors co-evolve with host immune effectors and chemokines through a process that has been described as immune editing (Dougan and Dranoff, 2009; Dunn et al., 2002; Dunn et al., 2006; Reiman et al., 2007; Swann et al., 2008). Immune editing has been dramatically illustrated in several models of carcinogenesis (Bui et al., 2006; Shankaran et al., 2001; Willimsky and Blankenstein, 2005). Host immune effectors also contribute to the initiation of tumorigenesis through profound effects on the tumor microenvironment (Coussens and Werb, 2002; de Visser et al., 2006). Thus, the immune system appears to play a complex role in both the initiation and restraint of tumorigenesis.
The role of the immune system in mediating tumor regression upon targeted oncogene inactivation is not known. Hosts that are immune compromised have a markedly increased incidence of many different types of cancers (Birkeland et al., 1995; Dunn et al., 2002; Pham et al., 1995). The host immune system is intimately involved not only in the promotion and prevention of neoplasia but also in determining the therapeutic response to treatment of cancer (Andreu et al., 2010; Boshoff and Weiss, 2002; Dave et al., 2004; Galon et al., 2006; Gatti and Good, 1971; Kohrt et al., 2005; Zitvogel et al., 2008). However, experimental study of new therapeutics for cancer is usually performed in vitro or in vivo in immune compromised hosts, circumstances in which immune effectors are necessarily absent. These models do not account for the role of host-tumor interactions and the role of the immune system.
The MYC oncogene has been implicated in the pathogenesis of many human tumors (Meyer and Penn, 2008). MYC is involved in the etiology of many types of lymphoma including Burkitt’s large cell and T-cell acute lymphoblastic lymphoma (TALL) (Boxer and Dang, 2001; Pelengaris et al., 2002). We have previously described our conditional transgenic mouse models of MYC-induced T-cell acute lymphoblastic lymphoma (T-ALL) (Felsher and Bishop, 1999). The inactivation of MYC is sufficient to induce sustained tumor regression associated with proliferative arrest, apoptosis, differentiation, cellular senescence and the shut down of angiogenesis (Felsher and Bishop, 1999; Giuriato et al., 2006; Wu et al., 2007). Here, we show that defects in the host immune system have a profound influence on the ability of oncogene inactivation to elicit oncogene addiction.
Results
Immune System is Required for Rapid, Complete and Sustained Tumor Regression
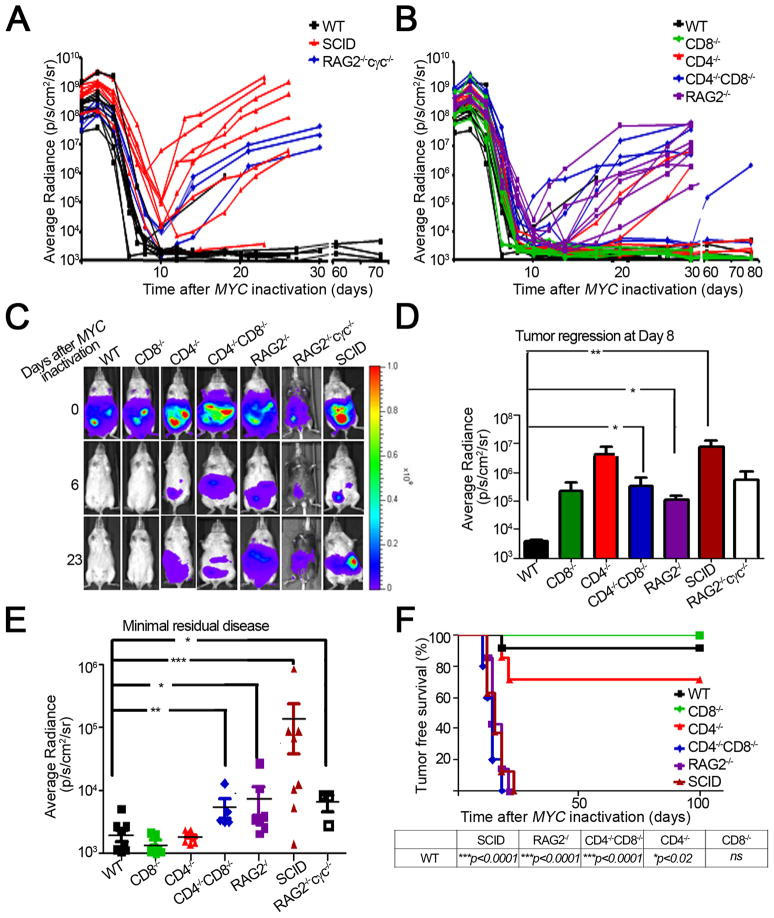
To interrogate if the immune system is required to elicit oncogene addiction upon MYC inactivation, we transplanted luciferase labelled tumors from our conditional transgenic mouse model of MYC-induced hematopoietic tumorigenesis into wild-type hosts and in hosts with specific defects in immune compartments: SCID, RAG2−/−cγc−/−, RAG2−/−, CD4−/−CD8−/−, CD4−/−CD8+/+, CD4+/+CD8−/− (Figure 1A, B). By using bioluminescence imaging, we could measure the kinetics of tumor regression upon MYC inactivation.
Figure 1. An intact immune system is required for sustained tumor regression.
1(A, B): Graphical representation of tumor regression and relapse kinetics as measured by bioluminescence imaging. Luciferase-labeled tumor cell lines from our conditional mouse T-ALL model were injected s.c. into different cohorts of mice (WT n = 11, CD8−/− n = 7, CD4−/− n = 6, CD4−/−CD8−/− n = 5, SCID n = 8, RAG2−/− n = 7, RAG2−/−cγc−/− n = 3). MYC was inactivated by administering doxycycline (dox) to the mice when tumors reached a comparable bioluminescence signal (108p/s/sr/cm2). 1(C): Bioluminescence images of tumors regressing in the different immunodeficient hosts. Data is representative of 3 experiments. 1(D): Quantitative analysis of tumor regression in the indicated hosts, 8 days post MYC inactivation. 1(E): Quantification of minimum residual disease in the indicated hosts. Data is presented as the minimum bioluminescence signal after MYC inactivation. 1(F): Kaplan Meier curves of tumor-free survival in the various immunodeficient genotypes. A mouse was scored as a relapse when its tumor bioluminescence signal first began to increase after tumor regression. The log-rank test was used to compare survival curves. Data is representative of 3 experiments using 2 cell lines and 1 primary tumor. Statistical significance was analyzed by pooling data from all experiments (WT n = 43, CD8−/− n = 20, CD4−/− n = 24, CD4−/−CD8−/− n = 15, SCID n = 15, RAG2−/− n = 46) (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are +/− SEM. See also Figure S1
Tumors initially exhibited regression regardless of the host immune status (Figure 1A, B, C). However, severely immune compromised hosts (SCID and RAG2−/−cγc−/− mice deficient in the adaptive immune system and NK cells) demonstrated significantly delayed kinetics of tumor regression upon MYC inactivation compared to wild-type (WT) hosts (Figure 1D, SCID versus WT, p < 0.001) and failed to execute complete tumor elimination with up to 1,000-fold more minimal residual disease (MRD) after MYC inactivation (Figure 1E, SCID versus WT, p < 0.001; RAG2−/− cγc−/− versus WT, p = 0.01 at the nadir of luciferase activity upon MYC inactivation). Similarly, less severely immune compromised hosts also exhibited delayed kinetics (Figure 1B, D, RAG2−/− versus WT, p = 0.02; CD4−/−CD8−/− versus WT, p = 0.02) and a significantly increased MRD (Figure 1E, RAG2−/− versus WT, p = 0.01; CD4−/−CD8−/− versus WT, p < 0.01). Hence, an intact immune system is required for rapid and complete tumor regression.
