Abstract
Galactosamine induces a dose-dependent hepatic injury in rats and many other animals. The toxicity of D-galactosamine appears to be a consequence of the loss of hepatic UTP. It has previously been reported that regenerating liver cytosol is able to prevent, at least in part, the lethal effect of this substance by stimulating hepatic regeneration. Recently, we have separated a fraction using alcohol precipitation (80%) from regenerating liver cytosol and from weanling rat liver cytosol prepared in acetate buffer (100 mM, pH 6.5). We named this fraction hepatic stimulatory substance because of its ability to stimulate DNA synthesis in vivo when injected intraperitoneally in 40% hepatectomized rats and in vitro in the presence of hepatocytes isolated and maintained in monolayer cultures. The stimulatory activity of the hepatic stimulatory substance is fully evident in subfractions of molecular weight up to 300,000 and 50,000 daltons of the crude material obtained using Amicon Ultra membrane filters. The present report describes the ability of hepatic stimulatory substance and its subfractions to stimulate hepatocyte proliferation and the application of these hepatic extracts in successfully reversing the lethality of D-galactosamine-induced hepatic necrosis in rats. D-Galactosamine (2.6 gm per kg of body weight) was administered intraperitoneally to 438 male Lewis strain rats. The animals were divided into six groups according to the type of treatment: Group 1 (n = 131) saline; Group 2 (n = 40) cytosol (75 mg total protein); Group 3 (n = 75) hepatic stimulatory substance (20 mg total protein); Group 4 (n = 42) 300,000 subfraction (4 mg total protein); Group 5 (n = 68) 300,000 subfraction (2 mg total protein), and Group 6 (n = 82) 50,000 subfraction (0.6 mg total protein). All rats received 4 ml of the test solution intraperitoneally at 48 hr after D-galactosamine administration. The percentage of rats surviving in each group was determined daily for 20 days. Although hepatic stimulatory substance and 50,000 subfraction tended to improve survival in intoxicated rats, only those rats treated with the 300,000 subfraction attained statistical significance with respect to the saline control.
A major objective of clinical hepatology is the development of a successful method for the treatment of fulminant acute hepatic failure, a disease which presently has an extremely poor prognosis and carries a high mortality (1). Various treatment modalities, including plasmapheresis, exchange transfusion, cross-circulation, heterotopic liver transplantation and charcoal hemoperfusion have been developed and used for this condition in humans. More recent attempts to study this entity and evaluate new methods of treatment have utilized reproducible, potentially reversible, animal models of fulminant hepatic failure which duplicate, at least in part, the pathophysiological conditions found in human fulminant hepatic failure. One of these models (2–4), that of D-galactosamine (D-GAL) intoxication, has received wide attention because of its reproducible dose/response relationship, its reversibility and its histologic similarity to that described in human fulminant hepatic failure. Numerous reports in the past years have indicated that, in rats with experimental acute hepatic failure, the administration of various liver fractions is able to significantly improve animal survival by stimulating hepatic regeneration (5–11).
We have recently described a protein fraction which is derived by alcohol precipitation of regenerating liver cytosol (RLC), which is able to stimulate DNA synthesis and cell proliferation both in vivo, when injected intraperitoneally into 40% hepatectomized rats, and in vitro when added to hepatocytes maintained in monolayer cultures (Francavilla, A. et al., Hepatology 1982; 2:701, Abstract). The stimulatory activity of this hepatic stimulatory substance (HSS) is fully evident in subfractions of molecular weight uup to 300,000 and 50,000 daltons of the crude material obtained using Amicon ultra membrane filters. The present report further describes the ability of HSS and its subfractions to stimulate hepatocyte proliferation and the application of these hepatic extracts in successfully reversing the lethality of D-GAL-induced hepatic necrosis in rats.
