SUMMARY
Macrophage apoptosis in advanced atheromata, a key process in plaque necrosis, involves the combination of ER stress with other pro-apoptotic stimuli. We show here that oxidized phospholipids, oxidized LDL, saturated fatty acids (SFAs), and lipoprotein(a) trigger apoptosis in ER-stressed macrophages through a mechanism requiring both CD36 and toll-like receptor 2 (TLR2). In vivo, macrophage apoptosis was induced in SFA-fed, ER-stressed wild-type but not Cd36−/− or Tlr2−/− mice. For atherosclerosis, we combined TLR2 deficiency with that of TLR4, which can also promote apoptosis in ER-stressed macrophages. Advanced lesions of fat-fed Ldlr−/− mice transplanted with Tlr4−/−Tlr2−/− bone marrow were markedly protected from macrophage apoptosis and plaque necrosis compared with WT → Ldlr−/− lesions. These findings provide insight into how atherogenic lipoproteins trigger macrophage apoptosis in the setting of ER stress and how TLR activation might promote macrophage apoptosis and plaque necrosis in advanced atherosclerosis.
INTRODUCTION
Atherothrombotic vascular disease is the leading cause of death in the industrialized world, and its prevalence is increasing world-wide (Braunwald, 1997, 2007). The key clinico-pathologic event is the conversion of a relative small percentage of subclinical atherosclerotic lesions to necrotic, disrupted “vulnerable” plaques that trigger acute lumenal thrombosis (Libby, 2000; Virmani et al., 2003). Thus, understanding the cellular and molecular factors that lead to this conversion is a key goal in our attempt to understand and identify potential therapeutic targets for this disease. Importantly, lesion size per se is not a parameter associated with conversion (Libby, 2000). Rather, the plaque morphology properties of intimal necrosis, cellular apoptosis, and fibrous cap thinning appear to be most important (Libby, 2000; Virmani et al., 2003). Thus, factors that promote these particular features will likely give important clues to how plaques progress to the clinical stage.
Plaque necrosis is a key feature of clinically dangerous lesions because it promotes inflammation, fibrous cap thinning, plaque destabilization, and thrombosis (Libby, 2000; Virmani et al., 2003). Plaque necrosis forms through the combination of lesional macrophage apoptosis and defective phagocytic clearance of the apoptotic cells, which leads to post-apoptotic cellular necrosis (Tabas, 2005). Work in our laboratory and others has provided evidence that advanced lesional macrophage apoptosis is enabled through endoplasmic reticulum (ER) stress, acting through the Unfolded Protein Response (UPR) effector CHOP (Feng et al., 2003; Thorp et al., 2009). For example, there is a close, positive correlation among CHOP expression, apoptosis of lesional cells, and progression of plaques to the vulnerable stage in human coronary arteries (Myoishi et al., 2007), and studies with CHOP-deficient mice show that CHOP contributes to advanced lesional apoptosis and plaque necrosis in both fat-fed Ldlr−/− and Apoe−/− mice (Thorp et al., 2009).
Although extreme ER stress in vitro can directly cause cell death, ER stress at the more subtle levels that occur in vivo is unlikely to trigger apoptosis by itself. Rather, ER stress renders macrophages susceptible to apoptosis in the face of other pro-apoptotic stimuli (DeVries-Seimon et al., 2005; Seimon et al., 2006; Manning-Tobin et al., 2009). Therefore, understanding which athero-relevant stimuli conspire with ER stress to cause advanced lesional macrophage apoptosis and plaque necrosis, and the mechanism of their action, is a key goal in deciphering the cellular biology of vulnerable plaque formation. In that context, we show here that a number of lipids and lipoproteins associated with atherosclerotic disease are potent inducers of apoptosis in ER-stressed macrophages through a pathway involving CD36, TLR2, NADPH oxidase, and oxidative stress. While CD36 and TLRs have been implicated previously in early atherogenesis, e.g. in foam cell formation and endothelial activation (Park et al., 2009; Mullick et al., 2008), their role in specific cellular processes related to the critical and distinct process of plaque necrosis is not known. Moreover, one of the lipoproteins that we found to activate the CD36-TLR2 apoptosis pathway is lipoprotein(a) [Lp(a)]. Lp(a) is a well-recognized risk factor for coronary artery disease (CAD) and has been shown recently to be strongly linked genetically to CAD (Erqou et al., 2009; Tregouet et al., 2009; Brazier et al., 1999; Holmer et al., 2003), but how Lp(a) might be linked to one or more cellular events specifically involved in advanced plaque progression is uncertain. These findings may provide insight into how Lp(a) contributes to atherothrombotic vascular disease.
RESULTS
Oxidized Phospholipids Trigger CD36-Dependent Apoptosis in ER-Stressed Macrophages
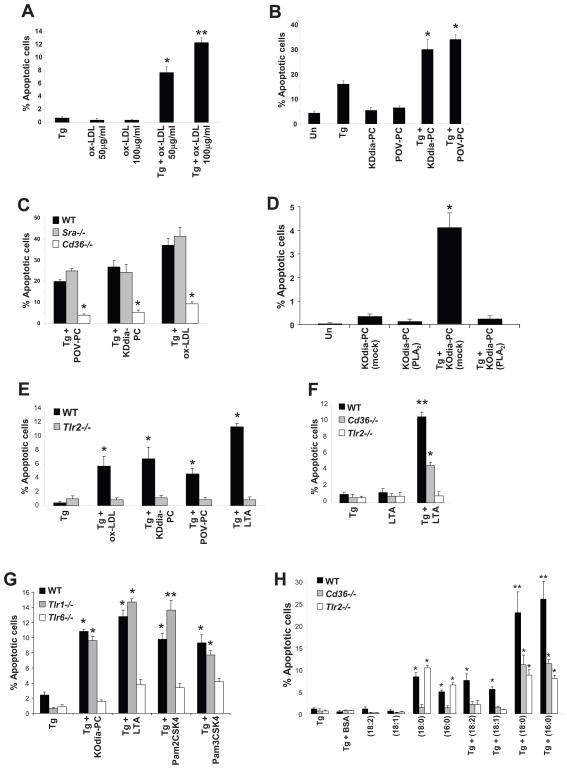
To discover athero-relevant triggers of apoptosis in ER-stressed macrophages, we investigated ligands for CD36, which we recently showed plays a role in plaque necrosis (Manning-Tobin et al., 2009). To determine whether CD36 ligands trigger macrophage apoptosis under conditions of ER stress, we treated primary mouse peritoneal macrophages with the ER stress-inducing agent thapsigargin alone or in the presence of oxLDL, a CD36 ligand (Endemann et al., 1993). Whereas oxLDL or thapsigargin alone had little effect, both reagents together synergistically induced apoptosis (Figure 1A). To test if oxidized phospholipids (oxPLs), which are atherosclerosis-relevant CD36 ligands present in oxLDL, also triggered macrophage apoptosis during ER stress, we incubated macrophages with two types of oxPLs found in oxLDL, KDdia-PC and POV-PC (Podrez et al., 2002). As shown in Figure 1B, while KDdia-PC or POV-PC alone had no significant effect, they synergistically triggered macrophage apoptosis when combined with thapsigargin. Similar results were observed when ER-stressed macrophages were incubated with two other oxPLs, KODia-PC and oxidized PAPC (below), and apoptosis was also observed when human macrophages derived from THP1 cells were exposed to KODia-PC and thapsigargin (Figure S1A). OxPL-induced apoptosis in ER-stressed macrophages was dependent on CD36 but not the type A scavenger receptor (SRA), as shown by experiments using macrophages from wild-type, Sra−/−, and Cd36−/− mice (Figure 1C). In a parallel experiment, we found that SRA-deficiency did block apoptosis induced by thapsigargin plus the SRA ligand AcLDL (data not shown) (DeVries-Seimon et al., 2005).
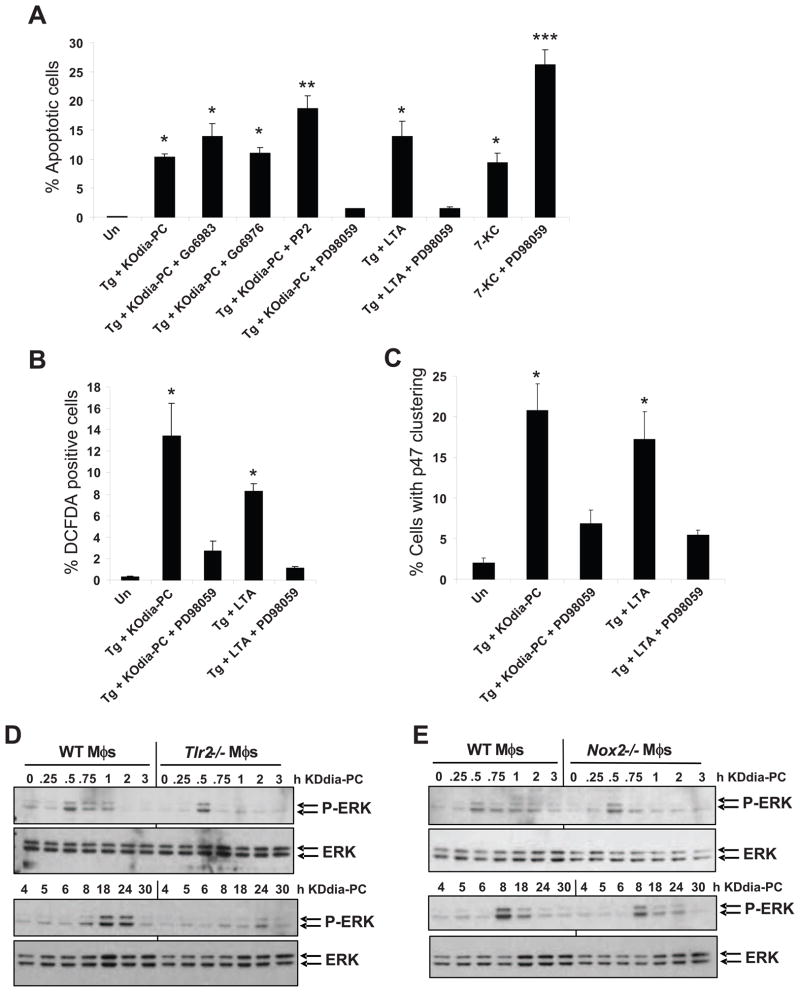
Figure 1. OxPLs, oxLDL, and Lp(a) Induce CD36- and TLR2/6-Dependent Apoptosis in ER-Stressed Macrophages.