To determine if host immune status influenced the frequency of tumor recurrence, we continued to observe mice for 80 days after MYC inactivation noting that tumors recurred at a statistically significant higher frequency in SCID, RAG2−/−cγc−/−, RAG2−/−, and CD4−/−CD8−/− hosts (87.5%, 100%, 100%, and 80% respectively) compared to WT hosts (9%) (immune compromised hosts versus WT, p < 0.0001, also see Figure 1F). CD4−/− but not CD8−/− deficient hosts exhibited a significant influence on tumor recurrence (28.5%, 0% respectively) (Figure 1F). Correspondingly, CD4+, but not CD8+ T-cell deficiency alone was sufficient to impede sustained tumor regression compared to WT mice (Figure 1F, WT versus CD4−/−, p = 0.02). Similar results could be obtained using non-luciferase labelled tumors (Figure S1A). By qPCR analysis it was confirmed that doxycycline treatment resulted in similar suppression of transgenic MYC expression regardless of host immune status (Figure S1B). Hence, defects in the host immune system prevented sustained tumor regression upon MYC inactivation.
Immune System is not Required to Induce Proliferative Arrest or Apoptosis
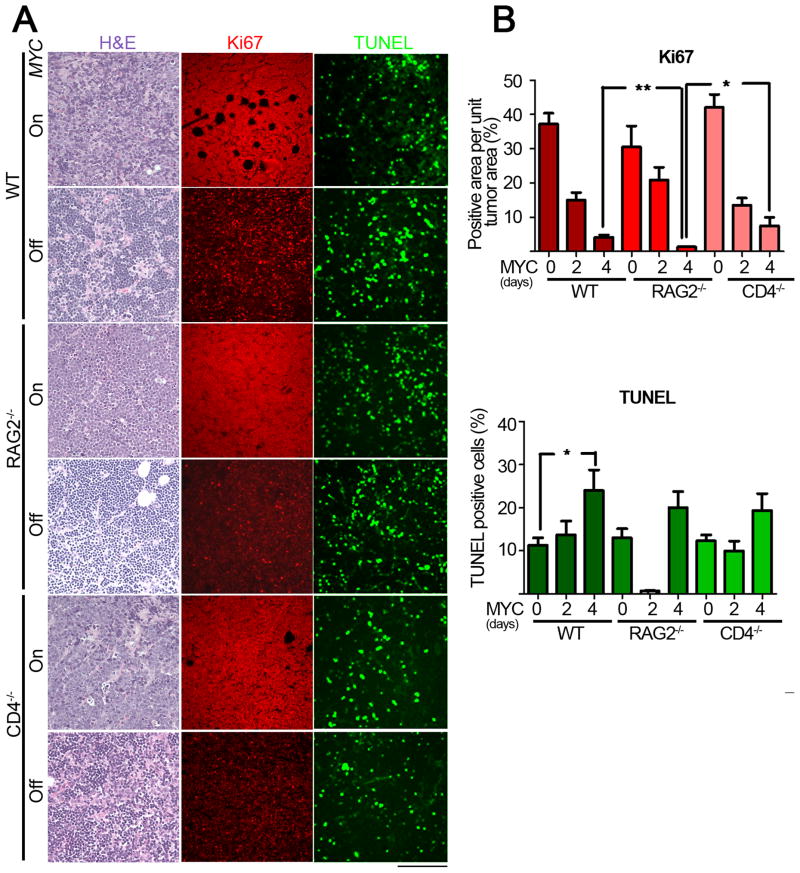
Previously, we have shown that upon MYC inactivation in a transgenic model of TALL, tumor cells undergo proliferative arrest and apoptosis (Felsher and Bishop, 1999). We determined if the mechanism by which immune cells were contributing to the process of tumor regression was through effects on proliferation and apoptosis of tumor cells before and after MYC inactivation (Figure 2A, B). After 4 days of MYC inactivation, tumors from wildtype and immunodeficient hosts exhibited an overall loss of pleomorphic characteristics evidenced by a similarly marked reduction in cell size and nuclear to cytoplasmic ratio in both cohorts. Importantly, upon MYC inactivation, we observed marked changes in the total number of cells per field and carefully controlled for these changes in our quantification of TUNEL and Ki67 staining.
Figure 2. The immune system does not influence apoptosis or proliferative arrest upon MYC inactivation.
2(A): Micrographs of Hematoxylin and Eosin staining (left panel), TUNEL (middle panel) and Ki67 (right panel) immunostaining of tumors derived from untreated (MYC On), two-day and four-day dox treated mice (MYC Off) from WT (top panel) RAG2−/− (middle panel) and CD4−/− (bottom panel) hosts. Scale Bar = 100 μm. 2(B): Quantitative representation of Ki67 (top panel) and TUNEL (bottom panel) immunostaining shown in 2(A) for 0, 2 and 4d after MYC inactivation. Quantification of TUNEL and Ki67 immunostaining is presented as the average percentage of TUNEL-positive cells and area of Ki67-positive regions, respectively, within the tumors. At least five different fields from three different tumors injected with at least two different tumor cell lines for each different condition. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are represented as +/− SEM.
To measure apoptosis, TUNEL staining was performed. Apoptosis occurred similarly upon MYC inactivation regardless of host immune status (Figure 2A) suggesting that initial tumor regression occurs similarly regardless of the presence or absence of an immune system. Quantification of TUNEL staining revealed a 2-fold increase in the extent of apoptosis upon MYC inactivation in tumors from WT hosts (Figure 2B, WT MYC On versus Off, p = 0.05). Moreover, the apoptosis in regressing tumors from WT hosts was not significantly different from that of regressing tumors in either RAG2−/− or CD4−/− hosts (Figure 2B, WT versus RAG2−/−, CD4−/− MYC Off, p = 0.3 and 0.3 respectively). Finally there was a small but statistically insignificant increase in the levels of apoptosis upon MYC inactivation in RAG2−/− or CD4−/− hosts (Figure 2B, RAG2−/−, CD4−/− MYC On versus Off, p = 0.07 and 0.09 respectively). Hence, the absence of the immune system may slightly increase but certainly does not impede apoptosis of tumor cells upon MYC inactivation.
Next, changes in cellular proliferation upon MYC inactivation were measured by Ki67 staining. MYC inactivation in tumors from WT and immunodeficient hosts resulted in a significant reduction in Ki67 staining (Figure 2A, B, WT, RAG2−/−, CD4−/− MYC On versus MYC Off, p < 0.01). Interestingly, in comparison to WT hosts, RAG2−/− but not CD4−/− hosts, underwent a statistically significant further decrease in Ki67 staining upon MYC inactivation (WT versus RAG2−/− or CD4−/− MYC Off, p = 0.02 or p < 0.05, respectively). Thus, the absence of the host immune system either has no effect or modestly enhances the effect of MYC inactivation in inducing proliferative arrest.