MATERIALS AND METHODS
Animals
Male Lewis strain rats (200 to 230 gm) purchased from Hilltop Lab Animals (Scottdale, Pa.) were kept in a temperature- and light-controlled room (6 a.m. to 6 p.m.), and received food and water ad libitum. Forty per cent and 70% partial hepatectomy (PH) were performed between 7:30 and 9:00 a.m. using the method of Higgins and Anderson (13). All rats were fasted for 12 hr prior to the injection of D-GAL and prior to the performance of PH. Food and water were then offered ad libitum 3 hr after the performance of the above.
Materials
Collagenase Type I (125 to 250 units per mg) was obtained from Worthington Diagnostic Systems (Freehold, NJ). Minimum essential medium and fetal calf serum were purchased from GIBCO Laboratories (Grand Island, NY). Insulin, epidermal growth factor (EGF), 3,3″-5-triiodothyronine, glucagon, HEPES and pyruvic acid were purchased from Sigma Chemical Company (St. Louis, Mo.) Methyl-[3H]thymidine (50 to 80 Ci per mmole) was obtained from New England Nuclear (Boston, Mass.). D-GAL hydrochloride (Sigma Chemical Co.) was reconstituted in 5% dextrose immediately prior to use, and the pH was adjusted to 6.88.
Preparation of Hepatic Extracts
RLC
Twenty-four hours after 70% PH in normal adult Lewis rats, the regenerating liver remnants were excised and placed in 4 volumes (w/v) of ice-cold buffer [0.27 M sucrose, 12 mM Tris-HCl, 1 mM EDTA (pH 7.6)], and a crude homogenate was prepared using a Potter-Elveljein tissue grinder. This crude homogenate was centrifuged for 10 min at 10,000 × g at 4°C, and the resultant supernatant was further ultracentrifuged for 1 hr at 100,000 × g. The resultant pellet contained membrane components, and the supernatant contained the cytosolic components (RLC).
HSS and Its Subtractions (300,000 and 50,000)
HSS was prepared essentially by the method previously reported by LaBrecque (14, 15). A 35% (w/v) homogenate in ice-cold acetate buffer (100 mM, pH 4.65) was prepared from pooled regenerating livers and was heated at 65 °C for 15 min with further centrifugation at 30,000 × g for 20 min at 4°C. The resulting supernatant was added to 6 volumes of cold ethanol. This mixture was stirred for 2 hr at 4°C and centrifuged at 37,000 × g for 20 min at 4°C. The resulting pellet, which represented HSS, was dissolved in H2O and stored frozen at −70°C when not used immediately. The subfractions (300,000 and 50,000) were prepared separately by filtering HSS through Amicon ultramembrane filters with molecular weight limit exclusion of 300,000 (XM 300,000) and 50,000 (XM 50,000) using the filtrate for the injection in the rats. Although identical fractions have been prepared from normal intact livers, these were not used in the present studies, since we have already demonstrated their lack of hepatic stimulatory activity in vitro and in vivo (Francavilla A, et al, Hepatology 1982; 2:701, Abstract).
Determination of Proliferative Activity of HSS and 300,000 Subfraction
In Vivo Experiments
In vivo experiments were performed using methods previously described by LaBrecque and Pesch (14). A heightened background of in vivo DNA synthetic activity was induced in host rats by 40% PH. Six hours later, these rats received 4 ml of saline, HSS (20 mg of protein) and 300,000 (2 mg of protein) subfraction solution intraperitoneally. Eighteen hours later 50 μCi of [3H]thymidine (specific activity = 76.6 Ci per mmole) was administered intraperitoneally. Rats were killed 2 hr later, and liver samples were taken for the determination of DNA content (16). [3H]thymidine incorporation (12), the percentage of labeled nuclei and the mitotic index, as previously described (12). The augmentation of these parameters in the liver beyond the modest response that usually follows 40% hepatectomy was considered to be a demonstration of the proliferative activity of liver extracts. Augmentation of proliferation resulting from liver extract administration was considered present when all three parameters were significantly elevated when compared to the response observed following 40% PH alone.
