Abstract
Morphogens act during development to provide graded spatial information that controls patterning and cell lineage specification in the nervous system. The role of morphogen signaling in instructing the expression of downstream effector transcription factors has been well established. However, a key requirement for morphogen signaling is the existence of functional intracellular machinery able to mediate the appropriate response in target cells. Here we suggest that dynamic changes in the temporal responses to Shh in the developing ventral telencephalon occur through alterations in progenitor competence. We suggest these developmental changes in competence are mediated by a transcriptional mechanism that intrinsically integrates information from the distinct signaling pathways that act to pattern the telencephalic neuroepithelium.
Introduction
During mouse embryonic brain development a number of distinct morphogens act in concert to provide the spatial information necessary to establish general patterning within the neuroepithelium. These events initiate the formation of distinct germinal territories that will later give rise to the different substructures that compose the adult nervous system. The role of morphogen signaling in directing the expression of key downstream effector transcription factors has been well characterized in the spinal cord and more recently in the forebrain [1-3]. Through the actions of such transcriptional regulators, functional intracellular signaling cascades are initiated that ultimately culminate in the establishment of restricted progenitor cell lineages in the developing neural tube. However, within the ventral telencephalon, an important mechanistic aspect that has not been extensively considered is the regulation of the responsiveness of target cells to extrinsic signals. The competence of the neuroepithelium to respond to environmental cues presupposes the expression of all the components required for proper reception, transduction and transcriptional response of such signals. Moreover, the interpretation of a specific signal is context dependent and will vary according to the functional state of these components.
Work over the past decade has increasingly suggested that the changing competence in tissue responses to morphogens is central to their proper development. With regard to the ventral telencephalon, we hypothesized over a decade ago that the emergence of distinct proliferative zones results more from intrinsic changes in the response of the ventral neuroepithelium to extrinsic factors than the absolute concentration of spatially distributed morphogens [4,5]. Despite considerable further examination of this region, it remains unclear whether the specification of progenitor fates in the area is dependent on the concentration of morphogens, regulated by dynamic alterations in the intrinsic responses of progenitors throughout development or some combination of the two. In this review we discuss evidence for temporal shifts in competence within the ventral telencephalon that appear to shape patterning in this region and speculate as to the cell intrinsic mechanisms by which these dynamic changes are regulated. We will focus specifically on the patterning events mediated by downstream transcriptional effectors of the sonic hedgehog (Shh) pathway, and ultimately how this bears on FGF signaling.
The temporal genesis of the ventral ganglionic eminences is marked by shifts in the domains of Shh expression
The ventral telencephalon is characterized during embryonic development by the emergence of the medial, lateral and caudal ganglionic eminences (the MGE, LGE and CGE, respectively). Each of these eminences will produce distinct populations of neurons and understanding how developmental patterning results in the production of different neuronal subtypes from these embryonic structures is the primary goal of a number of laboratories including our own. We have focused specifically on the production of cortical GABAergic interneurons, which appear to collectively arise almost entirely from the MGE and CGE. In addition, a plethora of other cell types including the long- range projection neurons that will populate the striatum, globus pallidus and amygdala are generated from these structures. A common misconception regarding the origin of the ventral telencephalon is that the three ganglionic eminences arise simultaneously, as suggested by their co-existence at around E13.5 of mouse development. As recognized by the earliest investigators of these structures [6], the three eminences are produced sequentially with the MGE appearing first around E9.0, followed by the LGE at E10 and the CGE around E11. However, as the LGE and CGE are anatomically continuous with one another, it is difficult to precisely determine their relative times of origin. Nonetheless, the temporal ordering in the appearance of these progenitor zones is likely a reflection of the mechanism by which they are generated.
Hedgehog (Hh) signaling is centrally involved in the patterning of the nervous system. Studies in the spinal cord have demonstrated that ventral sources of Shh establish a long-range graded signal that controls spatial patterning along the dorsal-ventral axis [3,7,8]. Notably, it has been shown that expression of Shh is required during distinct developmental windows for the specification of neuronal identity [9] and its activity triggers the sequential temporal expression of the transcriptional determinants involved in patterning the ventral spinal cord [10,11]. With regard to telencephalic development, expression of Shh is first observed between E8.5 and E9 in the mesendoderm and diencephalon, structures adjacent to the ventral telencephalon [12]. By E9.5 Shh is expressed broadly in the MGE and by E12.5 expression is observed in the preoptic area, the mantle of the MGE and the amygdala region [13,14]. The onset of Shh expression in each of these domains follows a precise sequential order that parallels the temporal appearance of the MGE, LGE and CGE (Fig.1). Furthermore, these events are closely accompanied by the sequential emergence of distinct Shh-dependent homeodomain transcription factors that are involved in ventral patterning and in specifying each of these structures [15]. After ventral patterning becomes established, Hh signaling continues to be required for a multitude of processes such as the regulation of mitosis, the maintenance of neurogenesis and the generation of both neuronal and glial cell types (reviewed in [16]). Cell types in the ventral ganglionic eminences that are dependent on Shh-signaling for their generation include both oligodendrocytes and ventrally born GABAergic populations, such as striatal projection neurons and cortical interneurons [14,17-20].