Immune System is Required to Induce Cellular Senescence and the Shut Down of Angiogenesis
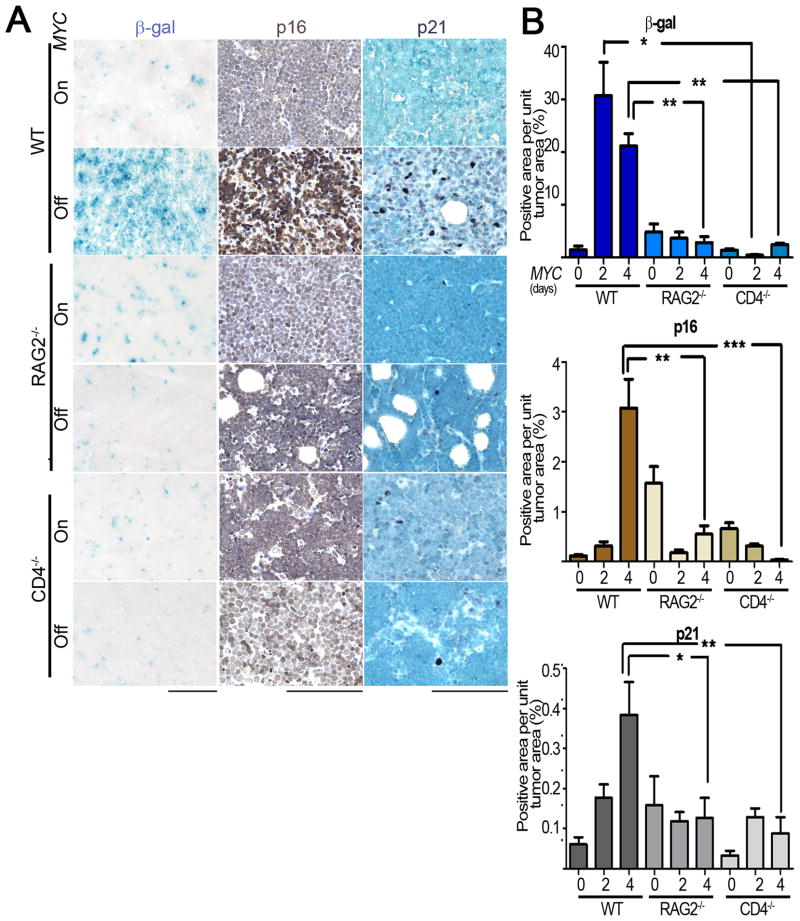
We have reported that upon MYC inactivation tumor cells undergo cellular senescence (Wu et al., 2007) and the shut down of angiogenesis (Giuriato et al., 2006). We examined the role of both processes. Tumors from WT hosts expressed a 20-fold increase in senescence-associated acidic β-gal (SA-β-Gal) activity upon MYC inactivation and demonstrated a 26- and 6-fold increase in senescence-associated markers, p16INK4a and p21, respectively, upon MYC inactivation (Figure 3A, B). In contrast, MYC inactivation in tumors in RAG2−/− and CD4−/− mice did not result in increased SA-β-Gal or the induction of p16INK4a or p21 (Figure 3A, B, WT versus RAG2−/− MYC Off SA-β-Gal, p = 0.01, p16 staining p = 0.002, p21 staining p = 0.01; WT versus CD4−/−, MYC Off SA- β-Gal, p = 0.009, p16 staining, p = 0.0005, p21 staining, p = 0.004). Thus, in immune deficient mice, MYC inactivation is impeded from inducing cellular senescence in tumor cells. Notably, CD4+ T-cells specifically appeared to be required.
Figure 3. An intact immune system is required for the induction of cellular senescence upon MYC inactivation.
3(A): Micrographs of Senescence Associated β-galactosidease (SA β-gal, left panel), p16 (middle panel) and p21 (right panel) immunostaining of tumors derived from untreated (MYC On), two-day and four-day dox treated (MYC Off) mice of the indicated genotypes. Scale Bar = 100 μm. 3(B): Quantification of SA-β-gal (top panel), p16 (middle panel) and p21 (bottom panel) staining shown in 3(A) for 0, 2 and 4 days after MYC inactivation. Quantification is presented as the average percentage of positively stained regions within the tumors. At least five different fields from three different tumors injected with at least two different tumor cell lines were analyzed for each different condition. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are +/− SEM.
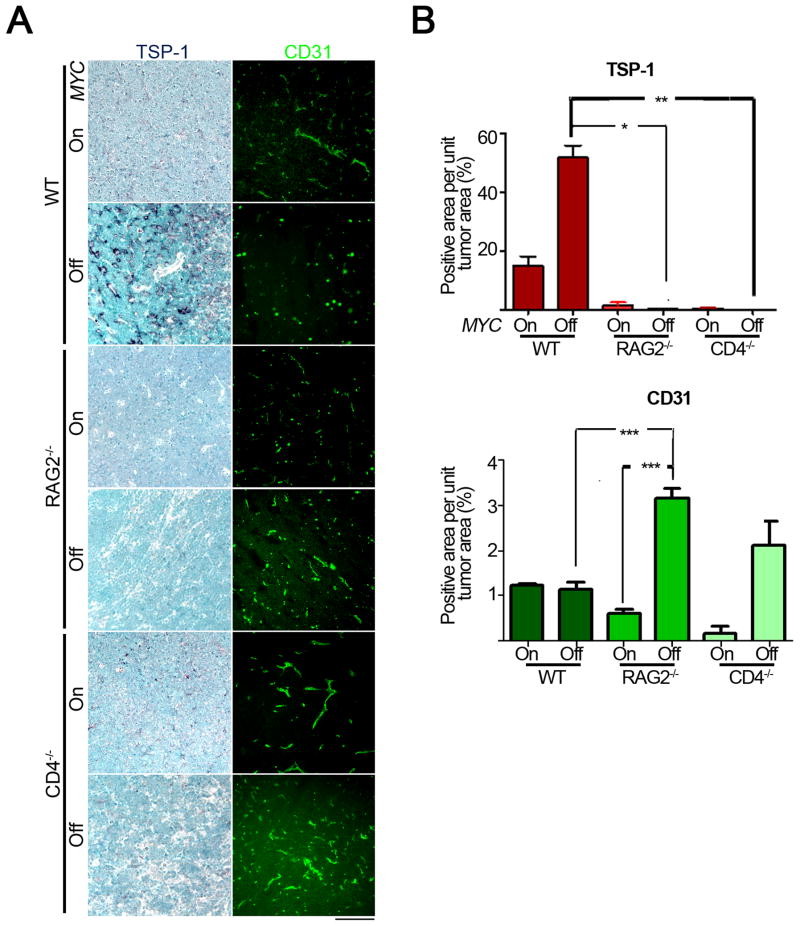
We determined if an intact immune system was required for MYC inactivation to induce the shut down of angiogenesis associated with the secretion of TSP-1, a potent anti-angiogenic protein (Giuriato et al., 2006; Kazerounian et al., 2008; Lawler, 2000). Upon MYC inactivation there was a 3.5-fold induction of TSP-1 in tumors from WT hosts but not in RAG2−/− or CD4−/− hosts (Figure 4A, WT versus RAG2−/−, CD4−/− MYC Off, p = 0.001). Furthermore, while tumors in WT mice demonstrated very little change in mean vascular density (MVD) as measured by CD31 staining upon MYC inactivation (Figure 4B), RAG2−/− and CD4−/− mice exhibited a 5- and 12-fold, respectively, increase in tumor MVD upon MYC inactivation (Figure 4A, B, RAG2−/− MYC On versus Off, p < 0.0001; CD4−/− MYC On versus Off, p = 0.07). Thus, the absence of CD4+ T-cells impairs the ability of MYC inactivation to induce cellular senescence as well as shut down angiogenesis.
Figure 4. An intact immune system is required for the inhibition of angiogenesis upon MYC inactivation.
4(A): Micrographs of TSP-1 (left panel) and CD31 (right panel) immunohistochemical and immunofluorescence staining of tumors derived from untreated (MYC On) and 4 day dox treated (MYC Off) mice of the indicated genotypes. Scale Bar = 100μm. 4(B): Quantification of TSP-1 (top panel) and CD31 (bottom panel) staining shown in 4(A). Quantification is presented as the average percentage of positively stained regions within the tumors. At least five different fields from two different tumors were analyzed for each different condition. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are +/− SEM. See also Figure S2
Finally, TSP-1 expression requires host immune cells and specifically CD4+ T cells. Indeed, we found that TSP-1 protein expression is markedly decreased in spleens of immune compromised versus wild type hosts (Figure S2A). Further, we show that activated CD4+ T-cells express TSP-1 (Figure S2B).