In Vitro Experiments
These experiments evaluated the effect of liver extracts on DNA synthetic activity in hepatocytes maintained in primary cultures. Hepatocytes were prepared by a modification of the two-step collagenase perfusion technique of Seglen (17) as reported by Jirtle et al. (18) The liver was perfused retrograde via cannula in the inferior vena cava with 250 ml buffer [142 mM NaCl, 6.7 mM KCl, 10 mM HEPES (pH 7.4)] followed by 250 ml of the same buffer containing in addition 5.7 mM CaCl2 and 0.5 mg per ml collagenase. Hepatocytes were dispersed and washed twice with cold Ca++-free perfusion buffer and resuspended in minimal essential medium supplemented with pyruvate (1 mM), aspartate (0.2 mM), serine (0.2 mM), gentamycin (40 μg per ml) and, in addition, insulin (10−7 M) and 5% fetal calf serum. Hepatocyte viability was determined by trypan blue exclusion. Only preparations having greater than 90% viability were used. Cell number was determined using a hemocytometer.
Hepatocytes were plated at a cell density of 2 × 105 per 35 mm Falcon “Primaria” tissue culture dish in 1.5 ml medium and maintained at 37°C in a 5% CO2 atmosphere. Following a 3-hr period to allow for attachment of the hepatocytes, the medium was changed and the cells were left for 72 hr. [3H] Thymidine (7.5 μCi per dish) was added for the last 24 hr. Each experimental group comprised four dishes so that when the cells were harvested, one dish was used to determine DNA concentration using a microfluorometric method (19). Two dishes were used to determine [3H]thymidine incorporation using the method described by Michalopoulos et al. (20) and one dish was used to determine labeling index by autoradiography (20).
Model of D-GAL-Induced Acute Hepatic Failure and Preventing Action of Hepatic Extracts
Development of Model
In preliminary experiments, 4 ml of D-GAL were administered intraperitoneally to Lewis strain rats using a range of dosages (1.8 to 2.6 gm per kg of body weight). Survival in such animals was observed during a period of 20 days (Figure 1). A dose of 2.6 gm per kg of body weight of D-GAL resulted in consistent, reproducible mortality as a result of massive hepatic necrosis and was therefore used for all experiments reported here.
Fig. 1.
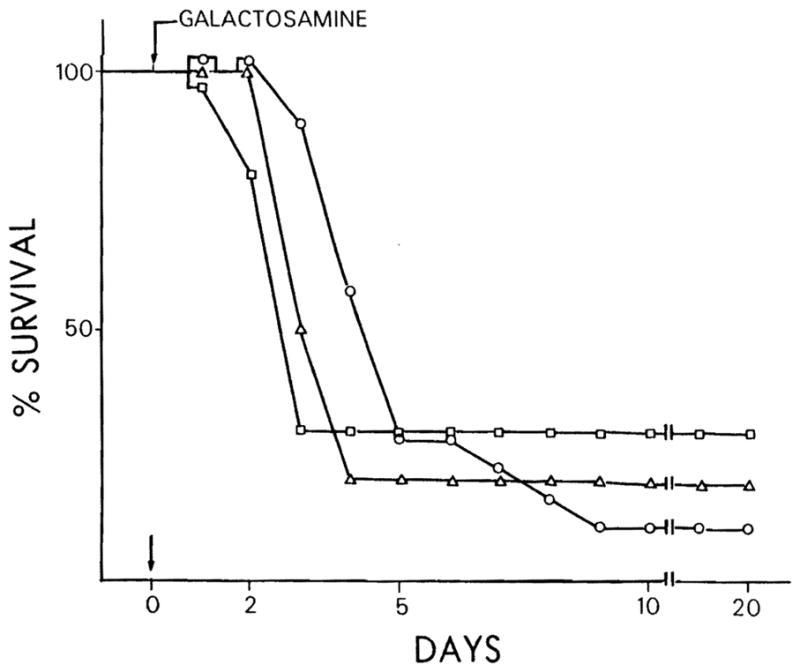
Per cent survival of Lewis strain rats receiving different concentrations of D-GAL. Sixty rats (20 per each group) were injected intraperitoneally with different dosages of D-GAL; 1.8 gm per kg of body weight = □; 2 gm per kg of body weight = △; 2.6 gm per kg of body weight = ○.
