Although adrenocortical steroids have often been used clinically in an attempt to prevent the rejection of renal homografts, the sphere of usefulness of these drugs has not been clearly defined. The potentiation of graft survival with steroids alone is a feeble effect, as is demonstrated clearly only under special experimental circumstances.
The present study is concerned with the evaluation of a different use of steroid therapy. Renal homografts were performed in dogs and antirejection therapy provided with a cytotoxic drug. When evidence of rejection developed, prednisolone was added to the pre-existing treatment. Evidence has been obtained which demonstrates that prednisolone can halt and reverse a rejection process under these circumstances. The data suggest that a rational and consistently successful program for clinical use can be evolved, with the use of steroids as an emergency measure at the time of rejection.
METHODS
Eight mongrel dogs were used, weighing 10 to 20 kilograms. In a one-stage operation, bilateral nephrectomy was performed, and in each case a renal homograft obtained from an unrelated dog was transplanted to the pelvis. Donor left kidney was placed into the right pelvis and donor right kidney into the left pelvis. The donor animals were cooled to 30° C. before operation and given heparin intravenously, 3 mg. per kilogram, before removal of the homograft. The periods of renal ischemia lasted 17 to 30 minutes.
All 8 animals were treated with azathioprine (BW 57–322, Imuran) for the entire period of survival, in a dose of 2 to 7.5 mg. per kilogram per day. In addition, 5 of the 8 animals received preliminary thymectomy 5 to 10 days before transplantation. All but 1 of the dogs had splenectomy performed at the time of homografting and bilateral nephrectomy (Table I).
Table I.
| Dog no. | Adjuvant surgery | Drug therapy | Survival (days) | Histologic rejection (0 to 4+) | Onset of uremia after transplant (days) | Response to steroids | Gastro-intestinal hemorrhage | Cause of death |
|---|---|---|---|---|---|---|---|---|
| KP5 | Splenectomy | Imuran | 36 | 1 + | 11 | Yes | No | Undetermined |
| KH6 | Splenectomy | Imuran | 14 | 2+ | 7 | No | Yes | Gastrointestinal hemorrhage |
| TK22 | Splenectomy and thymectomy | Imuran | 54 | 2+ | 26 | Yes | Yes | Gastrointestinal hemorrhage |
| TK23 | Splenectomy and thymectomy | Imuran | 53 | 0 | 32 | Yes | Yes | Gastrointestinal hemorrhage |
| TK26 | Splenectomy and thymectomy | Imuran | 11 | 2+ | 4 | Yes | No | Lobar pneumonia |
| TK27 | Splenectomy and thymectomy | Imuran | 27 | 2+ | 13 | Yes | Yes | Gastrointestinal hemorrhage |
| TK36 | Splenectomy and thymectomy | Imuran | 32 | 2+ | 19 | Yes | Yes | Rejection, gastrointestinal hemorrhage |
| KI 1 | None | Imuran | 43 | 1 + | 19 | Yes | Yes | Gastrointestinal hemorrhage, Pulmonary emembolus |
Prednisolone was started after it became certain that the process of rejection had begun. The chief criteria used to determine this were rising blood urea nitrogen (BUN) levels, reduction of urine volume, and the development of proteinuria. After biochemical evidence of rejection had become clear, prednisolone therapy was started with a dose of 50 to 200 mg. per day given intramuscularly. In 2 of the animals, prednisolone was withdrawn after the BUN level had returned to normal. Complete autopsies were performed on all dogs, including histologic studies, in order to rule out factors other than rejection which might have accounted for death.
RESULTS
Onset of uremia
Despite the antirejection therapy, uremia developed from 4 to 32 days after renal homotransplantation (Table I). Before therapy with prednisolone was begun, the rising trend of the BUN level was confirmed on at least 2 occasions.