CD4+ T-cells Home to the Tumor and are Sufficient to Restore Sustained Tumor Regression
We examined if CD4+ T-cells were homing to the tumor site upon oncogene inactivation. Upon adoptive transfer into RAG2−/− hosts, luciferase+ CD4+ T-cells rapidly localized to the tumor site upon MYC inactivation as seen by bioluminescence imaging of these tumors before and after MYC inactivation (Figure 5A). Inactivating this oncogene causes CD4+ T-cells to localize at the tumor site as early as 4 days after oncogene inactivation, peak at day 12 and persist up to 3 weeks after MYC inactivation. Thus, MYC inactivation is associated with trafficking of CD4+ T-cells to sites of tumor involvement. Notably, CD4+ T-cell depleted luciferase+ splenocytes also localized to the site of the tumor upon MYC inactivation, suggesting the recruitment of additional host immune effector populations (Figure S3A).
Figure 5. CD4+ T-cells home to the tumor and are sufficient to induce sustained tumor regression upon MYC inactivation.
5(A): Bioluminescence signal of luciferase+CD4+ T-cells that home to the tumor microenvironment. RAG2−/− mice were reconstituted with luciferase+ CD4+ T-cells and unlabeled tumor cell lines were injected s.c. 8 days post reconstitution. MYC was inactivated when tumors grew to a size of 1000 mm3. Data is represented as bioluminescence signal (average radiance) plotted against time after MYC inactivation (n=3). 5(B): Tumor regression and relapse kinetics measured by bioluminescence imaging. RAG2−/− mice were reconstituted with CD4+ (RAG2−/−reconst. CD4+Tcells, n=5) or CD8+ (RAG2−/−reconst. CD8+Tcells, n=6) T-cells from WT mice. 8 days after reconstitution, luciferase+ tumor cell lines were injected s.c. MYC was inactivated when tumors in all hosts reached a comparable bioluminescence signal. Data is presented as bioluminescence signal (average radiance) plotted against time after MYC inactivation. WT (n=3) and RAG2−/− (n=3) mice were used as positive and negative controls. 5(C): Quantification of minimum residual disease. Bioluminescence signals of tumors at their maximally regressed state are plotted against genotype. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are +/− SEM. 5(D): Kaplan Meier curves of tumor-free survival in the reconstituted RAG2−/−, RAG2−/− and WT mice. Log-rank test was used compare the survival curves. Data is representative of 3 experiments. Statistics were performed including all data: n = 14, RAG reconstituted with CD8+ T-cells: n = 12. reconst. = reconstituted with. See also Figure S3.
Next, we evaluated if we could restore the ability of MYC inactivation to induce sustained tumor regression in immune compromised hosts by adoptively transferring specific lymphocyte populations into RAG2−/− mice. By FACS analysis, we confirmed reconstitution of effector cells (Figure S3B). As expected, RAG2−/− mice adoptively transferred with splenic lymphocytes exhibited sustained regression (Figure 6B). RAG2−/− hosts demonstrated a significant amount of MRD after MYC inactivation compared to WT hosts (Figure 5B, C, RAG2−/− versus WT, p = 0.007). Reconstitution of immunodeficient hosts with naive CD8+ T-cells continued to have a significant burden of MRD (Figure 5C, RAG2−/−CD8+ versus WT, p = 0.03) whereas reconstitution of RAG2−/− hosts with naive CD4+ T-cells completely eliminated MRD, similar to WT hosts upon MYC inactivation (Figure 5C, RAG2−/−CD4+ versus WT, p = 0.09). Moreover, RAG2−/− hosts adoptively transferred with CD4+ T-cells exhibited statistically significant prolonged tumor-free survival compared to RAG2−/− or RAG2−/− hosts reconstituted with CD8+ T-cells (Figure 5B, D, RAG2−/− versus RAG2−/−CD4+, p = 0.007, RAG2−/−CD4+ versus RAG2−/−CD8+, p = 0.03). Hence, restoration of CD4+ T-cells alone was sufficient for the ability of MYC inactivation to eliminate MRD and induce sustained tumor regression.
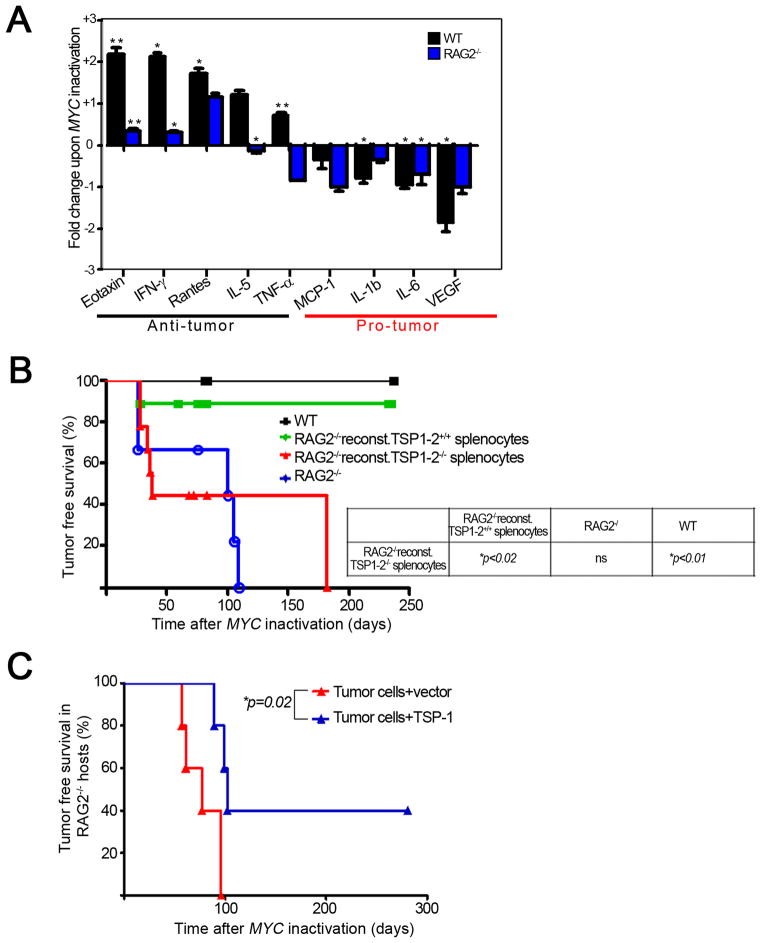
Figure 6. Cytokines produced by the immune system contribute to sustained tumor regression upon MYC inactivation.