Experimental Protocol
D-GAL (2.6 gm per kg of body weight) was administered intraperitoneally to 438 male Lewis strain rats. The animals were divided into six groups according to the type of treatment as reported in Table 1. The doses of all fractions used are related to the total protein concentration of the starting cytosol, so that, 75 mg of cytosol contain 20 mg of HSS, 2 mg of 300,000 subfraction solution and 0.6 mg of 50,000 subfraction solution.
Table 1.
Groups of animals, number of rats and treatment used after 48 hr injection of D-GAL (2.6 gm per kg of body weight)
| Groups | No. of animals | Intraperitoneal treatment | Total protein injected (mg) |
|---|---|---|---|
| 1 | 131 | 4 ml of saline | – |
| 2 | 40 | 4 ml of cytosol | 75 |
| 3 | 75 | 4mlofHSS | 20 |
| 4 | 42 | 4 ml of 300,000 subfraction | 4 |
| 5 | 68 | 4 ml of 300,000 subfraction | 2 |
| 6 | 82 | 4 ml of 50,000 subfraction | 0.6 |
Protocol for studying the effect of liver fractions on survival after D-GAL-induced acute hepatic failure. The doses used were related to the starting cytosol; 75 mg of cytosol protein dose contain 20 mg of protein of HSS, 2 mg of protein of 300,000 subfraction and 0.6 mg of protein of 50,000 subfraction.
All rats received 4 ml of the test solution intraperitoneally at 48 hr after D-GAL administration. This time of injection of an hepatic stimulatory factor had already been shown to be optimal in an identical model of hepatic failure (7). The percent of rats surviving in each group was determined daily for 20 days. The DNA synthetic activity and hepatic nuclear-labeling index were evaluated in a separate group of 30 rats intoxicated with D-GAL (2.6 gm per kg of body weight). Three groups of 10 intoxicated rats treated with saline (control), HSS and 300,000 subfraction received [3H]thymidine intraperitoneally at 48 hr after extract administration and 96 hr after D-GAL administration. The rats were sacrificed 2 hr later, and liver samples were taken and processed for nuclear incorporation of [3H]thymidine, labeling index and autoradiography as described previously.
Other Assays
The protein concentration of each fraction was determined using Lowry et al.’s (21) method and using bovine serum albumin as standard. The radioactive content of samples was determined in a Packard Tri-carb liquid scintillation spectrometer. Results are expressed as mean ± S.E.
Statistical Analysis
The odds ratio was used to assess relative risk or the odds in favor of survival associated with specific treatments. The confidence intervals were constructed using logarithms (to the base e) (22). When the confidence interval excluded unity (odds ratio equal to one), indicating a significant degree of association, the significance of the association was then tested using χ2; the continuity correction was used when the n was less than 50 or an expected value was less than 5. A p value of less than 0.05 was considered statistically significant.
RESULTS
The ability of HSS and its subfractions to stimulate hepatic regeneration was evaluated both in vivo and in vitro. Table 2 demonstrates the effect of HSS and 300,000 subfraction on in vivo DNA synthesis and hepatocyte proliferation in 40% hepatectomized rats. Hepatic incorporation of tritiated thymidine (DNA synthesis) was significantly increased from 14,520 ± 856 in the control group to 46,750 ± 15,820 in the group treated with HSS, and to 31,150 ± 4,850 in the group treated with 300,000 subfraction.
Table 2.