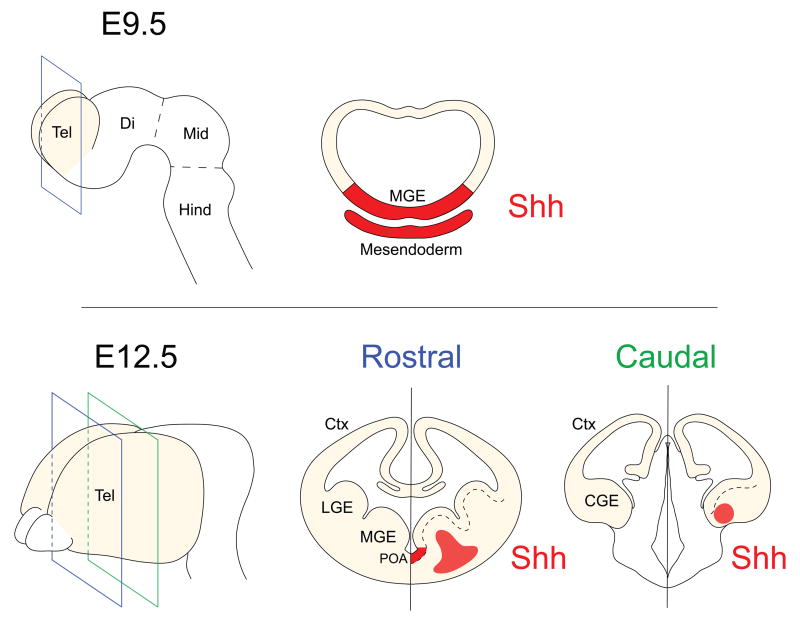
Figure 1. Developmental changes in the expression of Shh in the developing ventral telencephalon.
(top panel) Sources of Shh (red) in the mesendoderm induce expression of Shh in the most ventral aspect of the telencephalon (Tel), as observed in coronal sections of E9.5 embryos.
(bottom panel) As development progresses, the expression of Shh becomes progressively confined to restricted regions of the ventral telencephalon. By E12.5 it can be detected at rostral levels in the preoptic area (POA) and the mantle of the medial ganglionic eminence (MGE). Shh expression is also observed at this age in the amygdala region of the caudal ganglionic eminence (CGE). Ctx, Cortex; Di, Diencephalon; Hind, Hindbrain; Mid, Midbrain.
Responses to Shh in the ventral telencephalon define sequential temporal developmental windows
The telencephalic neuroepithelium is derived from the alar portion of the anterior neural plate and is first characterized by the expression of the forkhead transcription factor FoxG1 [21,22] and the paired-homeobox factor Pax6 [23,24]. However, during the early stages of development, Pax6 becomes repressed in the ventral telencephalic midline through the action of Shh secreted from the underlying axial mesendoderm (Fig. 1) [25,26]. This initial inductive event defines the first temporal response window which we designate as C1 (Competence window 1) (Fig. 2A) that leads to overlapping expression of Shh and Nkx2-1 at around E9.5 in a ventral domain and results in the formation of a sharp boundary with the dorsal Pax6 domain (Fig. 2A) [15]. This is similar to the notocord-mediated Shh-dependent induction of Shh expression in the floor plate of the spinal cord, an event mediated by the transcription factor FoxA2/HNF-3β [27-29]. Indeed, in the ventral telencephalon the homeodomain transcription factor Nkx2-1 is required for the expression of Shh in the early MGE through a process analogous to the homogenetic induction of Shh in the spinal cord mediated by FoxA2/HNF-3β [12,30-33]. As the expression of Nkx2-1 at the molecular level largely defines the MGE [30,34], this is consistent with the MGE being the first eminence to emerge from the ventral telencephalon.