6(A): Graphical representation of fold change of indicated cytokines upon MYC inactivation in tumors from WT and RAG2−/− hosts. Tumors from WT and RAG2−/− mice were harvested at tumor onset and 4 days after MYC inactivation and run on a luminex platform to check for protein expression of 21 different cytokines. The significant fold changes in the various cytokines upon MYC inactivation were log2 transformed and plotted for various pro- and anti-tumor cytokines. *p < 0.01, ** p < 0.001. * above the bars represents significance in cytokine expression upon MYC inactivation in the indicated host. Error bars are +/− SEM. 6(B): Kaplan-Meier curves of tumor free survival of reconstituted RAG2−/−, RAG2−/− and WT mice. RAG2−/− mice were reconstituted with splenocytes from WT (n=18) or TSP-1,2−/− (n=16) mice i.v. 8 days post reconstitution mice were transplanted with lymphoma cells s.c. MYC was inactivated when tumors were 1000 mm3. WT (n=8) and RAG2−/− (n=11). Log-rank test was used to analyze survival of indicated genotypes. Data is representative 3 experiments. 6(C): Kaplan-Meier curves of tumor free survival of RAG2−/− mice injected with TSP-1 transfected tumor cell lines (n = 5) or vector transfected control tumor cell lines (n = 5). A p53−/− conditional MYC lymphoma cell line was used. See also Figure S4
Host Immune System is Required to Elicit Changes in Chemokine Expression
We measured relative fold-changes in cytokine production in tumors growing in WT or RAG2−/− hosts after MYC inactivation (Figure 6A). MYC inactivation in tumors from WT compared to RAG2−/− hosts revealed an up regulation of anti-proliferative and anti-angiogenic (“anti-tumor”) cytokines that suggest potential involvement by other immune effectors. Eotaxin-1 and IL-5 (Figure 6A, WT versus RAG2−/− fold change upon MYC inactivation p = 0.02 and p = 0.003 respectively) are potent TH2 cytokines that have been implicated in the recruitment of an eosinophil-mediated anti-tumor inflammatory response (Simson et al., 2007). IFN-γ was observed to increase over 4-fold upon MYC inactivation in the WT hosts with virtually no change in the absence of the host immune system (WT versus RAG2−/− fold change upon MYC inactivation p = 0.03) while TNF-α was significantly downregulated in RAG2−/− hosts (RAG2−/− MYC On versus Off, p = 0.02), its upregulation was close to statistical significance in the WT hosts (WT MYC On versus Off, p = 0.07). Both cytokines have been shown by many to be critical mediators of potent CD4+ anti-tumor activity (Qin and Blankenstein, 2000; Thomas and Hersey, 1998). Interestingly, MCP-1, a potent chemo-attractant of inflammatory tumor-associated macrophages (TAMs), specifically, tumor-promoting M2 macrophages (Allavena et al., 2008; Hu et al., 2009), was significantly downregulated in the tumors from immunodeficient hosts compared to WT (WT versus RAG2−/− fold change upon MYC inactivation p = 0.008).
Also, the downregulation of “pro-tumor” cytokines was measured in tumors from WT and RAG2−/− hosts. Vascular endothelial growth factor (VEGF) was downregulated almost 4-fold in WT hosts (WT MYC On versus Off, p = 0.01) whereas no change in its expression could be detected in tumors from immunodeficient hosts. IL-β decreased significantly close to 2-fold (WT versus RAG2−/− fold change upon MYC inactivation, p = 0.02); downregulation of these two cytokines suggests enhanced suppression of angiogenesis in the presence of an intact host immune system upon MYC inactivation (Kowanetz and Ferrara, 2006; Shchors et al., 2006).
Finally, RAG2−/− hosts that had been reconstituted with CD4+ T-cells exhibited similar changes in chemokine expression to WT hosts upon MYC inactivation (Figure S4B). The anti-tumor cytokines (eotaxin, IFN-γ and RANTES) increased, while the pro-tumour cytokine, VEGF decreased in protein expression (Figure S4B). Thus, the host immune status is responsible for the regulation of changes in cytokine expression.
TSP Expression is Required for Sustained Tumor Regression upon MYC Inactivation
Our results suggested to us the possibility that specific cytokines may be critical to the remodeling of the tumor and the tumor microenvironment upon MYC inactivation. We used two approaches to investigate the role of TSP-1. First, we reconstituted RAG2−/− mice with splenocytes from either TSP-1,2+/+ (WT) or TSP-1,2−/− mice. Both TSP-1 and 2 have been implicated in the inhibition of angiogenesis and have similar structural domains (Kazerounian et al., 2008; Lawler, 2000). By FACS analysis, we verified equivalent immune reconstitution (Figure S4A). Indeed, RAG2−/− mice reconstituted with TSP-1,2−/− splenocytes completely failed to protect from sustained tumor regression upon MYC inactivation compared to RAG2−/− mice reconstituted with WT splenocytes (Figure 6B, relapse rate WT versus TSP-1,2−/−, 10% versus 100%, p = 0.02). We conclude that TSP expression in immune effectors is important for sustained tumor regression upon MYC inactivation.
Next, we addressed whether we could bypass the requirement for host TSP-1 expression from host immune cells by artificially introducing TSP-1 into tumor cells. We compared tumor recurrence upon MYC inactivation in RAG2−/− hosts of tumors infected with a vector control versus tumors infected with a TSP-1 expression vector. TSP-1 overexpressing tumors exhibited a delay in the kinetics (mean latency 80 versus 102 days) and a decreased frequency of tumor recurrence (40% versus 100%) resulting in a statistically significant survival advantage (Figure 6C, RAG2−/− TSP-1+ versus RAG2−/−, p = 0.02). Thus, TSP-1 overexpression of tumor cells is sufficient to increase the duration and frequency of sustained tumor regression upon MYC inactivation in immune compromised hosts.
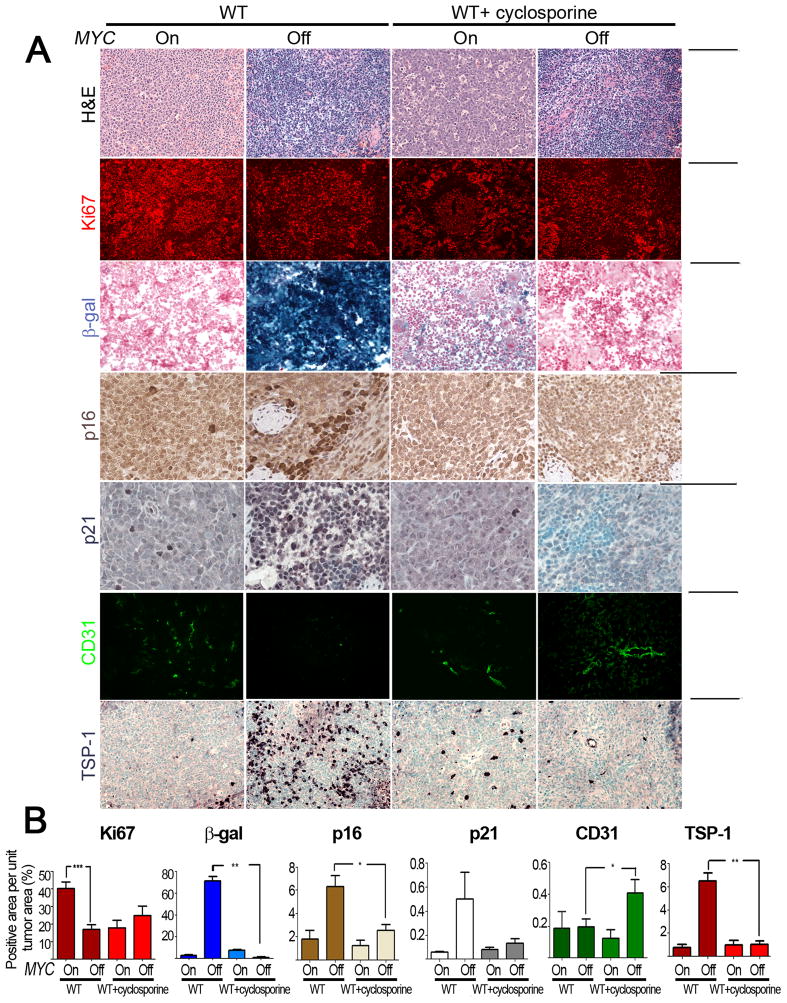
Cyclosporine A Treatment of Primary Tumors Impedes Senescence and Shut Down of Angiogenesis
To examine if similar results would be observed in primary transgenic tumors, we determined the influence of the pharmacological suppression of the host immune system with cyclosporine A (Shevach, 1985) on the consequences of MYC inactivation. Cyclosporine A did not have any direct effects on the proliferation of tumor cells in vitro (Figure S5). Cyclosporine A treated primary transgenic mice illustrated a marked inhibition on the ability of MYC inactivation to induce both cellular senescence as measured by staining for SA β-galactosidase (70% versus 1%; p < 0.01), p16 (6% versus 2%; p < 0.05) and p21 (0.5% versus 0.1%, p < 0.01) as well as the suppression of angiogenesis as measured by decrease in staining for CD31 (0.2% versus 0.4%, p = 0.05) and the induction of TSP-1 (6% versus 1%, p = 0.0006) (Figure 7A, B). Thus, cyclosporine A blocked the ability of MYC inactivation to induce senescence and shut down angiogenesis. We observed no effects on apoptosis as measured by TUNEL staining (data not shown). However, cyclosporine A treatment may suppress the ability of MYC inactivation to induce proliferative arrest. Interestingly, cyclosporine A seemed to inhibit proliferation during tumor progression when MYC was still activated. This suggests, that when MYC is on, T-cells might promote tumor formation indicating the dual nature of the immune response in cancer (de Visser et al., 2006).