Effect of HSS on hepatocyte proliferation activity in vivo
| Treatment | Protein concentration (mg) | DNA synthesis (cpm/mg of DNA) | p< | % labeled nuclei | p< | % mitoses | p< |
|---|---|---|---|---|---|---|---|
| Saline | — | 14,520 ± 856 | — | 11.5 ± 3 | — | 0.6 ± 0.2 | — |
| HSS | 20 | 46,750 ± 15,820 | 0.05 | 35.8 ± 5 | 0.05 | 2.6 ± 0.8 | 0.05 |
| 300,000 subfraction | 2 | 31,150 ± 4,850 | 0.05 | 28.8 ± 5 | 0.05 | 2.2 ± 0.3 | 0.05 |
Thirty rats with 40% hepatectomy were used. Six hours following hepatectomy, 10 rats received 4 ml of HSS (20 mg), 10 rats 4 ml of 300,000 subfraction (2 mg) and 10 rats 4 ml of saline solution. Eighteen hours following the injection of the extract, 50 μCi of [3H]thymidine was injected intraperitoneally, and 2 hr later the rats were sacrificed. DNA synthesis, per cent labeled nuclei and per cent mitoses were determined as reported in “Materials and Methods.” The values are expressed as mean ± SE.
Similarly, following HSS and 300,000 subfraction treatment, the per cent of labeled nuclei increases from a value of 11.5 ± 3 to values of 35.8 ± 5 and 28.8 ± 5, and the percentage of mitoses increased from 0.6 ± 0.2 to 2.6 ± 0.8 and 2.2 ± 0.3.
The ability of HSS and its subfractions to stimulate hepatocyte proliferation was confirmed also in vitro and is listed in Table 3. HSS, 300,000 subfraction and 50,000 subfraction stimulated incorporation of [3H] thymidine into hepatocyte nuclei, and enhanced the labeling index of hepatocytes in primary cultures. It should be noted that the in vitro stimulatory activity was observed in the presence of insulin and EGF. We have previously demonstrated no stimulatory activity when these two components were removed from the culture medium (23). A comparison of the stimulatory activity of 300,000 and 50,000 subfractions, in vivo and in vitro, showed that the filtrate retained 80% of the stimulatory activity as compared with the membrane-retained activity.
Table 3.
Effect of HSS on hepatocyte proliferation in vitro
| Medium additions | Protein (μg/ml) | DNA synthesis (cpm/μg of DNA) | p< | % labeled nuclei | p< |
|---|---|---|---|---|---|
| -0- | -0- | 1,630 ± 210 | 1 ± 0.3 | ||
| Insulin + EGF | -0- | 19,231 ± 1,270 | 15 ± 4.3 | ||
| Insulin + EGF + HSS | 10 | 30,028 ± 1,280 | 0.02 | 26 ± 3.8 | 0.02 |
| Insulin + EGF + 300,000 subfraction | 5 | 29,128 ± 983 | 0.05 | 28 ± 3.6 | 0.05 |
| Insulin + EGF + 50,000 subfraction | 5 | 31,322 ± 1,480 | 0.05 | 29 ± 5.8 | 0.05 |
Hepatocytes were incubated as described in “Materials and Methods.” Cells were plated in minimal essential medium supplemented with fetal calf serum and insulin. After 3 hr, the medium and the floating cells were removed and serum-free minimal essential medium added, plus the hormones or extracts as indicated. Cultures were exposed to 5 μCi of [3H]thymidine/ml for 24 hr and processed after 72 hr for determination of DNA synthesis. The hormone concentrations were: insulin, 10−7 M and EGF, 10 ng/ml. Triplicate culture dishes were set up for each treatment. The results are expressed as the mean ± S.E. of 12 dishes for four different experiments. Labeling of nuclei was determined by autoradiography and expressed as per cent of total nuclei. The statistical analysis was made comparing the value of each extract with that observed in the presence of insulin and EGF. The values are expressed as mean ± S.E.
The effect of varying the dose of D-GAL administration on rat survival (dose-response relationship) is depicted in Figure 1. Three groups of 20 rats each received D-GAL at concentrations of 2.6, 2.0 and 1.8 gm per kg of body weight. Although there was no statistical difference in rat survival among the 3 doses, the reproducible mortality was greatest at the highest dose (2.6 gm per kg of body weight), and no mortality was observed during the first 40 to 48 hr. The dosage of 2.6 gm per kg of body weight was used for all subsequent experiments. Animal death was evaluated daily for 20 days, but no additional change in mortality was observed after the tenth day.