For all the experiments described below, the experimental endpoint was macrophage apoptosis; the data are expressed as the percent of total cells that stained with annexin V and propidium iodide (mean ± SEM; n=4). Differences between values with symbols and no symbols, or between values with different symbols, are statistically significant (p values ranged from < 0.05 to < 0.001). (A) Macrophages were incubated for 28 h with 0.25 μM thapsigargin (Tg) alone or thapsigargin in combination with the indicated concentrations of ox-LDL. (B) Macrophages were left untreated (Un) or incubated for 30 h with 0.25 μM thapsigargin alone or thapsigargin in combination with 10 μg/ml of KDdia-PC or POV-PC, or the phospholipids alone. (C) Macrophages from WT, Sra−/−, or Cd36−/− mice were incubated for 18 h with thapsigargin alone or thapsigargin in combination with 10 μg/ml POV-PC or KDdia-PC or with 50 μg/ml of ox-LDL. (D) Macrophages were left untreated or incubated for 23 h with 50 μg/ml KOdia-PC without (mock) or with (PLA2) pretreatment with phospholipase A2, alone or in combination with 0.5 μM thapsigargin (Tg). (E) Macrophages from WT or Tlr2−/− mice were incubated for 30 h with 0.25 μM thapsigargin alone or thapsigargin combined with KDdia-PC, POV-PC, ox-LDL, or LTA. (F) Macrophages from WT, Cd36−/−, or Tlr2−/− mice were incubated for 24 h with thapsigargin alone or thapsigargin combined with 10 μg/ml LTA. (G) Macrophages from WT, Tlr1−/−, or Tlr6−/− mice were incubated for 24 h with thapsigargin with or without KOdia-PC, LTA, or 10ug/ml Pam3CSK4 or Pam2CSK4. (H) Macrophages from WT, Cd36−/−, or Tlr2−/− mice were incubated for 18 h with 0.25 μM thapsigargin (Tg) alone; 0.25 mM of the indicated fatty acids in complex with BSA; or thapsigargin plus either the fatty acids or BSA control. The unsaturated fatty acids were linoleic acid (18:2) and oleic acid (18:1), and the saturated fatty acids were stearic acid (18:0) and palmitic acid (16:0).
ER stress and induction of the UPR occurs in macrophages of atherosclerotic plaques in humans and mice (Feng et al., 2003; Zhou et al., 2005; Gargalovic et al., 2006; Thorp et al., 2009; Myoishi et al., 2007). Although the cause of ER stress in vivo is unknown, substances such as peroxynitrite and 7-ketocholesterol have been shown to accumulate in atherosclerotic plaque and are known inducers of ER stress (Dickhout et al., 2005; Pedruzzi et al., 2004; Myoishi et al., 2007). We therefore tested whether oxPLs could trigger apoptosis when combined with peroxynitrite and 7-ketocholesterol. As shown in Figure S1B, 7-ketocholesterol and SIN-1, a peroxynitrite donor, were poor inducers of macrophage apoptosis when given at low concentrations. However, the addition of KDdia-PC to either of these ER stress-inducing agents resulted in a marked increase in macrophage apoptosis. These results indicate that oxPLs can trigger apoptosis in macrophages exposed to various types of ER stressors, including those that may be relevant to advanced atheromata.
CD36-mediated internalization of oxLDL and the bacterial product LTA are dependent on the molecule dynamin, a GTPase that mediates the constriction of clathrin coated pits (Sun et al., 2007; Nilsen et al., 2008). To test the role of dynamin in CD36-dependent macrophage apoptosis, macrophages were transduced with adenovirus carrying dominant negative dynamin (DN-dyn) or LacZ as a negative control. The effectiveness of DN-dyn was demonstrated by its ability to block labeled oxLDL uptake by >90% compared to cells transduced with LacZ control (data not shown). DN-dyn completely protected macrophages from apoptosis induced by OxLDL and thapsigargin, KOdia-PC and thapsigargin, or KOdia-PC and 7-ketocholesterol (Figure S1C). Note that DN-dyn did not block the small but statistically significant increase in apoptosis induced by 7-ketocholesterol alone demonstrating that this construct is not a general inhibitor of apoptosis. These results suggest that CD36 ligands must be internalized, as opposed to functioning solely at the cell surface, to trigger apoptosis in ER-stressed macrophages.
The pro-apoptotic effect of oxPLs requires the sn2 oxidized fatty acid
Both the phosphocholine head group and the sn2 fatty acid in oxPLs are recognized by CD36 (Boullier et al., 2005; Greenberg et al., 2008). We therefore tested the effect of removing each of these two groups. Removal of phosphocholine with phospholipase D (PLD), which went to >92% completion as assessed by HPLC-MS analysis, had no effect on the ability of the oxPL to induce apoptosis in ER-stressed macrophages (Figure S1D-E). In contrast, treatment of KODia-PC with phospholipase A2 (PLA2), which cleaves the sn2 fatty acyl moiety of PLs, almost completely suppressed its ability to trigger apoptosis in ER-stressed macrophages (Figure 1D). Moreover, whereas oxPAPC induced apoptosis in these cells, non-oxidized PAPC did not (Figure S1F). These results indicate that the pro-apoptotic effect of oxPLs requires the sn2 oxidized fatty acid and not the phosphocholine head group.
TLR2 is Necessary For Apoptosis Induced by CD36 Ligands in ER-Stressed Macrophages
Different classes of pattern recognition receptors can participate in cooperative interactions, and previous work has shown that CD36-TLR2 interaction participates in the response to Staphylococcus aureus infection (Stuart et al., 2005; Hoebe et al., 2005). To test the role of TLR2 in CD36-induced apoptosis in ER-stressed macrophages, we conducted a series of experiments comparing macrophages from wild-type and Tlr2−/− mice. As shown in Figure 1E, TLR2 deficiency markedly protected ER-stressed macrophages from oxLDL-, POV-PC-, and KDdia-PC-induced apoptosis. Moreover, lipoteichoic acid (LTA), a staphylococcal lipid that activates TLR2 in a CD36-dependent manner (Stuart et al., 2005; Hoebe et al., 2005), also induced apoptosis in ER-stressed wild-type macrophages, but not in Tlr2−/− or Cd36−/− macrophages and not in the absence of ER stress (Figure 1E and F). Tlr4−/− macrophages exposed to KDdia-PC, POV-PC, and oxLDL in combination with thapsigargin showed similar levels of apoptosis to that of wild-type macrophages (data not shown). These results demonstrate that TLR2, but not TLR4, contributes to apoptosis induced by CD36 ligands during ER stress.
MyD88 is a signaling adaptor utilized by many of the TLRs and is considered a primary adaptor for TLR2 (Kenny and O'Neill, 2008). To determine whether apoptosis in the CD36/TLR2/ER stress model was MyD88-dependent, WT and Myd88−/− macrophages were incubated with the KOdia-PC, a CD36 oxPL ligand similar to KDdia-PC (Podrez et al., 2002), POV-PC, or LTA in combination with thapsigargin. As shown in Figure S1G, apoptosis induced by each of these ligands was markedly suppressed in Myd88-deficient macrophages. In contrast, apoptosis was not blocked in macrophages lacking the other TLR adaptor, TRIF (data not shown).
TLR2 has been shown to heterodimerize with either TLR1 or TLR6 to initiate signaling (Palsson-McDermott and O'Neill, 2007). To determine the possible role of TLR1 and TLR6 in our model, macrophages from wild-type, Tlr1−/−, or Tlr6−/− mice were treated with thapsigargin alone or in combination with KOdia-PC and LTA (Figure 1G). We found that macrophage apoptosis was highly dependent on TLR6, but not TLR1. We also compared the apoptosis-inducing potential of Pam3CSK4, a lipid that has been reported to be relatively specific for the TLR1/2 heterodimer, with Pam2CSK4, which is reported to activate the TLR2/6 heterodimer (Palsson-McDermott and O'Neill, 2007). As expected, the latter ligand induced apoptosis in ER-stressed macrophages in a TLR2/6-dependent manner. Unexpectedly, Pam3CSK4 also induced apoptosis, and its effect was also blocked in TLR6-deficient, but not TLR1-deficient, macrophages. These combined data suggest that the active heterodimer in the CD36/ER stress apoptosis pathway is TLR2/6.