Figure 7. Cyclosporine A treatment inhibits induction of senescence and inhibition of angiogenesis in tumors from primary MYC induced T-ALL.
7(A): Micrographs of Hematoxylin and Eosin, Ki67, SA-β-gal, p16, p21, CD31 and TSP-1 immunostaining (ordered from top to bottom) of tumors derived from untreated and cyclosporine A treated primary tumor bearing mice (MYC On and 4 day dox treated MYC Off). Scale Bar = 100μm. 7(B): Quantification of immunostaining shown in 7(A) above. Ordered from left to right, the graphs represent quantification of Ki67, SA-β-gal, p16, p21, CD31 and TSP-1 expression. Quantification is the average percentage of positively stained regions within the tumors. At least five different fields from two different tumors were analyzed. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. Error bars are +/− SEM. See also Figure S5.
Immune System is Required for Sustained Regression of BCR-ABL induced B- cell Acute Lymphocytic Leukemia (B-ALL)
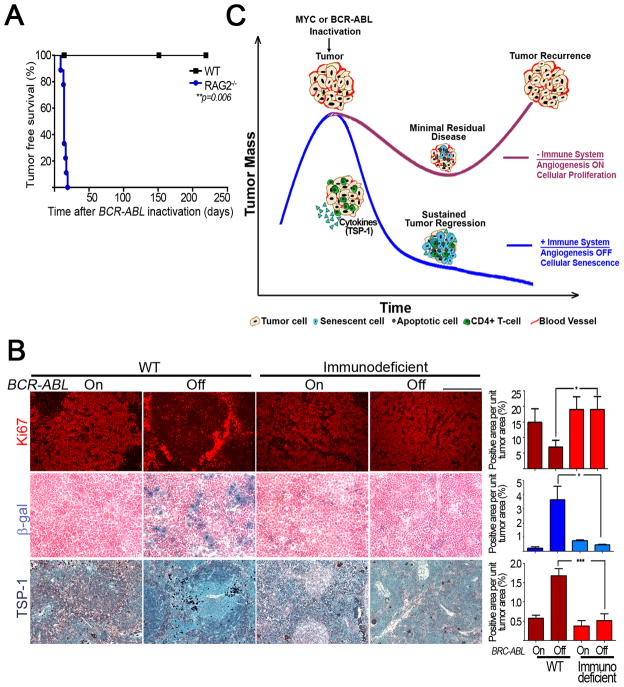
To determine if our results would generalize to another model of hematopoietic tumorigenesis, we used a conditional transgenic model of BCR-ABL induced pro-B- cell lymphocytic leukemia (B-ALL) (Huettner et al., 2000). First, we determined if host immune status influenced the ability of BCR-ABL inactivation to induce sustained tumor regression. Similar to MYC inactivation, tumors upon BCR-ABL inactivation underwent sustained tumor regression in wild type hosts while 100% of the immunodeficient hosts relapsed within 14 days of BCR-ABL inactivation (Figure 8A, WT versus RAG2−/−, p = 0.006). Hence, BCR-ABL inactivation also induces sustained tumor regression only in immune intact hosts.
Figure 8. An intact immune system is required for sustained regression of tumors in a conditional mouse model of BCR-ABL-induced B-ALL.
8(A): Kaplan-Meier curves of tumor free survival of RAG2−/− (n=9) and WT (n=4) mice transplanted with unlabelled leukemia cells i.p. When mice were moribund with tumor, BCR-ABL was inactivated, and mice were scored for relapse. 8(B): Micrographs and quantification of Ki67, SA-β-gal and TSP-1 immunostaining (ordered from top to bottom) of tumors derived from untreated (BCR-ABL On) and doxycycline treated (BCR-ABL Off) wildtype and immunodeficient tumor bearing mice. Scale Bar = 100μm. Quantification is average percentage of positively stained regions. At least five different fields from two different tumors were analyzed for each different condition. Statistical significance (p value evaluated by unpaired Student’s t-test) is shown. * p < 0.01, ** p < 0.001, *** p < 0.0001. 8(C): Model for role of immune system in eliciting oncogene addiction. See also Figure S6.
Upon BCR-ABL inactivation, Ki67 expression showed a non-significant decrease in tumors transplanted into wild type hosts but no change in tumors transplanted into immunodeficient hosts. Ki67 expression was higher in tumors transplanted into immunodeficient hosts compared to those transplanted into immune intact hosts (Figure 8B, WT BCR-ABL off versus immunodeficient BCR-ABL off p = 0.03). Cellular senescence increased upon BCR-ABL inactivation in tumours from wild type hosts versus immunodeficient hosts as measured by increased SA-β-gal staining (4% versus 0.4%, p = 0.05). Finally, there was a 3-fold increase in TSP-1 upon BCR-ABL inactivation in tumors from immunocompetent hosts while TSP-1 expression did not change upon BCR-ABL inactivation in immunodeficient hosts (Figure 8B, TSP-1 panel, WT BCR-ABL on versus BCR-ABL off, p < 0.0001; WT BCR-ABL Off versus immunodeficient BCR-ABL Off, p = 0.0001). We were unable to measure any significant CD31 expression in any of the tumors. Hence, BCR-ABL inactivation also induces sustained tumor regression only in immune intact hosts.
Discussion
Oncogene addiction has been presumed to be a cell autonomous process. Here we have shown that interactions between the tumor microenvironment and the immune system are essential for sustained tumor regression upon oncogene inactivation. In the absence of an intact immune system, we see a 10–1000-fold reduction in the rate, extent, and duration of tumor regression upon MYC inactivation. The absence of CD4+ T-cells alone was sufficient to markedly impede sustained tumor regression. Thus, oncogene addiction is not necessarily cell autonomous. CD4+ T-cells may play a critical role in enabling MYC inactivation to elicit changes in the microenvironment and in cytokine expression that appear to be required for cellular senescence and the shut down of angiogenesis. TSP-1 must be expressed by immune effectors to cooperate with MYC inactivation to induce tumor regression. Our results generalized to primary tumors from MYC-induced T-ALL bearing hosts that had been treated with the immunosuppressive agent cyclosporine A and a conditional transgenic model of BCR-ABL induced B-ALL. Oncogene inactivation generally may induce tumor regression through immune cell dependent mechanisms.
Our observations are consistent with a multitude of reports that document the role of the immune system in neoplasia (de Visser et al., 2006; de Visser et al., 2005; Dunn et al., 2002; Soucek et al., 2007). Tumors co-evolve in the context of an intact immune system through the process of immune editing, resulting in tumor elimination, dormancy or evolving to escape the immune system and progress to full malignancy (Dunn et al., 2002; Guerra et al., 2008; Teng et al., 2008). Upon MYC inactivation, a massive recruitment of CD4+ T-cells occurs that is associated with marked changes in cytokine production in the tumor microenvironment leading to cellular senescence and the shut down of angiogenesis. TSP-1 is one of the critical chemokines. Interestingly, immune effector recruitment and associated changes in chemokines occur upon restoration of the tumor suppressor p53 in both liver cancer (Xue et al., 2007) and upon MYC inactivation in lymphoma (Giuriato et al., 2006).