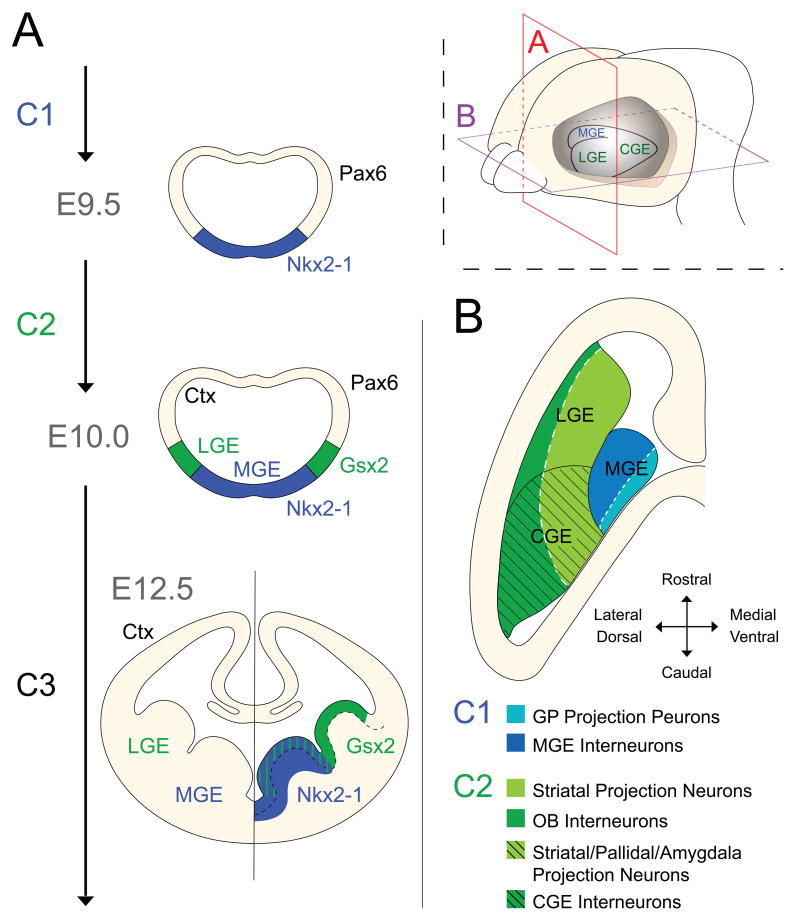
Figure 2. Early telencephalic development is characterized by the dynamic expression of distinct homeobox transcription factors that reflect the sequential appearance of the ventral eminences.
(A) At E9.5, Nkx2-1 expression appears within a ventral domain that is induced to express Shh. These events define the first temporal competence window (C1). This event defines the MGE at the molecular level and expression of Nkx2-1 persists in this region throughout development. Around E10.0 the second competence window is initiated (C2), during which the expression of Gsx2 accompanies the emergence of the more dorsally positioned lateral domain that will give rise to the LGE.
(B) After E10.0, ventral patterning is already established and Shh activity is predominantly required for proliferative control of progenitors. A horizontal view of the ventral ganglionic eminences reveals a repeated pattern in both C1 and C2-derived structures where the ventral aspect of each region gives rise to early-born projection neurons (light colors) while the more lateral/dorsal domains mainly generate later-born interneurons (darker colors).
After induction of Nkx2-1 and Shh and the onset of MGE morphogenesis, the spatial expression of developmental genes is highly dynamic. At E10.0 a small gap appears between the domains of Nkx2-1 and Pax6 expression where Gsx2, another downstream target of Shh is first observed [15]. The domain of Gsx2 expression subsequently widens and is initially associated with tissues possessing LGE-identity. These events provide hallmarks for a second temporal window involved in the patterning of the ventral telencephalon (Competence window 2 – C2) (Fig. 2) [4,5]. Gsx2 expression may also indicate the appearance of a nascent CGE during this developmental window. However, the relationship between Gsx2 expression and the onset of CGE development has proved more elusive due to the present paucity of CGE-specific molecular markers. The observation that peak neurogenesis within the CGE is substantially later than that within the MGE and LGE [35] suggests that while the CGE ultimately expresses Gsx2, early expression reflects only the LGE anlage. Recent findings promise to clarify the temporal and spatial development of the CGE [36-38].
The two initial temporal windows of ventral telencephalic development described to this point (C1 and C2, Fig. 2) are characterized by patterning responses, as indicated by the induction of Nkx2-1 and Gsx2 expression, respectively. Explant studies using tissue from mid-embryogenesis suggest that the telencephalon while losing the ability to express patterning genes in response to Shh-signaling, maintains a sustained proliferative response to this signaling molecule [4]. Consistent with these observations, while early E9.5 removal of Hh-responsiveness from the ventral telencephalon through a conditional deletion of the obligatory Hh-signaling mediator Smoothened (Smo) gives rise to extensive patterning alterations, removal of Hh-signaling from E12.5 onwards results in a reduction in the number of neural progenitors located in the postnatal subventricular zone but only minor patterning abnormalities [18,19]. Therefore, after ventral patterning is established, a third developmental window of Shh function (Competence window 3 – C3, Fig.2) is characterized by its influence on proliferation within the neuroepithelium. While the control of cell proliferation is an important factor in the morphogenesis of the telencephalon and has been demonstrated to affect the development of ventrally-derived cell types [39], it is less clear that proliferation per se during this period directs the specification of distinct cell fates. Hence, although the expression of Shh and its downstream targets has been observed until late developmental stages [40] a clear link between late Shh expression and the regulation of cell diversity has not been established in the ventral telencephalon. In addition, the control of cell proliferation likely involves the coordinated convergence of several signaling pathways in addition to Shh. Therefore, for the remainder of this review we will focus on how patterning during C1 and C2 is regulated.