Saturated Fatty Acids Trigger CD36- and TLR2-Dependent Apoptosis During ER Stress
Although the sn2 oxidized fatty acid in oxPLs is required for apoptosis (above), oxPLs and bacterial products such as LTA, Pam3CSK4, and Pam2CSK4 all have saturated fatty acids residues at the sn1 and/or sn2 position of the glycerol backbone. In addition to the role of CD36 in oxLDL uptake and cell signaling, the receptor also functions as a fatty acid transporter (Ibrahimi and Abumrad, 2002). Moreover, several studies have suggested that saturated fatty acids might activate TLR2 and TLR4-dependent signaling (Nguyen et al., 2007; Shi et al., 2006; Shi et al., 2006; Senn, 2006; Lee and Hwang, 2006), although this is not a consistent finding (Erridge and Samani, 2009). We therefore tested whether different types of fatty acids could trigger apoptosis in macrophages undergoing ER stress and, if so, whether such an effect was CD36- and/or TLR2-dependent. As shown in Figure 1H, oleic acid and linoleic acid did not trigger apoptosis when given alone. A small but significant increase in apoptosis was observed when these unsaturated fatty acids, but not BSA carrier alone, were combined with thapsigargin. Moreover, this effect was suppressed in macrophages lacking either CD36 or TLR2. When the saturated fatty acids palmitic and stearic acids were given alone, apoptosis was induced, and this effect was dependent on CD36 but not TLR2. However, apoptosis by these fatty acids was increased markedly in the setting of thapsigargin-induced ER stress, and this increment was dependent on both CD36 and TLR2. Some studies (Miller et al., 2003; Oka et al., 1998; Hazzard and Ettinger, Jr., 1995), though not all (Erridge and Samani, 2009), have suggested that saturated fatty acids can also trigger certain TLR4-dependent signaling reactions. We found that palmitic acid-induced apoptosis was not blocked significantly in TLR4-deficient ER-stressed macrophages (data not shown). In summary, the combined CD36/TLR2 pathway of apoptosis plays a role in two scenarios of fatty acid-induced apoptosis: unsaturated fatty acids in ER-stressed macrophages; and the increment in saturated fatty acid-induced apoptosis in ER-stressed vs. non-ER-stressed cells. Finally, we found that dipalmitoyl PC or dipalmitoyl glycerol, which have saturated fatty acids in both the sn1 and sn2 positions, can trigger apoptosis in ER-stressed macrophages (data not shown). These findings raise the possibility that saturated fatty acids in PLs, in the context of either an sn2 oxidized fatty acid (above) or sn1 and sn2 saturated fatty acids, might play a role in the apoptotic response in ER-stressed macrophages.
Ligand Internalization is Necessary for Macrophage Apoptosis During ER Stress
Reactive Oxygen Species Contribute to Macrophage Apoptosis by CD36 Ligands During ER Stress
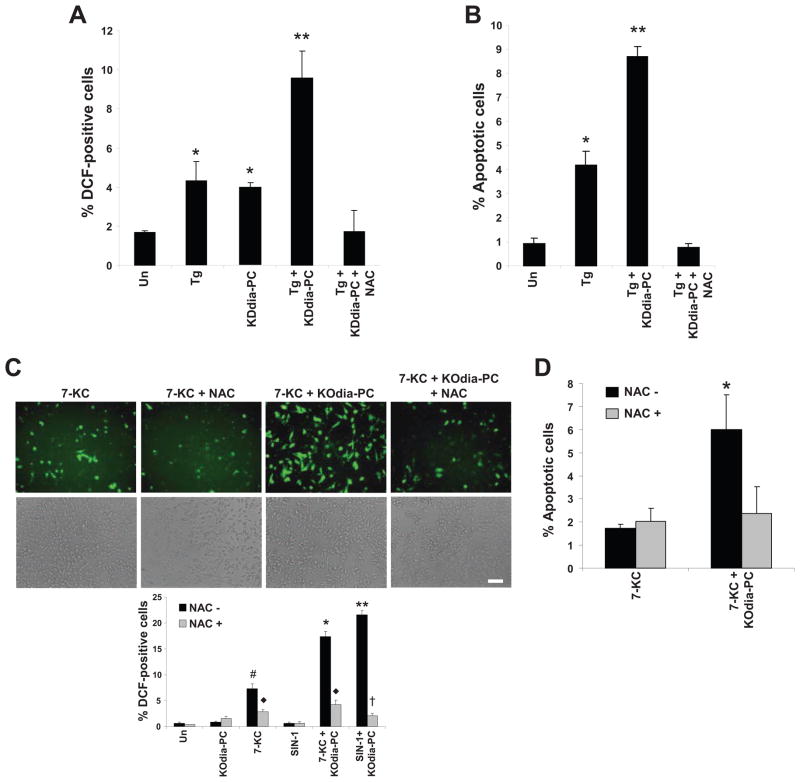
ROS can contribute to apoptosis under physiologic and pathologic conditions (Li et al., 2009; Simon et al., 2000), and a CD36-ROS pathway has been implicated in oxLDL-induced inactivation of SHP-2 phosphatase in macrophages (Park et al., 2009). We therefore tested whether ROS are generated by CD36 ligands during ER stress and, if so, whether this process plays a role in apoptosis. Treatment of cells with KDdia-PC, thapsigargin, SIN1, or 7-ketocholesterol alone resulted in a low level of 2',7'-dichlorofluorescin diacetate (DCF) staining, a measure of cellular oxidative stress (Frank et al., 2000) (Figure 2A,C). However, when the oxPLs KOdia-PC or KDdia-PC were combined with the ER stressors, DCF staining was substantially increased. Similar results were obtained when thapsigargin was combined with other TLR2 ligands, including LTA, Pam2CSK4, and Pam3CSK4 (data not shown). Furthermore, pretreatment of macrophages with the anti-oxidant N-acetylcysteine (NAC) suppressed DCF staining and, most importantly, apoptosis under these conditions (Figure 2B,D). NAC also decreased apoptosis in response to palmitic acid plus thapsigargin (data not shown). Thus, ROS is an important component of the CD36/TLR2/ER stress pathway of apoptosis.
Figure 2. CD36 Ligand-Induced Apoptosis in ER-stressed Macrophages is Dependent on Reactive Oxygen Species (ROS).
(A–B) Macrophages were pre-incubated for 60 min in the absence or presence of 100 μM N-acetylcysteine (NAC) and then incubated for 24 h in medium alone (Un) or containing 0.25 μM thapsigargin (Tg), 20 μg/ml KDdia-PC or Tg and KDdia-PC, as indicated. The cells were then assayed for ROS, using the DCF fluorescence assay, and apoptosis. (C–D) As above, but here the ER stressor was 5 μg/ml 7-ketocholesterol or 2 mM SIN-1 instead of thapsigargin. Differences between values with symbols and no symbols, or between values with different symbols, are statistically significant (p values ranged from < 0.05 to < 0.01).
Lp(a) Triggers Apoptosis in ER-Stressed Macrophages in a CD36-TLR2-Oxidative Stress-Dependent Manner
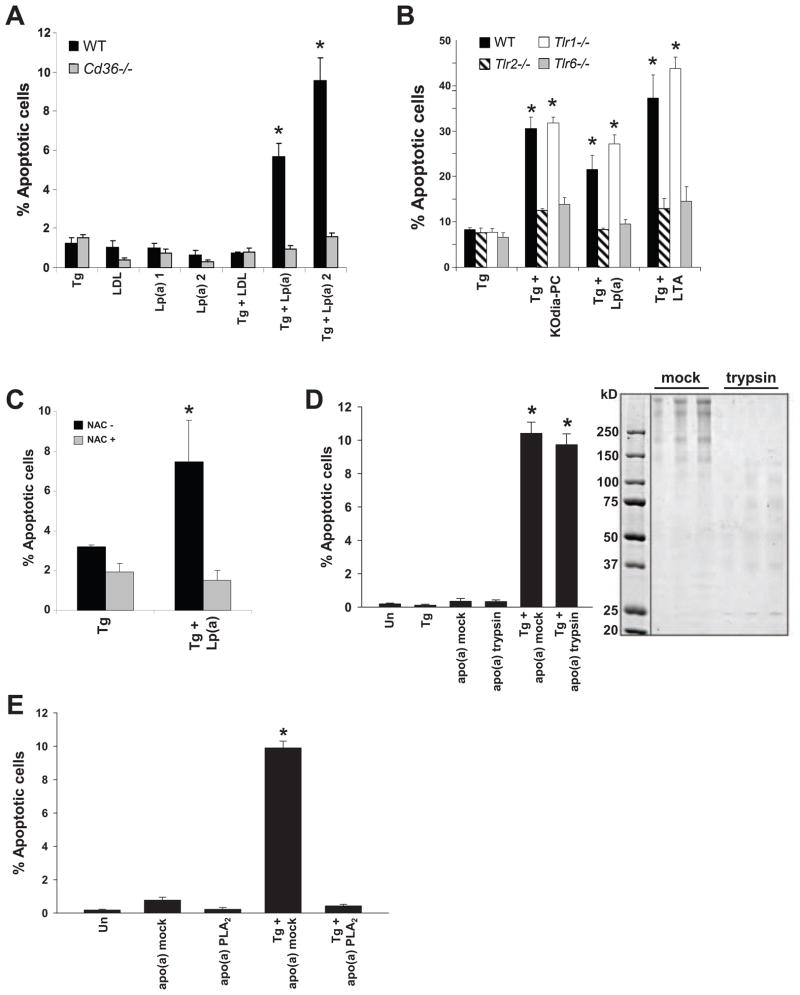
Lipoprotein(a) [Lp(a)] is a modified LDL-like lipoprotein in which a kringle-containing protein called apolipoprotein(a) is covalently bound to apolipoprotein B of LDL (Koschinsky and Marcovina, 2004). Epidemiological and human genetic studies have shown that Lp(a) is a potent, independent risk factor for advanced atherothrombotic vascular disease. Moreover, in a transgenic rabbit model, modest plasma levels of Lp(a) (~15 mg/dl) markedly promoted plaque necrosis without affecting en face lesion area (Sun et al., 2002). However, a clear mechanism linking Lp(a) with a cellular event specifically associated with advanced plaque progression has been elusive (Tregouet et al., 2009; Brazier et al., 1999; Holmer et al., 2003; Erqou et al., 2009). In the context of the data herein, a potential clue to this mystery is the recent finding that Lp(a) is a major carrier of oxPLs in human plasma (Bergmark et al., 2008). We therefore tested whether Lp(a) could trigger apoptosis in ER-stressed macrophages (Figure 3A). The Lp(a) was verified to contain oxPLs by anti-oxPL antibody (E06) reactivity (data not shown). As expected, neither LDL nor Lp(a) induced apoptosis when given alone. However, Lp(a) from two separate subjects, but not LDL, markedly induced apoptosis when added in combination with thapsigargin. Moreover, apoptosis under these conditions was CD36-dependent. We then compared Lp(a) with KOdia-PC and LTA in ER-stressed macrophages from WT, Tlr2−/−, Tlr6−/−, or Tlr1−/− mice to determine whether apoptosis induced by Lp(a) was also TLR-dependent. As shown in Figure 3B, all ligands triggered apoptosis in a TLR2- and TLR6-dependent manner. Also similar to oxPL-induced apoptosis, Lp(a)-induced apoptosis in ER-stressed macrophages could be suppressed by NAC (Figure 3C).