Provocatively, CD4+ T-cells emerged as the critical host effector population for sustained tumor regression upon MYC inactivation. Notably, hosts deficient in CD4+ T-cells exhibited impaired kinetics, degree and durability of tumor regression as well as reduced senescence and suppression of angiogenesis upon MYC inactivation. The reconstitution of CD4+ T-cells into RAG2−/− hosts alone was capable of restoring the ability of MYC inactivation to induce sustained tumor regression. The reconstitution of CD4+ T cells into RAG2−/− hosts had more potent effects on tumor regression compared with the depletion of these cells, perhaps reflecting that in hosts that are congenitally defective for a specific immune compartment there may be compensation from other immune effectors (Xing et al., 1998).
CD4+ T-cells have been previously implicated in the restraint of tumor growth through regulation of antigen dependent mechanisms involving either macrophages or cytotoxic T-cells (Corthay et al., 2005; Dranoff et al., 1993; Qin and Blankenstein, 2000). Intriguingly, host CD4+ T-cells sculpted the tumor’s response to MYC inactivation, likely not by their modest influence upon apoptosis or proliferation, but by dramatically inducing cellular senescence and the shut down of angiogenesis, processes previously suggested by us to be integral to the ability of MYC inactivation to effect sustained tumor regression. Two of the hallmarks of oncogene addiction, both the induction of cellular senescence and the suppression of angiogenesis, have been linked to the expression of cytokines known to be expressed by CD4+ T-cells (Acosta et al., 2008; Beatty and Paterson, 2001; Kuilman et al., 2008; Muller-Hermelink et al., 2008).
Thus, CD4+ T-cells are one important component of the mechanism of tumor regression upon oncogene inactivation. Other host immune effectors are likely to contribute and we recognize that other innate and adaptive immune compartments are also likely to be involved including macrophages, NK cells, mast cells, and B-cells. Recent work suggests that mast cells and macrophages both may be critical (Soucek et al., 2007; Xue et al., 2007). Indeed, it is possible that CD4+ T-cells are mediating part of the effects we have observed by recruiting these effector populations.
TSP-1 is critical for the mechanism by which host immune effectors mediate tumor regression upon MYC inactivation. TSP-1 is a potent cytokine that has been implicated in the regulation of many cellular processes including the regulation of angiogenesis (Jimenez et al., 2000; Kazerounian et al., 2008; Lawler, 2000; Short et al., 2005; Zaslavsky et al., 2010). Furthermore, TSP-1 also has been suggested to regulate lymphocyte homing and function (Li et al., 2006) and appears to be required for the ability of CD4+ T-cells to contribute to sustained regression upon oncogene inactivation.
Additionally, other cytokines including eotaxin-1, IL-5, IFN-γ and TNF-α are possible candidates for mediating the changes in cellular senescence and angiogenesis upon MYC inactivation, consistent with reports that these chemokines may be involved in these processes (Beatty and Paterson, 2001; Beyne-Rauzy et al., 2004). The downregulation of other cytokines such as VEGF, IL-1β, and MCP-1 could also contribute (Kowanetz and Ferrara, 2006; Shchors et al., 2006; Su et al., 2010). IFN-γ and TNF-α have been previously implicated in the regulation of cellular quiescence and angiogenesis (Beatty and Paterson, 2001; Beyne-Rauzy et al., 2004; Kuilman et al., 2008; Muller-Hermelink et al., 2008), and eotaxin-1 and IL-5 have demonstrated potent anti-tumor activity in numerous mouse models of cancer (Simson et al., 2007). Notably, tumor regression induced by the restoration of p53 expression was also associated with marked changes in chemokine expression (Xue et al., 2007).
In primary transgenic tumor hosts, an immune compromised state induced via treatment with cyclosporine A greatly impeded the consequences of oncogene inactivation. Therefore, our results generalize in the case when endogenous tumor-host interactions evolved throughout tumorigenesis. Cyclosporine A treatment is well known to increase the frequency of haematological malignancies in patients (Cockburn and Krupp, 1989; Opelz and Dohler, 2004). Hence, this agent may impede sensitivity to oncogene directed therapies.
An immune intact host was also found to be required for BCR-ABL inactivation to induce sustained tumor regression in B-ALL. Similar to MYC inactivation, inactivation of the BCR-ABL oncogene resulted in the induction of cellular senescence, the shutdown of tumor angiogenesis, and ultimately sustained tumor regression only in the presence of the host immune system. However, different from MYC inactivation, BCR-ABL inactivation appeared to be less capable of suppressing cellular proliferation. Hence, the host immune system appears to be generally important in mediating the consequences of oncogene inactivation. Thus, oncogene addiction is a consequence of both cell autonomous processes such as proliferative arrest and apoptosis as well as host-immune dependent mechanisms such as cellular senescence and angiogenesis (Figure 8C). Upon oncogene inactivation, tumor cells are eliminated primarily in a cell autonomous manner. However, the kinetics of tumor cell elimination and the extent of tumor elimination, or minimal residual disease, as well as the durability of sustained tumor regression are all dictated by the presence of an immune system and appear to be strongly associated with its ability to elicit cellular senescence and shut down angiogenesis. These processes may contribute to the constraint of minimal residual disease (Aguirre-Ghiso, 2007). CD4+ T-cells are a critical component to this phenomenon and TSP-1 emerges as a possible cytokine regulating these processes. Other immune effectors and chemokines/cytokines (including IFN-γ, eotaxin-1, IL-5, TNF-α, and MCP-1) are likely to be involved. Immune cells and inflammation can be important to the pathogenesis of cancer through many effects on the tumor microenvironment (Coussens and Werb, 2002; Greten and Karin, 2004; Xue et al., 2007)
In general, the deficiency in CD4+ T-cells may render the treatment of tumors in patients less efficacious and impede the complete elimination of tumor cells. Indeed, AIDS patients exhibit not only a more than 100-fold increased frequency of lymphomas often associated with MYC overexpression but are much less responsive to therapy (Boshoff and Weiss, 2002; Carbone, 2003). Hence, CD4+ T-cells may contribute to the efficacy of therapeutic agents.
Our results suggest that screening methods used to identify new therapies that rely on the in vitro study of cell lines or in vivo analysis of xenograft models in immune compromised hosts may underestimate the efficacy of a therapy by failing to faithfully recapitulate tumor-host interactions (Ronnov-Jessen and Bissell, 2009; Weigelt and Bissell, 2008). Moreover, the active modulation of CD4+ T-cell function may enhance the efficacy of therapeutics for cancer (Gattinoni et al., 2006; Lake and Robinson, 2005). Thus, a combination of targeted oncogene inactivation with immunotherapy may be a particularly efficacious anti-cancer therapy.
Experimental Procedures
Transgenic Mice
The generation and characterization of Tet system transgenic lines for conditional expression of MYC, have been described (Felsher and Bishop, 1999). CD4−/−, CD8−/−, CD4−/−CD8−/− and RAG2−/− mice were generously provided in the FVB/N background by Lisa Coussens (University of California, San Francisco). TSP-1,2−/− mice were generously provided by Ben Barres (Stanford University). Luciferase+L2G85 mice were generously provided by Robert Negrin (Stanford University). Tet-o-BCR-ABL mice were generously provided by Daniel Tenen (Harvard University). Genotyping was performed by PCR on genomic DNA from tails. All animal experiments were approved by Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) and in accordance with institutional and national guidelines.
Tumor Surveillance and Tumorigenicity Assays
Mice that were moribund with tumor were either humanely euthanized or treated with doxycycline in their drinking water (100 μg/ml) to follow tumor regression and relapse. Statistical comparison of Kaplan–Meier curves is based on the log-rank test. Further details can be found in supplementary methods.
Reconstitution of RAG2−/− mice
RAG2−/− mice were injected intravenously (i.v.) with either (i) 20X106 splenocytes from WT or TSP-1,2−/− mice or (ii) 4X106 CD4+ or CD8+ T-cells isolated from spleens and lymph nodes of WT mice using Magnetically Activated Cell Sorting (MACS). 8 days post reconstitution, mice were bled from the tail vein and CD4+ and CD8+ T-cell reconstitution was verified using FACS.