Cell type specification in structures derived from C1 (MGE) and C2 (LGE/CGE) developmental window
As discussed above, the emergence of the MGE during C1 precedes that of the LGE and CGE, which occurs in C2. Expression of Nkx2-1 (C1) and Gsx2 (C2) reveal that distinct transcriptional programs are set in place in the ventral telencephalon during both of these temporal windows. Interestingly, these two domains do not give rise to homogeneous populations of neurons [34,35,41-43]. Akin to what is observed in the spinal cord [7,8], this is likely due to a further subdivision of these broad domains by the spatially restricted expression of additional transcription factors [44]. In agreement with this, spatial biases have been found in the generation of individual neuron types in the MGE and LGE [40,45-49] However, a causal link between the function of these spatially restricted transcription factors and individual neuronal types has yet to be established.
From a combined temporal and spatial perspective, in both C1- and C2-derived structures, a pattern is recapitulated during which the earlier born ventral cells give rise to projection neurons, while the more dorsally positioned later born cells generate interneurons. During C1 the MGE generates early projection neurons from its most ventral aspect, while more dorsal domains give rise to subpopulations of interneurons at later timepoints (Fig. 2B) [40,45-47]. In contrast, during C2, projection medium spiny neurons (MSN) that populate the striatum arise from the ventral LGE (vLGE) and lateral olfactory bulb interneurons arise from the dorsal LGE (dLGE) [48,49]. In addition, the CGE, which appears during the later period of C2, gives rise to subsets of interneurons that are distinct from the ones generated in the MGE (Fig. 2B) [35,41,42,50,51]. These observations suggest that transcriptional codes induced at C1 (Nkx2-1) and C2 (indicated by the initial expression of Gsx2) impart distinct differentiation potentials to progenitors within the resultant MGE and LGE/CGE respectively. Recently, analyses of the temporal requirement of Nkx2-1 and Gsx2 during telencephalic development has generated support for these ideas. The early removal of Nkx2-1 from MGE progenitors respecifies them to acquire an early LGE medium spiny neuron (MSN) identity and a late removal leads to the acquisition of CGE interneuron profiles [34,52]. In complementary fashion, a gain of Gsx2 function during early development leads to an ectopic production of MSN neurons in the dorsal telencephalon, while the same approach at later timepoints gives rise mostly to olfactory bulb inteneurons [52].
The orchestrated production of different projection neuron and interneuron subtypes is central for appropriate morphogenesis and circuit assembly within the telencephalon. Recent work in the amygdala has shown how the integration of projection neurons and interneurons is highly regulated [53,54]. Similarly, transplantation and fate-mapping studies of interneuron populations have demonstrated that the differential production of cortical interneurons allows them to be appropriately allocated within specific cortical laminae [35,41,42]. Underlying these events is the precisely timed production of specific cell types.
Expression of Gli transcription factors correlates with differential responses to Shh in ventral telencephalic progenitors
What then are the intrinsic factors that regulate the dynamic and differential competence of MGE, LGE and CGE neural progenitors to specific morphogens? Central to the mechanism that imparts neural progenitors competence to respond to Hh signaling is the Gli family of transcriptional regulators – Gli1, Gli2 and Gli3 – homologous to the Drosophila factor Cubitus interruptus (Ci). Work in spinal cord has demonstrated that Gli transcription factors are collectively necessary and sufficient for all aspects of intracellular Shh-responses involved in ventral patterning [55-60].
Interpretation of the role of Gli proteins in the telencephalon is complicated by their non-uniform distribution and by the dynamic nature of Shh expression discussed above (Fig. 1 and Fig. 3). Moreover, the three Gli genes are expressed in distinct and partially overlapping spatial domains in the ventral telencephalon, which shift dramatically as development progresses. Initially, Gli2 and Gli3 have the widest expression patterns, being present in almost the entire telencephalic neuroepithelium (Fig. 3). This pattern is altered with the appearance of the MGE during C1, when Gli2 becomes excluded from the MGE and Gli3 expression is markedly reduced (Fig. 3) [61,62]. Consistent with this broad expression of Gli2 and Gli3, gain-of-function studies have shown that most regions of the telencephalon are able to respond to Hh-signaling [14,63]. However, detailed analysis of gene expression indicates that the response to Hh signaling during telencephalic development is region specific, as targets of Hh-signaling including Gli1, Nkx6-2 and Hhip are only expressed in the most dorsal aspect of the ganglionic sulcus (Fig. 3) [45,62,64]. Those targets become downregulated when expression of Shh or Gli2 is removed from the developing telencephalon [20,62,63].