Figure 2 demonstrates the effect of cytosol and HSS on the survival of rats receiving D-GAL at dosage of 2.6 gm per kg of body weight. Forty-eight hours following D-GAL intoxication, 4 ml of cytosol (protein content = 75 mg) were injected intraperitoneally into 40 animals, and 4 ml of HSS prepared as described above (protein content = 20 mg) were injected intraperitoneally into 75 animals. The mortality rate was determined daily and compared with that of a control group of 131 animals which had been injected intraperitoneally with 4 ml of saline. Both RLC and HSS administration improved the survival rate of the animals when compared to the control group. However, only the effect of HSS was statistically significant, during the first 5 days. In fact, at the fifth day, the odds of survival in this group were 2.5-fold more than that of the control group (odds ratio = 2.44; 95% CI: 4.96, 1.20; χ2 = 6.21; p < 0.02).
Fig. 2.
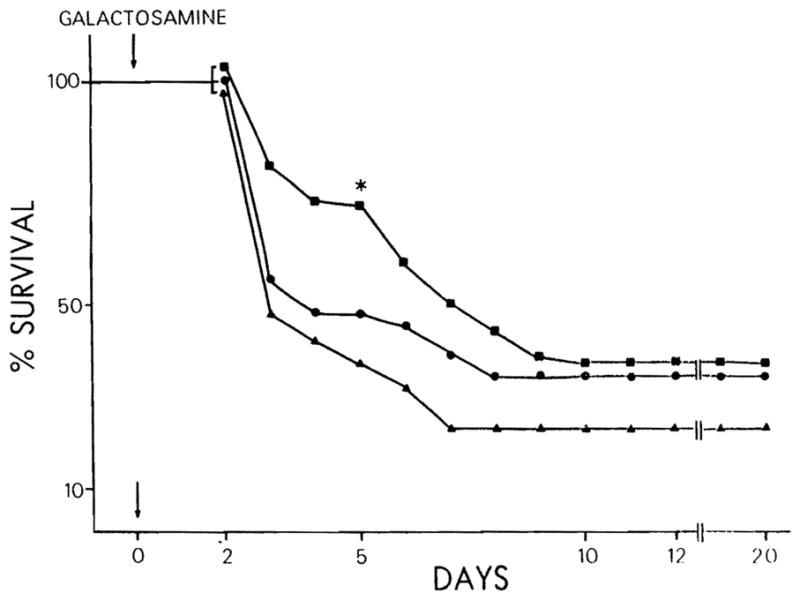
Per cent survival of D-GAL-intoxicated (2.6 gm per kg of body weight) rats treated with cytosol 75 mg = ● and HSS (20 mg) = ■, compared with control groups tested with saline = ▲. *, p < 0.02.
Figure 3 depicts the survival curves for rats receiving the 300,000 and 50,000 subfractions of HSS. Forty-eight hours following D-GAL intoxication, 4 ml of 300,000 subfraction (total protein content = 2 mg) were injected intraperitoneally in 68 animals, and 4 ml of 50,000 subfraction (total protein content = 0.6 mg) were injected in 82 animals. The mortality rate was determined daily and compared with that of the control group of 131 animals injected with 4 ml saline. The 300,000 subfraction injection resulted in a significant improvement in survival; the odds in favor of survival were significantly increased at all time points beyond 4 days (p < 0.005 and p < 0.001). To confirm these results, 4 ml of 300,000 subfraction with double concentration of protein (4 mg) were injected in 42 rats 48 hr after D-GAL intoxication. Also, in this case, at all points beyond 4 days, a significant improvement of survival was achieved compared to the control group (p < 0.005 and p < 0.001). Although the per cent of alive animals was higher in the latter group receiving 4 mg of 300,000 subfraction, a statistical difference with the group of animals receiving 2 mg of 300,000 subfraction was not detected. No significant improvement in survival was observed with the 50,000 subfraction.