Influence of steroids on uremia
In 7 of the 8 animals, the BUN level stopped rising after prednisolone treatment was begun and within 2 to 7 days began to fall. In 2 animals, the fall was temporary, and after several days, a secondary rise occurred despite continuation of prednisolone (Fig. 1). Death under these circumstances followed rapidly. In 2 other cases, steroids were slowly withdrawn after the rejection crisis had apparently passed, and the BUN level, which had dropped toward normal, did not manifest a secondary elevation (Fig. 2). In 4 animals, the uremia waxed or waned with the withdrawal or administration of prednisolone therapy.
Fig. 1.

Reduction in BUN in animal under concomitant azathioprine therapy. Note secondary rise of BUN when azathioprine was reduced, despite continuation of prednisolone. Dog died of gastric hemorrhage.
Fig. 2.
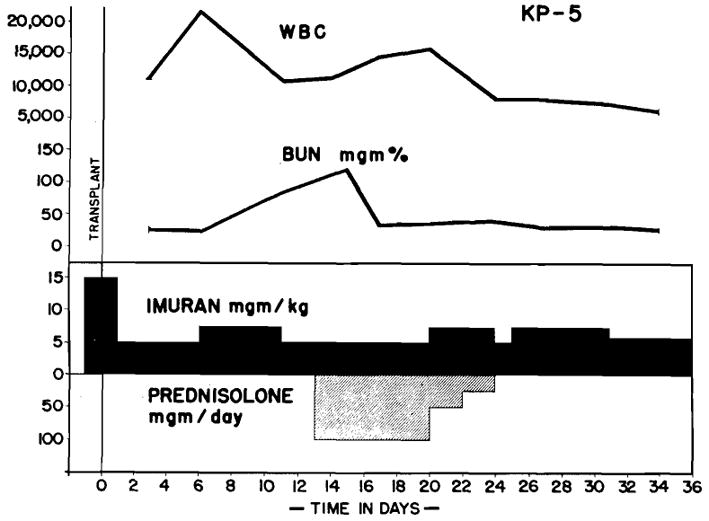
KP5. Effect of prednisolone on BUN in dog undergoing rejection despite therapy with azathioprine. Note reversal of azotemia. BUN level did not rise again when prednisolone was discontinued.
Survival
The animals lived from 7 to 28 days after uremia had become well established, and after the beginning of steroid therapy. The longest survival in this series of animals was 54 days.
Although the steroids apparently resulted in alleviation of the uremic state in 7 of the 8 animals, it appeared to impose limitations on the total survival time, inasmuch as 6 of the 8 animals eventually developed serious gastrointestinal hemorrhage, which was either the direct or contributing cause of death in each case.
Autopsy findings
All but one of the dogs showed some evidence of rejection of the renal homograft at the time of death. This was generally rather mild in nature, consisting chiefly of a scattered mononuclear infiltrate (Fig. 3). Ulceration and hemorrhage of the gastrointestinal tract was found in all but 2 of the animals, usually involving the gastric antrum (Fig. 4), the duodenum, and the upper jejunum, but involving the entire alimentary tract in 2 of the dogs. The ulcers were penetrating, with minimal histologic evidence of healing. The lymph nodes in all animals had depleted germinal centers (Fig. 5), a finding commonly observed by others after treatment with high doses of adrenocortical steroids.
Fig. 3.

KP5. Microscopic section of kidney in same animal as Fig. 2. Animal lived 25 days after onset of uremia. Rejection is minimal. (Original magnification ×160.)
Fig. 4.
TK27. Death resulted from gastrointestinal hemorhage while on steroid therapy. A, gross view of antral ulcer. B, microscopic view. Not minimal healing. (Original magnification ×250.)
Fig. 5.

Lymph node from TK27. Note depletion of follicles. (Original magnification ×I 60.)