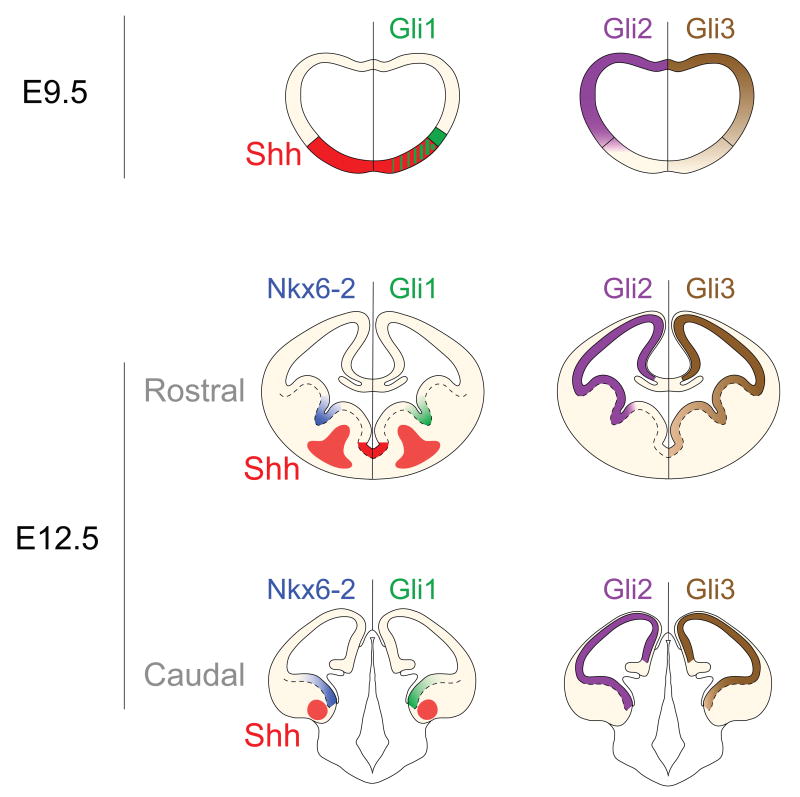
Figure 3. The differential expression of members of the Gli/Ci family of transcription factors controls the response of progenitors to Shh in the ventral telencephalon at distinct embryonic ages.
(top panel) The distribution of Gli protein expression within the telencephalon at E9.5 [72] and (middle and bottom panels) E12.5 in patterns dictates the differential responses of progenitors to Shh. At E12.5, the targets of Shh-signaling Nkx6-2 (blue) and Gli1 (green) can be observed only in the Gli2-expressing domains (purple) that are closest to the ventral sources of Shh (red) along the rostro-caudal axis of the telencephalon. Gli3 (brown) is expressed in a dorsal-to-ventral gradient in the entire telencephalon with a characteristic low level of expression in the MGE (that is apparently insufficient to provide enough Gli-activator function to allow for expression of Nkx6-2 or Gli1).
Fully understanding the function of Gli proteins in the ventral telencephalon is further complicated by the fact that Gli2 and Gli3 can function in Hh-responsive cells either as transcriptional repressors in the absence of a Hh signal or as activators when in the presence of Hh ligands (which inhibit the proteolytic truncation of these Gli proteins into repressor forms [56,65,66]). Gli1, whose expression in the telencephalon is dependent on Shh-signaling, functions uniquely as a transcriptional activator. With regard to Gli gene function, the current model focuses on the C1 period and postulates that Hh-signaling in the ventral telencephalon is solely required to counteract Gli3 repressor activity. Indeed, consistent with this idea, a Hh-independent signaling pathway seems to be able to establish the basic elements of dorsoventral patterning in the telencephalon of Gli3/Shh and Gli3/Smo double mutant embryos [63]. In this context, the activator functions of Gli proteins required for the transduction of quantitative information relayed by Shh gradients [55] have been understood as dispensable for overall telencephalic development. In support of this view, mutants for the two principal mediators of activating Hh signaling Gli1 and Gli2 have resulted in relatively minor defects in telencephalic development [57]. However, those aspects of telencephalic patterning associated with C2 have only begun to be deciphered in the last decade and already indicated that this phase of development is more sensitive to the loss of Gli activators (Sousa VH et al. 2009; Yu W et al., 2009). It seems likely that with the advent of conditional loss of function approaches the precise requirement for Shh-signaling during C1 and C2 can be fully determined [16].