Fig. 3.
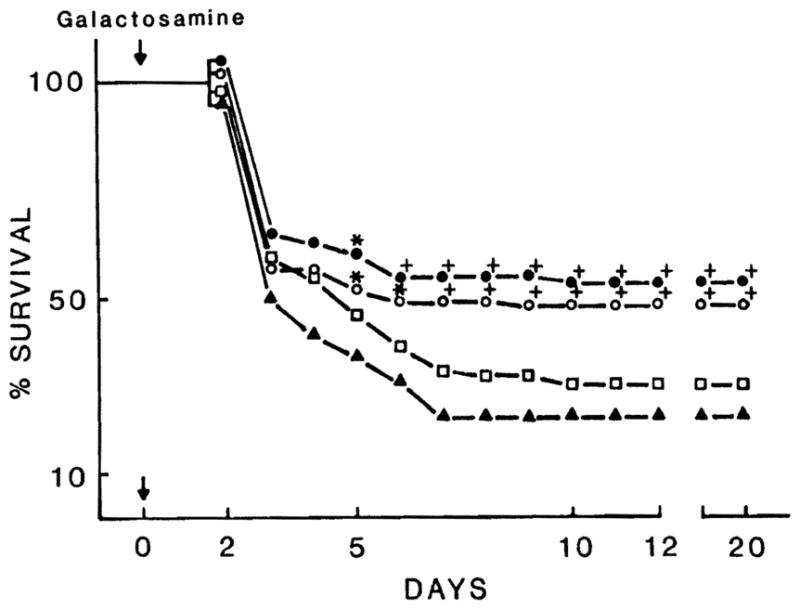
Per cent survival of D-GAL-intoxicated rats (2.6 gm per kg of body weight) treated with 300,000 subfraction (4 mg) = ●, 300,000 subfraction (2 mg) = ○ and 50,000 subfraction (0.65 mg) = □, compared with control groups treated with saline = ▲. *, p < 0.005; +, p < 0.001.
Table 4 demonstrates the DNA synthetic activity (cpm per mg DNA) and the labeling index found in rats intoxicated with D-GAL, and sacrificed 48 hr after the injection of HSS, 300,000 subfraction and saline. The results confirm that both of these fractions significantly increase DNA synthesis and labeling index.
Table 4.
Effect of HSS and 300,000 subfraction on hepatocyte proliferative activity in rats intoxicated with D-GAL
| Fraction | Protein (mg) | DNA synthesis (cpm/mg of DNA) | p< | % labeled nuclei | p< |
|---|---|---|---|---|---|
| Saline | — | 3,650 ± 685 | — | 0.8 ± 0.25 | — |
| HSS | 20 | 18,380 ± 1,958 | 0.01 | 8.0 ± 3 | 0.005 |
| 300,000 subfraction | 2 | 13,520 ± 1,150 | 0.01 | 7.5 ± 2.8 | 0.005 |
Three groups of rats, treated similarly to Groups 1, 3 and 5 listed in Table 1, were used for DNA synthesis determination and nuclear labeling index. Forty-eight hours following administration of saline and extracts (96 hr after galactosamine administration), all rats received 50 μCi of [3H]thymidine intraperitoneally. Animals were sacrificed 2 hr later and the determinations were performed as reported in “Materials and Methods.” The values are expressed as mean ± S.E.
DISCUSSION
The management of human fulminant hepatic failure remains very disappointing despite the evaluation of a multitude of treatment modalities. The goal of all these treatments is to support the patient temporarily in order to allow the injured host liver to regenerate.
Since initial reports suggesting a role for insulin and glucagon in liver regeneration (24, 25), these two hormones have been used as a potential treatment for acute hepatic failure both in animals and humans. Despite the encouraging initial data reported in animals (26), such treatments have shown no consistent effect in humans (27).