Figure 3. Lp(a) Triggers Apoptosis in ER-Stressed Macrophages in a CD36-TLR2-Oxidative Stress-Dependent Manner.
(A–B) Macrophages from WT, Cd36−/−, Tlr2−/−, Tlr1−/−, or Tlr6−/− mice were incubated for 24 h with 0.5 μM thapsigargin alone or in combination with 25 μg/ml LDL or Lp(a), 50 μg/ml KOdia-PC, or 10 μg/ml LTA. (C) Apoptosis data for macrophages that were pre-incubated with or without 500 μM N-acetylcysteine (NAC) and then incubated for 24 h with thapsigargin alone or with 50 μg/ml of Lp(a). (D) Macrophages were incubated for 22 h with thapsigargin alone or in combination with 25 μg/ml apo(a) that was pre-treated in the absence (mock) or presence of trypsin and then assayed for apoptosis. The image shows a Coomassie-stained SDS-polyacrylamide electrophoresis gel of mock and trypsin-treated apo(a); for each condition, three different amounts of proteins were loaded per lane, increasing from left to right. (E) As in D, but here the apo(a) was treated with ± PLA2 prior to trypsinization. *, p < 0.01 compared to other groups without the asterisk.
The oxPLs of Lp(a) are found both in the lipid phase and covalently bound to apo(a) (Bergmark et al., 2008; Edelstein et al., 2003). The next series of studies were conducted using recombinant 17-kringle apo(a) (Feric et al., 2008), which contains oxPLs as assessed by both anti-oxPL antibody (E06) reactivity and mass spectrometry (data not shown). These oxPLs are presumably acquired during secretion from the protein-producing cells. We found that apo(a) was a potent inducer of apoptosis in ER-stressed macrophages (Figure 3D). To determine whether apoptosis depended on an intact apo(a) protein, apo(a) was subjected to extensive digestion with trypsin or mock treatment in buffer alone. Trypsin treatment led to extensive digestion of apo(a), which normally migrates between ~250–500 kDa depending on the level of glycosylation (Koschinsky et al., 1991) (Figure 3D, gel image). The apoptosis data in Figure 3D show that the trypsin-treated protein had the same level of pro-apoptotic activity as the mock-treated protein, consistent with a role for apo(a)-associated lipids.
To gain further evidence that oxPLs on apo(a) are necessary for its pro-apoptotic activity, the apo(a) preparation was treated with PLA2 or buffer alone (mock). Because oxPLs are bound covalently to apo(a) through the sn2 oxidized fatty acyl moiety (Edelstein et al., 2003), PLA2 hydrolysis would be expected to release the lipids from the protein as lysoPC. After treatment, the apo(a)-PLA2 (or mock) reaction mixture was subjected to trypsin treatment to avoid an effect of the PLA2 on the cells. As shown in Figure 3E, PLA2 treatment completely abrogated apo(a)-induced apoptosis in ER-stressed macrophages, further supporting a role for apo(a)-associated phospholipids in the apoptotic response. In summary, oxPL-containing Lp(a) and apo(a) are potent inducers of macrophage apoptosis in the setting of ER stress. The mechanism, like that of oxPL, involves CD36, TLR2, TLR6, and oxidative stress in the macrophages.
NOX2 is Necessary for the Generation of ROS and Apoptosis by CD36 Ligands During ER Stress
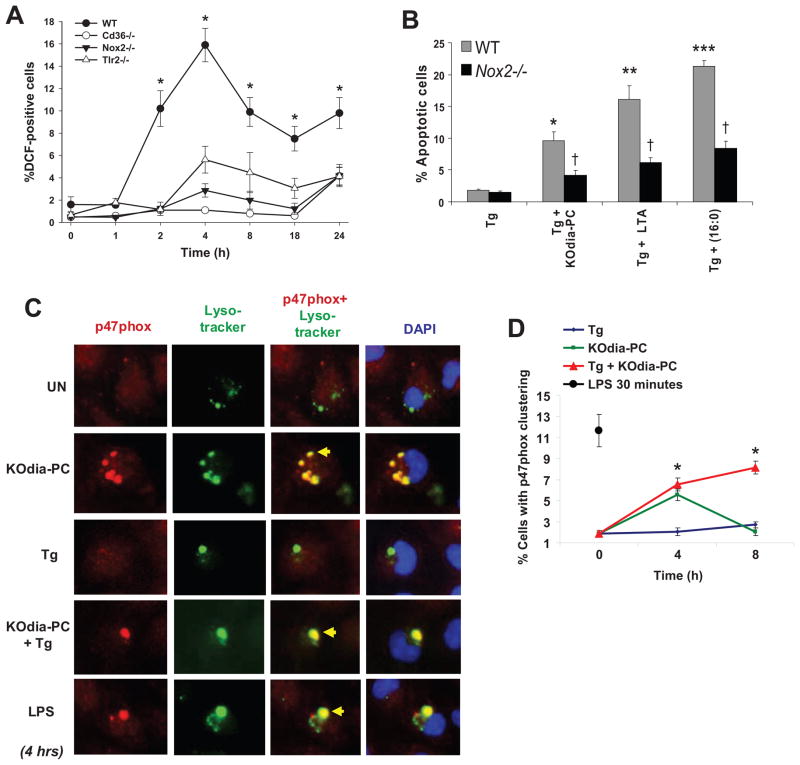
To determine whether NADPH oxidase could be a source of ROS and involved in apoptosis in ER-stressed macrophages exposed to CD36 ligands, we compared macrophages from wild type mice with those from mice lacking TLR2, CD36, or the macrophage NADPH oxidase subunit NOX2. Time course analysis revealed that DCF staining increased after 1 h of KDdia-PC/thapsigargin treatment, peaked at 4h, dropped slightly between 4 and 8 h, and then remained at this level for the next 16 h (Figure 4A). However, NOX2-, TLR2-, and CD36-deficient macrophages showed suppressed DCF staining throughout the entire time course. Most importantly, apoptosis was suppressed in NOX2-deficient macrophages treated with palmitic acid, KOdia-PC, or LTA in combination with thapsigargin (Figure 4B). Similar results were found when the combination of KOdia-PC and either 7-ketocholesterol or SIN-1 was used (data not shown). Thus, CD36 ligands promote ROS accumulation in ER-stressed macrophages in a CD36-, TLR2-, and NOX2-dependent manner, and both ROS and NOX2 are necessary for the full apoptotic response in this setting.
Figure 4. Sustained Activation of NADPH Oxidase by CD36 Ligands in ER-Stressed Macrophages.
(A) Macrophages from WT, Cd36−/−, Nox2−/−, or Tlr2−/− mice were incubated for the indicated times with 0.25 μM thapsigargin (Tg) alone or in combination with 20 μg/ml POV-PC and then assayed for the percent of DCF-positive cells (ROS). (B) Macrophages from WT or Nox2−/− mice were incubated for 24 h with thapsigargin alone or in combination with KOdia-PC, 10 μg/ml LTA, or 0.25 mM palmitic acid (PA). The cells were then assayed for apoptosis. (C) Macrophages were incubated for 4 h in medium alone or medium containing KOdia-PC, thapsigargin or the two reagents combined and then subjected to immunofluorescence for p47phox (red) and labeling with LysoTracker® Green and the nuclear stain DAPI (blue). Treatment with LPS for 30 min was used as a positive control. Bar, 5 μm. (D) Quantification of the data at two timepoints for the experiment in C, expressed as the percent of total cells that exhibited p47 clustering (mean ± SEM, n=4). Differences between values with symbols and no symbols, or between values with different symbols, are statistically significant (p < 0.05).