Nkx2-1 modulates the response of the MGE to Hh-signaling
Shh is initially expressed throughout the entire prospective MGE progenitor zone (window C1, Fig.2) but progressively becomes restricted within this structure (window C2, Fig. 2) [4,30]. Surprisingly once the MGE is formed, the regions that appear to display the strongest response to Shh-signaling based on their expression of Gli1 and Nkx6-2 are positioned some distance from areas of high Shh-expression, specifically in the most dorsal and ventral aspects of the MGE, i.e. the interganglionic sulcus, the ventral MGE and the preoptic area (POA) [19,20,46,64]. The proximal cause of this apparent anomaly is that the regions of high Shh-expression lack the full complement of Gli activators required for initiating expression of Shh targets. Surprisingly, the explanation for this lack of Shh-responsiveness appears to be a negative regulation exerted by Nkx2-1, whose expression is itself initially Shh-dependent [30]. Consistent with this idea, conditional removal of Nkx2-1 at E10.5 results in MGE progenitors upregulating targets of Hh signaling such as Gli1 and Nkx6-2 [34]. The same outcome has been observed after Nkx2-1 removal from a smaller domain in the vMGE [46]. Although to our knowledge never explicitly stated, it is not surprising that events of homogenetic induction of morphogens are accompanied by a loss of responsiveness to the induced morphogen itself, presumably to prevent the formation of a self-reinforcing autocrine loop. With regards to cell fate, the loss of Nkx2-1 gene function in these mutants is also accompanied by a respecification of MGE progenitors at the moment of their exit from cell cycle to adopt CGE/LGE character [34]. Whether the ectopic expression of Hh targets in these mutant cells is responsible for their altered postmitotic identity is not clear as a direct causal link between these events has not yet been established. However, such observations suggest that after Nkx2-1 removal, MGE progenitors acquire competence to respond to Hh-signaling in a way that replicates the development of the C2 window (i.e. production of the LGE/CGE). Indeed, MGE progenitors that become respecified in the absence of Nkx2-1 give rise to projection MSN neurons at early timepoints and to CGE interneurons during later development [34].
Shh-mediated competence appears to regulate FGF signaling
Contexts in which Shh or FGF signaling are perturbed result in a disruption in ventral telencephalic patterning [63,67,68]. Loss of function analysis indicates that within the ventral telencephalon the loss of Shh-signaling results in an attenuation of FGF-signaling, while compound loss of Shh and Gli3 gene function restores it [2,69]. Specifically, Shh-signaling attenuates the function of the Gli3- repressor that in turn acts to negatively regulate FGF signaling (Fig. 4). While the compound loss of Gli3 in Shh mutants restores ventral patterning in the telencephalon, mutants which both lack Gli3 and the two primary mediators of FGF-signalling FGFR1 and FGFR2 display almost a complete failure in ventral patterning [67].
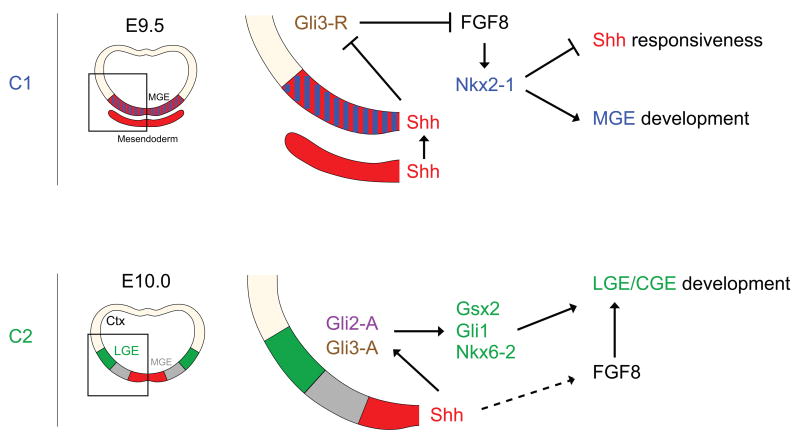
Figure 4. Key genetic interactions involving Shh signaling that pattern and determine the fate of ventral telencephalic progenitors during C1 and C2 temporal competence windows.
During C1, Shh is indirectly required to establish the ventral expression of Nkx2-1 in the MGE anlage through a derepression of FGF signaling. At later stages, gene expression reveals that mostly positive Shh-signaling mediated through Gli proteins is associated with LGE/CGE development. It is at present unclear whether FGF-signaling plays a role during C2 inductive events.