DISCUSSION
The effect of adrenal cortical extracts on mitigation of the homograft rejection process has been extensively studied. It would seem reasonable to expect a modification of the rejection reaction, inasmuch as some of the steroids decimate the lymphocyte population,5 and because steroids inhibit the synthesis of various antibodies.2, 16 Early investigators showed a definite prolongation of skin graft survival in animals treated with cortisone acetate.3, 8–11, 13, 14 The experiments were conducted under highly controlled conditions, employing inbred test animals. In general, cortisone and cortisol had the greatest efficacy, ACTH had a feeble effect, and corticosterone was without value.
Under less rigidly controlled conditions, the effect of steroid therapy on homograft survival has been difficult to demonstrate. Ellison and associates6 were unable to find prolongation of skin graft survival in humans under therapy with cortisone. Dempster,4 working with greyhound dogs, was unable to prolong the survival of renal homografts despite the administration of 150 to 200 mg. of cortisone acetate per day. His meticulous histologic and physiologic studies, however, provided a rationale for the use of steroid therapy for renal homotransplants undergoing rejection. He showed that the vascular endothelial reaction in the rejection of renal homografts was materially reduced, that the plasma cell and lymphocyte infiltration was moderated, and that the arteriolar constriction characterizing the active rejection process was reduced, which thereby provided a better peripheral blood supply to the kidney while it was under immunologic attack.
Most of the foregoing studies have suggested that steroid therapy in large doses modified homograft rejection slightly, but that this effect was so feeble as to be of little clinical value. A few isolated observations have suggested that steroids might be of more substantial value when used in combination with other agents. Baker and associates,1 on the basis of limited data, concluded that the combination of nitrogen mustard and cortisone had a greater potentiating effect on graft survival than either agent alone. Goodwin and co-workers7 recently reported a clinical case in which a vigorous rejection occurred 35 days after the transplantation of a kidney from a mother to her daughter. Forty days after operation, 200 mg. per day of prednisone was added to the pre-existing therapy of nitrogen mustard and Cytoxan. There was gradual clearing of the azotemia. Although the child died 4 months later, the state of histologic preservation of the homograft was surprisingly normal. Merrill and associates,12 in treating late rejection in a kidney donated by one fraternal twin to another, employed supplementary irradiation plus intermittent steroid therapy. Attacks of rejection were aborted but the roles of the irradiation and steroid therapy are difficult to separate.
The present study serves to focus attention on the value of steroids as supplementary therapy in patients receiving continuous treatment with more powerful agents. If, as in the present experimental series, day-to-day therapy is provided with azathioprine, the steroids can be withheld until the onset of rejection. With the advent of azotemia or proteinuria, the use of steroids in large doses has led to the consistent regression of uremia. In some of the animals, furthermore, this restoration of the BUN level to or toward normal persisted, even after a total withdrawal of the prednisolone.
The matter of timing of institution of steroid therapy has also been clarified to some extent by the present experiments. It is clear that withholding this adjuvant therapy until the onset of rejection does not diminish its therapeutic efficacy. This would appear to be an important feature in view of the high incidence of gastrointestinal ulcerations in the present series of animals. These observations have strongly influenced our policy in clinical cases. Large doses of steroids (200 to 300 mg. per day of prednisone) have been used only with the specific indication of rejection, and they have then been withdrawn as rapidly as possible after the threat of rejection has passed. In 9 clinical cases16 in which steroids have been used, there has been only 1 instance of acute gastrointestinal hemorrhage, probably because all the patients have been on vigorous concomitant antacid therapy. Nevertheless, the hazards involved in the clinical use of steroids are undoubtedly real.
SUMMARY
Renal homotransplantation was performed in 8 dogs. Antirejection therapy with azathioprine (BW 57–322, Imuran) was given in doses of 2 to 7.5 mg. per kilogram per day. Evidence of rejection developed in all animals from 4 to 32 days following transplantation.
When rejection had been established by at least 2 successive elevations of the BUN level, intramuscular injections of prednisolone (50 to 200 mg. per day) were added to the preexisting azathioprine therapy. Seven of the 8 animals had a favorable response evidenced by a falling BUN level, increased urine volumes, and decreasing proteinuria.