During NADPH oxidase activation, p47phox is recruited to membranes as part of the assembly of the NADPH oxidase complex. This process causes “clustering” of p47 at the plasma membrane or endocytic/phagocytic compartment (Laroux et al., 2005). While p47phox is localized diffusely in unstimulated cells, treatment with KOdia-PC for 4 h led to an increase of p47phox clustering (Figure 4C). Interestingly, p47 appeared to cluster in regions that stained with LysoTracker® Green, suggesting assembly on acidic organelles such as lysosomes. After 8 h, the percentage of cells exhibiting p47phox clustering in response to KOdia-PC alone dropped significantly (Figure 4D). Cells treated with KOdia-PC in combination with thapsigargin also exhibited an increase in p47phox clustering, but in this case clustering was sustained and did not diminish by 8 h. The combination of KOdia-PC with SIN-1 or 7-ketocholesterol also led to sustained clustering of p47phox (data not shown). Thapsigargin alone did not induce p47phox clustering at any of the timepoints examined. As a positive control, macrophages were treated for 30 min with 500 ng/ml of LPS to induce clustering (Figure 4C-D). Thus, while KOdia-PC alone induces transient clustering of p47phox within acidic compartments, the addition of an ER stressor causes sustained p47phox clustering, which is consistent with sustained ROS production under these conditions.
TLR2-Dependent ERK Activation is Necessary for Apoptosis, p47phox Clustering, and ROS Production
Several kinases such as PKC, ERK, and c-Src have been implicated in promoting p47 phosphorylation and NADPH oxidase activation (Dewas et al., 2000; Touyz et al., 2003; Bey et al., 2004). We therefore screened a variety of inhibitors for their ability to suppress apoptosis in ER-stressed macrophages exposed to CD36 ligands. We found that the conventional PKC inhibitor, Go6976, the pan-PKC inhibitor, Go6983, and the Src inhibitor PP2 had no effect on apoptosis. In contrast, PD98058, which inhibits MEK1 and its downstream substrate ERK, completely suppressed apoptosis induced by KOdia-PC or LTA in combination with thapsigargin (Figure 5A). Although ERK has been shown to promote cell survival in other models of ER stress (Hu et al., 2004), these data and others (Sim et al., 2005) suggest that ERK can mediate apoptosis in ER-stressed macrophages exposed to CD36 ligands. To directly demonstrate the concept of context-dependent effects of ERK inhibition, we treated macrophages with a dose of 7-ketocholesterol that induces apoptosis in the absence of a CD36 ligand (Figure 5A). We found that macrophages treated with the ERK inhibitor were markedly more susceptible to apoptosis in this scenario, demonstrating that ERK functions as a survival kinase in an ER stress model that does not involve CD36.
Figure 5. TLR2-Dependent ERK Activation Promotes Apoptosis, ROS Generation, and p47phox Clustering.
(A–C) Macrophages were left untreated (Un) or incubated for 24 h with the ER stressors thapsigargin or 7-ketocholesterol alone or in combination with KOdia-PC or LTA. Some of the samples were pre-treated for 1 h with 250 nM of the conventional or pan PKC inhibitors Go6976 and Go6983, respectively; 10 μM of the Src-like kinase inhibitor PP2; or 10 μM of the MEK1 inhibitor PD98059. The cells were then analyzed for apoptosis (A), DCF positivity (B), or p47phox clustering (C). (D–E) Macrophages from WT, Nox2−/−, or Tlr2−/− mice were incubated with KDdia-PC alone for the indicated times. Cell lysates were then immunoblotted for Thr202/Tyr204-phospho-ERK1/2 and total ERK. Differences between values with symbols and no symbols, or between values with different symbols, are statistically significant (p < 0.05).
To determine whether ERK plays a role in NADPH oxidase activation and ROS production, macrophages were exposed to KOdia-PC or LTA in combination with thapsigargin, with or without treatment with PD98058. The ERK inhibitor markedly suppressed DCF staining induced by KOdia-PC or LTA during ER stress (Figure 5B). Similar results were found when p47phox clustering was assayed (Figure 5C). Moreover, KDdia-PC promoted ERK phosphorylation, a measure of its activation, in two phases, peaking at 30 min and 18–24 h, respectively (Figure 5D). The latter phase was partially dependent on TLR2 but not on NOX2 (Figure 5D–E). Taken together, these results are consistent with a model in which ERK is activated downstream of TLR2, leading to p47 translocation and NADPH oxidase activation.
SFAs Trigger CD36 and TLR2-Dependent Apoptosis in Macrophages Undergoing ER Stress In Vivo
To test the relevance of the CD36-ER stress apoptosis pathway in vivo, we compared chow-fed mice with mice fed a diet containing 3.3% cocoa butter, which is rich in stearic and palmitic acids. After two weeks on the diet, the mice were given an intraperitoneal (i.p.) injection of concanavalin A to recruit macrophages to the peritoneal cavity. Two days later the mice were given another i.p. injection of 0.4 mg/kg of thapsigargin dissolved in 150 mM dextrose or, as a control, dextrose vehicle alone. After 24 h, freshly isolated peritoneal macrophages were assayed for apoptosis. As shown in Figure 6A, macrophages from mice fed the SFA diet and subsequently given vehicle control had very little apoptosis. Similar results were seen when mice were fed a regular chow diet for two weeks, followed by an i.p. injection of thapsigargin. In contrast, there was a marked increase in apoptosis in macrophages isolated from mice fed the SFA diet in combination with thapsigargin. Although SFAs can induce ER stress under certain conditions (Borradaile et al., 2006; Erbay et al., 2009), we found that pro-apoptotic CHOP was not induced in macrophages from the SFA-fed mice and was not increased above the thapsigargin-alone level in macrophages from SFA/thapsigargin-treated mice (data not shown). Using the TUNEL assay, apoptosis was also quantified in splenic macrophages from the same groups of mice. As shown in Figure 6B, a marked increase in apoptosis of Mac3-positive splenic macrophages was observed in ER-stressed mice fed the SFA diet but not in those fed the chow diet.
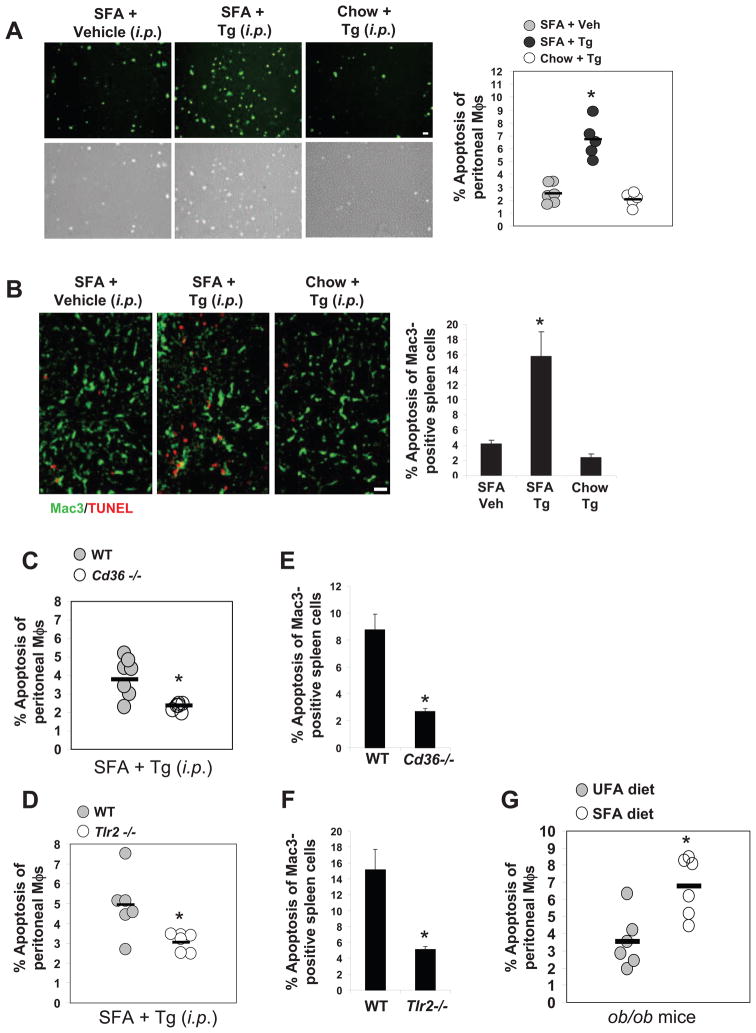
Figure 6. SFAs Trigger CD36- and TLR2-Dependent Macrophage Apoptosis In Vivo.
(A–B) Mice were fed a regular chow diet or a diet containing saturated fatty acids (SFAs) for two weeks. Concanavalin A was administered i.p., and two days later the mice were given an i.p. injection of 150 mM dextrose (vehicle) or 0.4 mg/kg of thapsigargin (Tg) in dextrose. (A) Macrophages were harvested from the peritoneum 24 h later and stained immediately with alexa-488 annexin-V and propidium iodide. Representative fluorescent and bright-field images are shown. Bar, 20 μm. The dot plot shows the quantified data for each individual mouse, which was obtained from 5 individual fields/mouse. The horizontal line represents the mean in each group (*p < 0.05). (B) Spleens harvested from these mice were sectioned and stained for the macrophage marker Mac3 (green) and for apoptosis by TUNEL (red) (Bar, 10 μm; *p < 0.05). (C–F) WT, Cd36−/−, and Tlr2−/− mice were fed a SFA-rich diet for 2 wks and then treated with thapsigargin as described above. Peritoneal macrophages (C–D) and splenic macrophages (E–F) were then assayed for apoptosis. (G) ob/ob mice were fed a diet rich in SFAs or unsaturated fatty acids (UFA) for 2 wks, and then freshly isolated peritoneal macrophages were assayed for apoptosis. The horizontal lines in C, E, and G represent the mean value in each group (*p < 0.05).