This suggests that FGF rather than Shh is the primary positive inducer of ventral patterning during C1. Such possibility raises the interesting question of what happens to FGF signaling subsequent to the initiation of Nkx2-1 expression within the MGE. Is it required to maintain Nkx2-1 expression at later timepoints? Does it regulate additional aspects of later MGE development independent of Nkx2-1? Specifically, does the loss of Shh-responsiveness in the MGE result in a concomitant downregulation of FGF in this region as well? Recent data indicates it does. Inhibition of the Hh pathway through a cell-autonomous loss of the obligatory Hh-signaling mediator Smoothened (Smo) can lead to a loss of Nkx2-1 expression during later development in a mechanism that appears to be independent of Gli3 repressor activity [70,71]. Although the nature of that mechanism is still unknown and could involve interactions with additional signaling pathways with a role in ventral patterning, it primarily suggests a continued regulation of the FGF pathway by Hh-signaling throughout development. Therefore, we hypothesize that during C2, diverse responses to Shh in these two regions could lead to differential activation of FGF-signaling in the MGE versus the LGE/CGE and may be functionally responsible for the differential fates of progenitors. If true, these must include the generation of distinct cortical interneuron subtypes. It will be interesting to test this hypothesis explicitly by examining whether FGF-target genes other than Nkx2-1 are differentially expressed within populations of neurons generated from the ventral telencephalon. Specifically, it will be of interest to see how FGF signaling regulates the later C2 window through targets that may be present in the LGE/CGE but absent from the MGE.
Conclusion
In conclusion, we suggest that ventral telencephalic patterning is largely mediated by two sequential periods of competence, which we designate as C1 and C2. The Shh-mediated induction of Nkx2-1 expression provides the hallmark of the initiation of MGE development and correspondingly the C1 period. We suggest that although Nkx2-1 involvement in the homogenetic induction of Shh establishes the MGE, it makes this territory non-responsive to the ligand and by proxy attenuates FGF-signaling in this structure. This is followed by the C2 competence period, during which Shh-signaling induces the LGE/CGE. Interestingly, both structures induced during C1 (MGE) and C2 (LGE/CGE) are characterized by the early production of projection neurons from their ventral aspects, followed by the late production of interneurons from their dorsal domains. Finally, we suggest that the negative regulation of Shh-signaling within the MGE coupled with the positive regulation of this pathway within the LGE/CGE creates zones in which FGF-signaling is differentially active. As a consequence, we hypothesize that FGF signaling may ultimately be one proximal pathway that influences the creation of cell diversity within the ventral telencephalon.
Acknowledgments
We would like to thank Renata Batista-Brito, Theofanis Karayannis, Rob Machold, Melissa McKenzie, Goichi Miyoshi, Sebnem Tuncdemir and other members of the Fishell laboratory for comments on this manuscript. Research in Fishell lab is supported by NIH grants (RO1NS039007, RO1MH071679) and generous support from both the Simons Foundation and New York State through their NYSTEM initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
-
*
of special interest
-
**
of outstanding interest
- 1.Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- 2.Hebert JM, Fishell G. The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci. 2008;9:678–685. doi: 10.1038/nrn2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- 4.Kohtz JD, Baker DP, Corte G, Fishell G. Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development. 1998;125:5079–5089. doi: 10.1242/dev.125.24.5079. [DOI] [PubMed] [Google Scholar]
- 5.Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- 6.Smart IH. A pilot study of cell production by the ganglionic eminences of the developing mouse brain. J Anat. 1976;121:71–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 8.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 9.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- **10.Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347.. This study suggests cumulative Shh signaling is integrated within spinal cord progenitors as a function of both the duration and concentration of exposure to this morphogen. This results in a temporal integration of net Shh-signaling in progenitor cells resulting in the initiation of differential gene expression.
- 11.Kutejova E, Briscoe J, Kicheva A. Temporal dynamics of patterning by morphogen gradients. Curr Opin Genet Dev. 2009;19:315–322. doi: 10.1016/j.gde.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- 13.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 14.Nery S, Wichterle H, Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- 15.Corbin JG, Rutlin M, Gaiano N, Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;130:4895–4906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- 16.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 17.Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH, Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- 18.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 19.Fuccillo M, Rallu M, McMahon AP, Fishell G. Temporal requirement for hedgehog signaling in ventral telencephalic patterning. Development. 2004;131:5031–5040. doi: 10.1242/dev.01349. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- 21.Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 22.Tao W, Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- 23.Bell E, Ensini M, Gulisano M, Lumsden A. Dynamic domains of gene expression in the early avian forebrain. Dev Biol. 2001;236:76–88. doi: 10.1006/dbio.2001.0301. [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Nakamura S, Osumi N. Fate mapping of the mouse prosencephalic neural plate. Dev Biol. 2000;219:373–383. doi: 10.1006/dbio.2000.9616. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- 26.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 27.Jeong Y, Epstein DJ. Distinct regulators of Shh transcription in the floor plate and notochord indicate separate origins for these tissues in the mouse node. Development. 2003;130:3891–3902. doi: 10.1242/dev.00590. [DOI] [PubMed] [Google Scholar]
- 28.Epstein DJ, McMahon AP, Joyner AL. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development. 1999;126:281–292. doi: 10.1242/dev.126.2.281. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz i Altaba A, Placzek M, Baldassare M, Dodd J, Jessell TM. Early stages of notochord and floor plate development in the chick embryo defined by normal and induced expression of HNF-3 beta. Dev Biol. 1995;170:299–313. doi: 10.1006/dbio.1995.1216. [DOI] [PubMed] [Google Scholar]
- 30.Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 31.Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993;117:205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 33.Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development. 1991;(Suppl 2):105–122. [PubMed] [Google Scholar]
- **34.Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031.. Demonstrates a requirement for Nkx2-1 in MGE progenitors as they exit mitosis for selection of C1- over C2- associated cell identities. This reveals that the induction of Nkx2-1 by Shh is the chief genetic hallmark distinguishing these two competence periods.