Gastrointestinal bleeding was a serious complication in 6 of the 8 dogs. It was a direct or contributing cause of death in all 6.
The rationale for the use of prednisolone in homotransplantation is discussed. From these experiments it would appear to be a valuable adjunct in reversing established rejection when added in large doses to preexisting antirejection therapy with azathioprine. Vigorous antacid therapy is indicated to prevent gastrointestinal ulceration and bleeding.
Acknowledgments
Aided by United States Public Health Service Grants A-6283, A-6344, AI-041542, AM-07772, OG-27, and HE 07735-01.
References
- 1.Baker R, Gordon R, Huffer J, Miller GH., Jr Experimental renal transplantation: I. Effect of nitrogen mustard, cortisone, and splenectomy. A M A Arch Surg. 1952;65:702. doi: 10.1001/archsurg.1952.01260020694008. [DOI] [PubMed] [Google Scholar]
- 2.Berglund K, Fagraeus A. Effect of cortisone on antibody formation. Proc Internal Cong Microbiol. 1953;2:231. [Google Scholar]
- 3.Billingham RE, Krohn PL, Medawar PB. Effects of cortisone on survival of skin homografts in rabbits. Brit M J. 1951;1:1157. doi: 10.1136/bmj.1.4716.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dempster WJ. The effects of cortisone on the homotransplanted kidney. Arch internat pharmacodyn. 1953;95:253. [PubMed] [Google Scholar]
- 5.Dougharty TF, Berliner ML, Berliner DL. Hormonal influence in lymphocyte differentiation from RES cells. Ann New York Acad Sc. 1961;88:78. [PubMed] [Google Scholar]
- 6.Ellison EH, Martin BC, Williams RD, Clatworthy HW, Hamwi G, Zollinger RM. The effect of ACTH and cortisone on the survival of homologous skin grafts. Ann Surg. 1951;134:495. doi: 10.1097/00000658-195109000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin WE, Kaufman JJ, Mims MM, Turner RD, Glassock R, Goldman R, Maxwell MM. Human renal transplantation. I Clinical experiences with 6 cases of renal homotransplantation. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 8.Krohn PL. The influence of ACTH on the homograft reaction. Transplantation Bull. 1953;1:20. [Google Scholar]
- 9.Krohn PL. The effect of steroid hormones on the survival of skin homografts in the rabbit. J Endocrinol. 1954;11:78. doi: 10.1677/joe.0.0110078. [DOI] [PubMed] [Google Scholar]
- 10.Krohn PL. The effect of ACTH on the reaction to skin homografts in rabbits. J Endocrinol. 1954;11:71. doi: 10.1677/joe.0.0110071. [DOI] [PubMed] [Google Scholar]
- 11.Krohn PL. The effect of cortisone on second set skin homograft in rabbits. Brit J Exper Path. 1954;35:535. [PMC free article] [PubMed] [Google Scholar]
- 12.Merrill JP, Murray JE, Harrison JH, Friedman EA, Dealy JB, Dammin GJ. Successful homotransplantation of the kidney between nonidentical twins. New England J Med. 1960;262:1251. [Google Scholar]
- 13.Morgan JA. The influence of cortisone on the survival of homografts of skin in the rabbit. Surgery. 1951;30:506. [PubMed] [Google Scholar]
- 14.Randall P, Brown JB, McDowell F. The effects of ACTH and cortisone on experimental skin homografts. S Forum. 1951;2:475. [PubMed] [Google Scholar]
- 15.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynec & Obst. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 16.Taliffaro WH. The possible role of cortisone in overcoming resistance to the growth of human tissues in heterologous hosts. Ann New York Acad Sc. 1957;59:745. doi: 10.1111/j.1749-6632.1955.tb45953.x. [DOI] [PubMed] [Google Scholar]