To determine whether the increase in apoptosis observed in vivo was dependent on CD36 and/or TLR2, wild-type, Cd36−/−, and Tlr2−/− mice were compared in the above experimental protocol. Peritoneal and splenic macrophages were protected from apoptosis in both CD36-deficient and TLR2-deficient mice (Figure 6C–F). To test SFA-induced apoptosis in a physiologic model of ER stress, we used the leptin-deficient ob/ob model, a model of ER stress and insulin resistance whose macrophages undergo a low level of ER stress in vivo (Liang et al., manuscript in revision) (Li et al., 2009). Apoptosis in peritoneal cavity macrophages was determined in ob/ob mice fed either the aforementioned SFA diet or a calorically matched safflower oil diet rich in the unsaturated fatty acids (UFA), which are relative weak inducers of apoptosis (see Fig. 1H). SFA- and UFA-treated mice had similar weights and plasma glucose and insulin levels, and the macrophages harvested from the two groups of mice showed similar induction of iNOS by LPS (data not shown). As shown in Figure 6G, apoptosis was significantly higher in the ob/ob mice fed the SFA diet. Thus, the level of ER stress that occurs in ob/ob mice is enough to trigger SFA-induced macrophage apoptosis in the absence of an exogenous ER stressor.
TLR2/4 Deficiency Suppresses Advanced Lesional Macrophage Apoptosis and Plaque Necrosis in Ldlr−/− Mice
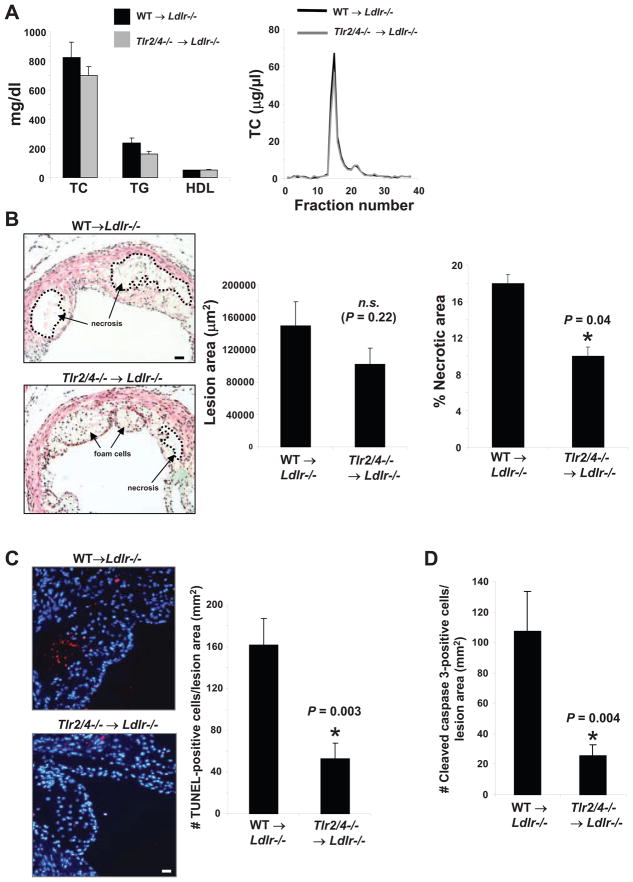
We have previously shown that Apoe−/− mice with a combined deficiency of SRA and CD36 are protected from advanced lesional macrophage apoptosis and plaque necrosis (Manning-Tobin et al., 2009). Importantly, plaque necrosis was not decreased in atherosclerosis models with deficiencies of SRA alone or CD36 alone (unpublished studies), indicating that both pathways of apoptosis need to be eliminated before effects are observed. Because fat-fed Ldlr −/− mice transplanted with bone marrow from mice deficient in TLR4 alone also did not demonstrate an effect on advanced plaques (unpublished studies), and because CD36 requires TLR2 while SRA requires TLR4 (Seimon et al., 2006), we determined whether combined deficiency of TLR2 and TLR4 suppresses advanced lesional macrophage apoptosis in a mouse model of atherosclerosis. Note that while a number of studies have shown roles for TLR2 and TLR4 in early atherogenesis (Michelsen et al., 2004; Mullick et al., 2008), their involvement in the key process of advanced plaque progression, specifically plaque necrosis, has not been examined. Thus, Ldlr−/− mice were reconstituted with wild-type or Tlr2−/−Tlr4−/− bone marrow and then placed on a Western-type diet for 10 wks. The two groups of mice showed no statistically significant difference in body weight (data not shown); total plasma cholesterol, HDL, and triglycerides; or FPLC lipoprotein profile (Figure 7A). Aortic root lesion area, which would not necessarily be altered by processes affecting advanced lesional macrophage apoptosis, was modestly decreased in the TLR2/4-deficient group, but the difference was not statistically different (Figure 7B). In contrast, there was a statistically significant 45% decrease in percent necrotic area in the Tlr2−/−Tlr4−/− → Ldlr−/− lesions. Plaque necrosis arises as a consequence of advanced lesion macrophage apoptosis (Tabas, 2005; Schrijvers et al., 2007). We therefore assessed the effect of TLR2/TLR4-deficiency on lesional macrophage apoptosis using TUNEL and activated caspase 3 staining. As shown in Figure 7C–D, there was an approximately 60% decrease in TUNEL positivity and a 75% decrease in activated caspase-3 staining in Tlr2−/−Tlr4−/− → Ldlr−/− lesions. The majority of the TUNEL-positive cells co-localized with the macrophage marker Mac3 as assessed by immunofluorescence microscopy (Figure S2). Thus, combined TLR2 and TLR4-deficiency in bone-marrow-derived cells of Ldlr−/− mice is partially protective against macrophage apoptosis and plaque necrosis in advanced atherosclerosis.
Figure 7. TLR2/4 Deficiency in Bone Marrow-Derived Cells Suppress Apoptosis and Plaque Necrosis in Advanced Aortic Root Lesions of Fat-Fed Ldlr−/− Mice.
Ldlr−/− mice were transplanted with Tlr2+/+Tlr4+/+ (wild-type, WT) or Tlr2−/−Tlr4−/− bone marrow and then fed a Western-type diet for 10 wks starting 4 wks after transplantation. (A) Plasma cholesterol, triacylglycerol, HDL, and FPLC lipoprotein profile of the 2 groups of mice. There were no statistical differences between the 2 groups in any of the values. (B–D) Images and quantification of lesion area and percent necrotic area (n=10 and n=9), percent of TUNEL-positive cells (n=10 and n=7), percent activated caspase-3 positive cells (n=8 and n=9) in the lesions of WT → Ldlr−/− and Tlr2−/−Tlr4−/− → Ldlr−/− mice respectively. Panel B shows hematoxylin and eosin staining of the sections with the necrotic area outlined (dotted black lines), and panel C shows TUNEL (red) and DAPI (blue) staining. The P values shown in panels B–D were derived from the Mann-Whitney test. Bars in B and C, 20 and 15 μm, respectively.
DISCUSSION
The vast majority of atherosclerotic lesions, even those that are relatively large, are clinically silent (Libby, 2000). Rather, acute atherothrombotic vascular disease, the leading cause of death in the industrialized world, is caused by a very small minority of lesions that are characterized not by large lesion volume but by plaque necrosis, plaque disruption, and susceptibility to acute thrombosis (Libby, 2000; Virmani et al., 2003). This report provides evidence in vitro and in vivo that a pathway involving the pattern recognition receptors CD36 and TLR2/6 plays an important role in a critical process leading to plaque necrosis, namely, the triggering of apoptosis in ER-stressed macrophages (Figure S3). Although these pattern recognition receptors have been implicated previously in very different processes related to early atherogenesis (below), their role in the late lesional event of apoptosis in ER-stressed macrophages provides a link of these receptors to the completely distinct and clinically relevant process of plaque necrosis. Moreover, these studies have revealed the surprising and fascinating finding that Lp(a), a oxPL-carrying lipoprotein specifically associated with acute atherothrombosis through an unknown mechanism, is a potent stimulator of this CD36-TLR2 apoptosis pathway in ER-stressed macrophages.
While this is the first study to examine the role of CD36 and TLR2 in processes related specifically to plaque necrosis, other studies have examined their roles in early atherogenesis. For example, several studies have shown that SRA and/or CD36 deficiency protects mice from macrophage foam cell formation in mouse models of atherosclerosis, although the magnitude of this effect can be quite modest and shows dependence on the level of oxLDL-associated CD36 ligands induced by certain diets (Silverstein, 2009; Zhao et al., 2005; Manning-Tobin et al., 2009; Kennedy et al., 2009). CD36 has also been implicated in macrophage trapping in lesions (Park et al., 2009). Similarly, a number of previous studies have examined the role of TLR2 on atherogenic processes relevant mostly to early lesion development (Mullick et al., 2005; Mullick et al., 2008; Liu et al., 2008). These studies have revealed that endogenous TLR2 ligands may be acting primarily on endothelial cells, while exogenous ligands exert their effect on macrophages (Mullick et al., 2005). Of interest, one study linked TLR2 to activation of NF-κB and the induction of inflammatory cytokines and matrix metalloproteases (MMPs) in mixed vascular cells cultured from human carotid atherectomy specimens (Monaco et al., 2009). If future in vivo studies provide evidence for TLR2-induced inflammation and MMP induction in advanced lesions in vivo, these actions of TLR2 would complement the pro-apoptotic role for TLR2 revealed here in terms of advanced plaque progression.