- 35.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willi-Monnerat S, Migliavacca E, Surdez D, Delorenzi M, Luthi-Carter R, Terskikh AV. Comprehensive spatiotemporal transcriptomic analyses of the ganglionic eminences demonstrate the uniqueness of its caudal subdivision. Mol Cell Neurosci. 2008;37:845–856. doi: 10.1016/j.mcn.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Long JE, Cobos I, Potter GB, Rubenstein JL. Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb Cortex. 2009;19(Suppl 1):i96–106. doi: 10.1093/cercor/bhp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glickstein SB, Moore H, Slowinska B, Racchumi J, Suh M, Chuhma N, Ross ME. Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development. 2007;134:4083–4093. doi: 10.1242/dev.008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 42.Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- 44.Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010.. Characterizes a spatial and temporal bias in the generation of pallidal projection neuron lineages during the earliest stages of neurogenesis from C1-derived progenitors, located in the most ventral aspect of the MGE. Also describes Nkx2-1’s role in suppressing Shh-responsive gene expression associated with C2 regions.
- 47.Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 51.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 2009;63:451–465. doi: 10.1016/j.neuron.2009.07.015.. Using a gain of function strategy this study reveals the temporal requirement for Gsx2 in generating distinct neuronal populations at different developmental stages. Striatal projection neuronal fates are generated during early stages of C2 development while olfactory bulb interneurons arise at later periods of C2-derived neurogenesis.
- 53.Cocas LA, Miyoshi G, Carney RS, Sousa VH, Hirata T, Jones KR, Fishell G, Huntsman MM, Corbin JG. Emx1-lineage progenitors differentially contribute to neural diversity in the striatum and amygdala. J Neurosci. 2009;29:15933–15946. doi: 10.1523/JNEUROSCI.2525-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–149. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Stamataki D, Ulloa F, Tsoni SV, Mynett A, Briscoe J. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev. 2005;19:626–641. doi: 10.1101/gad.325905.. An elegant study showing that the duration and strength of Gli activity in a cell mediates the concentration dependent effects of Shh to direct differential gene expression in progenitor cells, ultimately resulting in their acquisition of divergent neuronal fates.
- 56.Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 57.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 58.Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–2543. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- 59.Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- 60.Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fotaki V, Yu T, Zaki PA, Mason JO, Price DJ. Abnormal positioning of diencephalic cell types in neocortical tissue in the dorsal telencephalon of mice lacking functional Gli3. J Neurosci. 2006;26:9282–9292. doi: 10.1523/JNEUROSCI.2673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu W, Wang Y, McDonnell K, Stephen D, Bai CB. Patterning of ventral telencephalon requires positive function of Gli transcription factors. Dev Biol. 2009;334:264–275. doi: 10.1016/j.ydbio.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- 64.Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matise MP, Joyner AL. Gli genes in development and cancer. Oncogene. 1999;18:7852–7859. doi: 10.1038/sj.onc.1203243. [DOI] [PubMed] [Google Scholar]
- 66.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **67.Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465.. Demonstrated that the FGF pathway likely provides the positive signal for the establishment of ventral pattern in the telencephalon.
- 68.Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 69.Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- 70.Gulacsi A, Anderson SA. Shh maintains Nkx2.1 in the MGE by a Gli3-independent mechanism. Cereb Cortex. 2006;16(Suppl 1):i89–95. doi: 10.1093/cercor/bhk018. [DOI] [PubMed] [Google Scholar]
- *71.Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 65:328–340. doi: 10.1016/j.neuron.2010.01.004.. Provides evidence for a requirement for Hh-signaling in maintaining later expression of Nkx2-1. Also describes an intriguing non-autonomous cellular phenotype, resulting in enhanced Shh-signaling in wild type cells adjacent to mutant Shh-non-responsive progenitors.
- 72.Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]