In Vivo Bioluminescence Imaging
Mice with tumors were anesthetized with a combination of inhaled isoflorane/oxygen delivered by the Xenogen XGI-8 5-port Gas Anaesthesia System. The substrate d-luciferin (150 mg/kg) was injected into the animal’s peritoneal cavity 10 min. before imaging. Animals were then placed into a light-tight chamber and imaged with an IVIS-200 cooled CCD camera (Xenogen, Alameda, CA) (Contag et al., 1997). Living Image was used to collect, archive, and analyze photon fluxes and transform them into pseudocolor images by using Living Image software (Xenogen). At least 5 mice per group were injected with tumors expressing luciferase.
Luminex cytokine Assay
The concentration of 26 cytokines was measured from tumor tissue lysates from WT and RAG2−/− mice at tumor onset and 4 days post MYC inactivation. Concentrations were measured using Luminex xMAP technology. Data were obtained as mean fluorescence intensity based on a standard curve generated for each cytokine. Further details can be found in supplementary methods.
Significance.
Utilizing transgenic mouse models of conditional oncogene inactivation, we show that the absence of an intact immune system results in a 10–1000-fold reduction in the rate, extent, and duration of tumor regression upon oncogene inactivation. We uncovered an unanticipated role for CD4+ T-cell effectors in mediating cellular senescence and the shut down of tumor angiogenesis and discovered a critical role for the expression of thrombospondin-1 in immune effectors. Most strategies to identify therapeutic agents utilizein vitro models or in vivo xenograft models overlooking the effect of the immune system. Our results argue for the necessity of models that include an intact host immune system to properly evaluate the potential efficacy of targeted therapeutics for maximum clinical impact.
Highlights.
Oncogene addiction involves both cell autonomous and non-autonomous mechanisms
An immune system is required for sustained tumor regression upon inactivation of oncogenes (MYC or BCR-ABL)
CD4+ T-cells regulate cellular senescence and angiogenesis in the tumor microenvironment
TSP-1 secretion from immune cells is required for sustained tumor regression upon inactivation of an oncogene.
Supplementary Material
Acknowledgments
This manuscript is dedicated to the memory of Julie Do. We thank Ron Levy, Kwan Hyuck, Lisa Coussens, Peter Choi, and other members of the Felsher laboratory for their helpful suggestions; Robert Negrin, Lisa Coussens and Ben Barres for providing transgenic mice; Pauline Chu for generating histology samples; Anet James for assistance with microscopy; Yael Resenberg-Hasson for assistance with the luminex cytokine assay. This work was funded by the Burroughs Welcome Fund Career Award, the Damon Runyon Foundation Lilly Clinical Investigator Award, NIH RO1 grant number CA 089305, National Cancer Institute’s In-vivo Cellular and Molecular Imaging Center grant number CA 114747, Integrative Cancer Biology Program grant number CA 112973, NIH/NCI PO1 grant number CA034233, the Leukaemia and Lymphoma Society Translational Research grant number R6223-07 (D.W.F.), NIH R01 grant number CA 118374 (S.R.), Stanford Graduate Fellowship (K.R.) Lymphoma Research Foundation and the Leukemia and Lymphoma Society (A.F.). Additionally P.B. and A.K. were funded by a Howard Hughes Medical Institute Research Training Fellowship for Medical Students and a Stanford Medical Scholars Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature reviews. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166:2276–2282. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, Sabatier L, Mansat-De Mas V. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene. 2004;23:7507–7516. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frodin L, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. International journal of cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Weiss R. AIDS-related malignancies. Nature reviews. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene. 2001;20:5595–5610. doi: 10.1038/sj.onc.1204595. [DOI] [PubMed] [Google Scholar]
- Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer research. 2006;66:7301–7309. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- Carbone A. Emerging pathways in the development of AIDS-related lymphomas. The lancet oncology. 2003;4:22–29. doi: 10.1016/s1470-2045(03)00957-4. [DOI] [PubMed] [Google Scholar]
- Cockburn IT, Krupp P. The risk of neoplasms in patients treated with cyclosporine A. J Autoimmun. 1989;2:723–731. doi: 10.1016/s0896-8411(89)80010-1. [DOI] [PubMed] [Google Scholar]
- Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, Stevenson DK, Benaron DA. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997;66:523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. The Journal of experimental medicine. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. The New England journal of medicine. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Dougan M, Dranoff G. The immune response to tumors. Curr Protoc Immunol. 2009;Chapter 20(Unit 20):11. doi: 10.1002/0471142735.im2011s85. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nature immunology. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- Felsher DW. Cancer revoked: oncogenes as therapeutic targets. Nature reviews. 2003;3:375–380. doi: 10.1038/nrc1070. [DOI] [PubMed] [Google Scholar]
- Felsher DW. Oncogene addiction versus oncogene amnesia: perhaps more than just a bad habit? Cancer research. 2008;68:3081–3086. doi: 10.1158/0008-5472.CAN-07-5832. discussion 3086. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Molecular cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gatti RA, Good RA. Occurrence of malignancy in immunodeficiency diseases. A literature review. Cancer. 1971;28:89–98. doi: 10.1002/1097-0142(197107)28:1<89::aid-cncr2820280117>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuriato S, Ryeom S, Fan AC, Bachireddy P, Lynch RC, Rioth MJ, van Riggelen J, Kopelman AM, Passegue E, Tang F, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16266–16271. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, et al. Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15:5485–5493. doi: 10.1158/1078-0432.CCR-08-2491. [DOI] [PubMed] [Google Scholar]
- Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 2000;24:57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- Jain M, Arvanitis C, Chu K, Dewey W, Leonhardt E, Trinh M, Sundberg CD, Bishop JM, Felsher DW. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science (New York, NY. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature medicine. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700–712. doi: 10.1007/s00018-007-7486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS medicine. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nature reviews. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108:3112–3120. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nature reviews. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nature reviews. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez-Cancel I, Hardesty RL, Hattler BG, Griffith BP. Solid tumors after heart transplantation: lethality of lung cancer. The Annals of thoracic surgery. 1995;60:1623–1626. doi: 10.1016/0003-4975(95)00120-4. [DOI] [PubMed] [Google Scholar]
- Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Seminars in cancer biology. 2007;17:275–287. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Bissell MJ. Breast cancer by proxy: can the microenvironment be both the cause and consequence? Trends Mol Med. 2009;15:5–13. doi: 10.1016/j.molmed.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F, Nguyen C, Leserman L, Auphan-Anezin N, Schmitt-Verhulst AM. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179:6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes & development. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- Shchors K, Shchors E, Rostker F, Lawlor ER, Brown-Swigart L, Evan GI. q. Genes & development. 2006;20:2527–2538. doi: 10.1101/gad.1455706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, Smyth MJ, Parish CR. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunological reviews. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature medicine. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, Smyth MJ. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, Swann JB, Koebel CM, Schreiber RD, Smyth MJ. Immune-mediated dormancy: an equilibrium with cancer. Journal of leukocyte biology. 2008;84:988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (New York, NY. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Seminars in cancer biology. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB, Joe A. Oncogene addiction. Cancer research. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nature clinical practice. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Wang J, Croitoru K, Wakeham J. Protection by CD4 or CD8 T cells against pulmonary Mycobacterium bovis bacillus Calmette-Guerin infection. Infect Immun. 1998;66:5537–5542. doi: 10.1128/iai.66.11.5537-5542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky A, Baek KH, Lynch RC, Short S, Grillo J, Folkman J, Italiano JE, Jr, Ryeom S. Platelet-derived thrombospondin-1 (TSP-1) is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010 doi: 10.1182/blood-2009-09-242065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? The Journal of clinical investigation. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.