The identity of the molecular components on oxPLs, including oxPLs associated with Lp(a)/apo(a), that activate the CD36-TLR2/6 pathway remains to be fully elucidated. Our data thus far suggest that with free oxPLs, the sn2 oxidized fatty acid is a required element, but, in view of our data showing that free saturated fatty acids (SFAs) and dipalmitoyl PC can trigger the pathway, it may work in concert with the sn1 SFA of oxPLs. However, the situation with the oxPLs associated with apo(a) is likely to be different if the sn2 oxidized fatty acid is covalently bound to the protein, as suggested by previous work (Edelstein et al., 2003; Bergmark et al., 2008). In this case, the sn1 SFA may play a critical role, perhaps in concert with the phosphocholine headgroup. Another area for future investigation is to determine why oxPL internalization is necessary to trigger apoptosis in ER-stressed macrophages (Figure S5) and how this process might work in concert with cell-surface signaling through CD36 and/or TLR2/6 heterodimer.
The role of SFAs in the apoptosis pathway described here is an important finding both mechanistically and because of its potential relevance to obesity and insulin resistance. SFAs have been reported to directly stimulate TLR signaling (Shi et al., 2006; Senn, 2006; Nguyen et al., 2007), but these findings have recently been challenged by a study showing LPS contamination in the BSA used to complex the SFAs (Erridge and Samani, 2009). Contamination of BSA with TLR activators cannot account for the ability of SFAs to trigger apoptosis in ER-stressed macrophages because LPS in our model does not serve as a second TLR hit for apoptosis in ER-stressed macrophages (Seimon et al., 2006), and the pro-apoptotic effect of fatty acids in the current study was TLR4-independent. Moreover, the SFA-free BSA control did not trigger macrophage apoptosis when combined with thapsigargin, and SFAs were much more potent in inducing apoptosis than unsaturated FAs, which were also complexed with BSA. In addition to activating TLRs, SFAs can directly trigger pro-apoptotic ER stress (Wei et al., 2006), which likely accounts for the residual level of TLR2-independent macrophage death we observed with SFAs in the absence of thapsigargin (Fig. 1H). In this regard, a recent study showed that SFAs at a dose of 500 μM alone can cause ER stress-induced macrophage death through a pathway mediated by adipocyte-binding protein-4 (aP2), a cytosolic lipid chaperone that is also expressed in macrophages (Erbay et al., 2009). However, our in vivo studies with ob/ob and Ldlr−/− mice suggest that the TLR-dependent pathway of macrophage death revealed herein is relevant to pathological settings in which ER stress is present.
As mentioned above, our study may provide insight into how Lp(a) contributes to atherothrombotic vascular disease. Lp(a) is an independent risk factor for coronary artery disease, but the mechanism underlying this observation is not known (Tregouet et al., 2009; Brazier et al., 1999; Holmer et al., 2003; Bergmark et al., 2008; Erqou et al., 2009). Recent insight was provided by the finding that Lp(a) functions as a carrier of PC-containing oxPLs (Bergmark et al., 2008), and we show here that oxPL-containing Lp(a) and apo(a) can trigger apoptosis in ER-stressed macrophages in a CD36-, TLR2/6-, ROS-, and sn2 fatty acid-dependent manner. These findings may explain a report in 1994 showing that Lp(a) can induce oxidative stress in monocytes (Riis Hansen et al., 1994), although no mechanism was reported. Relevant to a role for Lp(a) in ER stress-induced macrophage apoptosis, transgenic hyperlipidemic rabbits with modest plasma levels of Lp(a) have a marked increase in plaque necrosis without a change in plasma lipids or en face lesion area compared with non-transgenic WHHL rabbits (Sun et al., 2002). This finding is consistent with the association between high plasma levels of Lp(a) and acute coronary syndromes. Thus, our findings raise the possibility of a specific cellular mechanism linking elevated plasma Lp(a) to advanced plaque progression and consequent atherothrombotic vascular disease.
The ultimate goal of studies on advanced plaque progression in general, and advanced lesional macrophage apoptosis in particular, is to suggest therapeutic strategies that specifically block the progression of the 2–3% of total human atherosclerotic lesions that actually cause acute atherothrombotic vascular disease. If one were to focus on interrupting the proximal components of the pathway described herein, i.e., CD36 or TLR2, lesional targeting would likely be required for specificity in view of the widespread role of these receptors in normal physiology and host defense. A further therapeutic clue from the data herein may come from the critical role of prolonged oxidative stress. On the one hand, we show here that ER stress is needed for prolongation of this response per se, and recent work using chemical chaperones to suppress ER stress has suggested potential promise of this approach in the setting of atherosclerosis (Erbay et al., 2009). The initial oxidant burst itself, which is triggered by a TLR2-NADPH oxidase pathway, is also a possible target through NADPH oxidase inhibitors (Yu et al., 2008) or anti-oxidants that partially suppress the response but do not compromise its role in host defense. The fact that vitamin E is not useful in preventing human cardiovascular disease may be due to the fact that it does not adequately target the specific types and mechanisms of oxidative stress that are key to plaque progression (Williams and Fisher, 2005). Indeed, the protective actions of NAC in our cell culture model of ER stress-induced macrophage apoptosis were not found with doses of vitamin E that have anti-oxidant actions in other settings (unpublished data). Of interest in this regard, NAC has been shown recently to suppress atherosclerosis in fat-fed Apoe−/− mice (Shimada et al., 2009). These types of findings and therapeutic strategizing provide strong rationale for understanding in depth the cellular and molecular mechanisms that specifically promote advanced plaque progression.
EXPERIMENTAL PROCEDURES
Animals and Diets
The following mice were purchased from Jackson Laboratory (Bar Harbor, Maine): C57BL/6J; Tlr2−/− (strain B6.129 Tlr2 tm1Kir/J) backcrossed to the C57BL/6J background; Tlr4-deficient (strain C57BL/10ScNJ); Nox2−/− (strain B6.129S6-Cybbtm1Din/J); Ldlr−/− (B6.129S7-Ldlrtm1Her/J); and ob/ob (B6.V-Lepob/J). The Myd88−/−, Sra−/− (Msr−/−)Cd36−/−, Tlr4−/−, Tlr1−/−, and Tlr6−/− mice were generated as described previously (Seimon et al., 2006; Moore et al., 2005; Adachi et al., 1998; Hoshino et al., 1999; Takeuchi et al., 2002). For the atherosclerosis study, Tlr2−/− and Tlr4−/− mice were crossed to generate Tlr2−/−Tlr4−/− mice as donors. Macrophages from female 8–10 week C57BL/6J mice were used as wild-type controls in all experiments. The saturated fatty acid and unsaturated fatty acid diets were purchased from Research Diets, Inc. Both the saturated fatty acid diet (SFA diet, formula # D06040503) and unsaturated fatty acid diet (UFA diet, formula # D09021101) were matched for caloric content and contain 20% protein and 70% carbohydrate. The SFA diet contains 3.5% cocoa butter (43% palmitic and stearic acids; 40% oleic and linoleic acids). The UFA diet contains 3.5% Safflower Oil (10% palmitic and stearic acids; 76% oleic and linoleic acids). For the atherosclerosis study, male mice, at 14 weeks of age and 4 weeks after the bone marrow transplant, were placed on a Western-type diet (TD88137; Harlan Teklad) for 10 wks.
Bone Marrow Transplantation
Ten-week-old male Ldlr−/− mice were lethally irradiated using a cesium γ source at a dose of 1000 rad 4–6 h before transplantation. Bone marrow cells were collected from the femurs and tibias of donor wild-type and Tlr2−/−Tlr4−/− mice by flushing with sterile medium as described previously (Lim et al., 2008). All animal procedures used in this study followed Columbia University’s institutional guidelines.
Macrophage Staining of Mouse Spleen Sections and Atherosclerotic Lesions
Specimens were immersion-fixed in 10% neutral-buffered formalin (for spleens) or 1% paraformaldehyde in PBS (for hearts) overnight followed by paraffin embedding. 7-μM sections on glass slides were deparaffinized in xylene and hydrated in water. Antigen retrieval was obtained by exposing the slides to 1 mM EDTA, pH 8.0, in a steamer for 20 min. The sections were permeabilized with 0.1% Triton X-100 and then incubated with anti-Mac-3 antibody overnight, followed by incubation with a biotinylated anti-rat IgG and then streptavidin-conjugated Alexa 488-labeled antibody. Sections were stained for TUNEL as described below, followed by counter-staining with DAPI to identify nuclei.
Apoptosis Assays
Apoptosis was assayed in cultured macrophages by staining with Alexa 488-conjugated Annexin V (green) and propidium iodide (PI; red), as described previously (DeVries-Seimon et al., 2005). Apoptotic cells in tissues were labeled by TUNEL (TdT-mediated dUTP nick-end labeling) using the in situ cell death detection kit TMR-red (Roche Diagnostics) according to the manufacturer’s protocol. Only TUNEL-positive cells that co-localized with DAPI-stained nuclei and Mac3 were counted as being positive. TUNEL and annexin staining were viewed using an Olympus IX-70 inverted fluorescent microscope. Representative fields (4–6 fields containing ~1000 cells) were photographed using an Olympus DP71 CCD camera. The number of Annexin V- and PI-positive cells were counted and expressed as a percent of the total number of cells in at least 4 separate fields from duplicate wells. For TUNEL analysis, DAPI, TUNEL, and/or Mac3 images were merged using Photoshop analysis software (Adobe Systems). The number of cells positive for TUNEL, DAPI, and Mac3 (in spleen sections only) were counted in two fields from each mouse and expressed as the percentage of total Mac3-positive cells in the image. Activated caspase 3 staining was performed using the SignalStain Cleaved Caspase-3 (Asp175) IHC detection kit according to the manufactures protocol (Cell Signaling Technology).
p47phox Clustering Assay
Macrophages were plated at 50% confluency and treated the following day as indicated in the figure legends. The media were removed, and the cells were incubated for 10 min at 37oC with 50 nM LysoTracker® Green DND25 in PBS. The cells were then rinsed with PBS twice, fixed in 4% paraformaldehyde in PBS for 20 min, rinsed again, and permeabilized using 0.5% Triton X-100 in PBS for 5 min. The permeabilized cells were incubated for 60 min in 5% normal goat serum and then overnight at 4°C with a rabbit anti-p47phox primary antibody. The cells were rinsed, stained with a goat anti-rabbit Alexa-Flour 594-labeled secondary antibody, and counterstained with DAPI to identify the nuclei. The number of cells exhibiting clustering was quantified from 4 independent fields of view and expressed as the percent of the total population.
Atherosclerotic Lesion Analysis
For morphometric lesion analysis, sections were stained with Harris’ hematoxylin and eosin. The total lesion area and necrotic area was quantified as previously described (Seimon et al., 2009). For plaque necrosis, boundary lines were drawn around regions that were free of hematoxylin and eosin (H&E) staining, and area measurements were obtained using image analysis software (Seimon et al., 2009). A 3,000-μm2 threshold was implemented in order to avoid counting regions that likely do not represent substantial areas of necrosis. Using this method, a 97% agreement in the percent necrotic area was calculated between our 2 independent observers.
Supplementary Material
Acknowledgments
This work was supported by an American Heart Association Scientist Development Grant (0735594T) to T.S. and an AHA Student Scholarship in Cardiovascular Disease and Stroke to M.N. The work was also supported by NIH grants HL087123 and HL075662 to I.T.; GM069338 (LIPID MAPS) to R.H, J.L.W., and S.T.; AG020255 and AG032349 to K.J.M.; GM54060 to D.T.G.; an NIH T32 training grant (HL007343) to M.J.N.; and an NIH Summer Research Training Grant to M.N. Other support includes US Army Medical Research and Materiel Command grant W81XWH-06-1-0212 to I.T; and the Fondation Leducq to J.L.W. and S.T. M.L.K. is a Career Investigator, Heart and Stroke Foundation of Ontario. We would like to thank Dr. Nancy Webb (University of Kentucky) for providing the DN-Dynamin adenoviral construct and Dr. Alan Tall for helpful advice during the course of this project.
Footnotes
Supplemental Information includes seven figures and Supplemental Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- NHLBI Morbidity and Mortality Chart Book. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- Brazier L, Tiret L, Luc G, Arveiler D, Ruidavets JB, Evans A, Chapman J, Cambien F, Thillet J. Sequence polymorphisms in the apolipoprotein(a) gene and their association with lipoprotein(a) levels and myocardial infarction. The ECTIM Study. Atherosclerosis. 1999;144:323–333. doi: 10.1016/s0021-9150(98)00333-5. [DOI] [PubMed] [Google Scholar]
- DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewas C, Fay M, Gougerot-Pocidalo MA, El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- Edelstein C, Pfaffinger D, Hinman J, Miller E, Lipkind G, Tsimikas S, Bergmark C, Getz GS, Witztum JL, Scanu AM. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J Biol Chem. 2003;278:52841–52847. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erqou S, Kaptoge S, Perry PL, Di AE, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Feric NT, Boffa MB, Johnston SM, Koschinsky ML. Apolipoprotein(a) inhibits the conversion of Glu-plasminogen to Lys-plasminogen: a novel mechanism for lipoprotein(a)-mediated inhibition of plasminogen activation. J Thromb Haemost. 2008;6:2113–2120. doi: 10.1111/j.1538-7836.2008.03183.x. [DOI] [PubMed] [Google Scholar]
- Frank J, Biesalski HK, Dominici S, Pompella A. The visualization of oxidant stress in tissues and isolated cells. Histol Histopathol. 2000;15:173–184. doi: 10.14670/HH-15.173. [DOI] [PubMed] [Google Scholar]
- Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- Hazzard WR, Ettinger WH., Jr Aging and atherosclerosis: changing considerations in cardiovascular disease prevention as the barrier to immortality is approached in old age. Am J Geriatr Cardiol. 1995;4:16–36. [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Holmer SR, Hengstenberg C, Kraft HG, Mayer B, Poll M, Kurzinger S, Fischer M, Lowel H, Klein G, Riegger GA, Schunkert H. Association of polymorphisms of the apolipoprotein(a) gene with lipoprotein(a) levels and myocardial infarction. Circulation. 2003;107:696–701. doi: 10.1161/01.cir.0000048125.79640.77. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Curr Opin Clin Nutr Metab Care. 2002;5:139–145. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Kuchibhotla SD, Guy E, Park YM, Nimako G, Vanegas D, Morton RE, Febbraio M. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:1481–1487. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny EF, O'Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15:167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Koschinsky ML, Tomlinson JE, Zioncheck TF, Schwartz K, Eaton DL, Lawn RM. Apoprotein(a): expression and characterization of a recombinant form of the protein in mammalian cells. Biochemistry. 1991;30:5044–5051. doi: 10.1021/bi00234a029. [DOI] [PubMed] [Google Scholar]
- Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21:174–185. [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. STAT1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, III, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, varez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Nilsen NJ, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von AS, Hartung T, Lien E, Bakke O, Espevik T. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson-McDermott EM, O'Neill LA. The potential of targeting Toll-like receptor 2 in autoimmune and inflammatory diseases. Ir J Med Sci. 2007;176:253–260. doi: 10.1007/s11845-007-0103-1. [DOI] [PubMed] [Google Scholar]
- Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- Riis Hansen P, Kharazmi A, Jauhiainen M, Ehnholm C. Induction of oxygen free radical generation in human monocytes by lipoprotein(a) Eur J Clin Invest. 1994;24:497–499. doi: 10.1111/j.1365-2362.1994.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci U S A. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–898. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Murayama T, Yokode M, Kita T, Uzui H, Ueda T, Lee JD, Kishimoto C. N-acetylcysteine reduces the severity of atherosclerosis in apolipoprotein E-deficient mice by reducing superoxide production. Circ J. 2009;73:1337–1341. doi: 10.1253/circj.cj-08-1148. [DOI] [PubMed] [Google Scholar]
- Silverstein RL. Inflammation, atherosclerosis, and arterial thrombosis: role of the scavenger receptor CD36. Cleve Clin J Med. 2009;76(Suppl 2):S27–S30. doi: 10.3949/ccjm.76.s2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Yong TS, Park SJ, Im KI, Kong Y, Ryu JS, Min DY, Shin MH. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J Immunol. 2005;174:4279–4288. doi: 10.4049/jimmunol.174.7.4279. [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Boyanovsky BB, Connelly MA, Shridas P, van der Westhuyzen DR, Webb NR. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res. 2007;48:2560–2570. doi: 10.1194/jlr.M700163-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun H, Unoki H, Wang X, Liang J, Ichikawa T, Arai Y, Shiomi M, Marcovina SM, Watanabe T, Fan J. Lipoprotein(a) enhances advanced atherosclerosis and vascular calcification in WHHL transgenic rabbits expressing human apolipoprotein(a) J Biol Chem. 2002;277:47486–47492. doi: 10.1074/jbc.M205814200. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der HP, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009;41:283–285. doi: 10.1038/ng.314. [DOI] [PubMed] [Google Scholar]
- Virmani R, Burke AP, Kolodgie FD, Farb A. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol. 2003;16:267–272. doi: 10.1034/j.1600-0854.2003.8042.x. [DOI] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Fisher EA. Oxidation, lipoproteins, and atherosclerosis: which is wrong, the antioxidants or the theory? Curr Opin Clin Nutr Metab Care. 2005;8:139–146. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Yu J, Weiwer M, Linhardt RJ, Dordick JS. The role of the methoxyphenol apocynin, a vascular NADPH oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr Vasc Pharmacol. 2008;6:204–217. doi: 10.2174/157016108784911984. [DOI] [PubMed] [Google Scholar]
- Zhao Z, de Beer MC, Cai L, Asmis R, de Beer FC, de Villiers WJ, van der Westhuyzen DR. Low-density lipoprotein from apolipoprotein E-deficient mice induces macrophage lipid accumulation in a CD36 and scavenger receptor class A-dependent manner. Arterioscler Thromb Vasc Biol. 2005;25:168–173. doi: 10.1161/01.ATV.0000149145.00865.d9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.