Nonetheless, the concept of promoting hepatic regeneration in an injured liver presents an attractive alternative and potentially beneficial approach to the management of this clinical problem. Recently, there has been a revived interest in the effect of liver cell fractions as a means of stimulating liver regeneration. LaBrecque and Pesch (14) described a regenerative stimulatory substance present in the cytosol of regenerating or weanling rat livers which had the ability to stimulate DNA synthesis in 40% PH rats. Subsequently Starzl et al. (28), using a canine model, identified the presence of hepatic growth stimulatory substance in an organelle-free, non-insulin or glucagon-containing, cytosolic fraction derived from livers after 70% PH. Recently, a fraction in rats with similar activity has been identified by Francavilla et al. (Hepatology 1982; 2:701, Abstract) and termed HSA (hepatic stimulatory activity). This substance is able to stimulate DNA synthesis and cellular proliferation both in vivo and in vitro as further reported in Tables 2 and 3. Moreover, the present experiments demonstrate that the same activities (i.e., enhanced DNA synthesis and cell proliferation) are exhibited by partially purified extracts of crude HSS having molecular weights of 300,000 and 50,000 daltons.
A recent extension of the research into these liver-derived growth factors has been the use of these fractions as experimental therapies for acute liver failure in animals and in humans. Makowka et al. (6) have demonstrated improved survival and restitution of hepatic histologic deterioration, following acute hepatic necrosis in rats induced either by D-GAL or anoxia, using the intraperitoneal administration of syngeneic, allogenic and xenogenic dispersed liver cells. The same authors reported the efficacy of regenerating liver cytosol in improving the survival of rats with acute D-GAL intoxication and in reversing the inhibitory effects of ethanol on hepatic regeneration following 70% PH (9). More recently, they have also reported the ability of hepatic cytosol to reduce the lethal effect of chemotherapeutically induced acute hepatic necrosis (10). Using cytosol preparations derived from 70% hepatectomized dogs, Starzl et al. (28) also have successfully reversed the liver atrophy and ultrastructural deterioration seen in dogs after portacaval shunting. All of these reports suggest that the beneficial effect achieved with the various preparations was due to their ability to promote hepatic DNA synthesis and hepatic cellular proliferation.
The data reported here clearly demonstrate that each of the fractions tested was able to stimulate DNA synthesis and hepatocyte proliferation both in vivo and in vitro in 60% hepatectomized rats and in D-GAL-intoxicated rats (Tables 2 to 4).
Although these fractions were able to stimulate hepatocyte proliferation as measured by autoradiography, it was only the 300,000 subfraction that resulted in a significant improvement in survival in intoxicated rats. Double the total protein concentration of the 300,000 subfraction (i.e., 4 mg) resulted in a further enhancement of survival. The apparent discrepancy that all the fractions were active in vivo and in vitro in their ability to significantly stimulate DNA synthesis but only the 300,000 subfraction significantly improved survival is open to speculation. One consideration is that as the purity of these substances increases, so does the potency and ability to influence the kinetics of regeneration. Further purification and characterization of these factors should resolve this issue.
These studies do not include serial histology after D-GAL poisoning. This has already been well characterized by various authors for rats receiving various doses of D-GAL (5–9). Furthermore, recent studies using liver cytosol in a comparable model have demonstrated serial improvement in liver morphology in rats that were treated with such fractions, at 48 hr after D-GAL poisoning (7, 9).We, as well as others (5, 7–9), have evaluated liver functions biochemically and have demonstrated that the degree of liver injury was equivalent in all groups receiving D-GAL at 48 hr regardless of additional treatment (unpublished data). Therefore, the differences in survival observed in these studies are related to the differences in treatment at 48 hr.
The timing of administration in the in vivo model of the various fractions tested in these studies is critical. It has already been demonstrated using a similar model and liver cytosol that 48 hr after D-GAL treatment was the optimal time and specifically that earlier administration of the fractions did not result in enhancement of survivorship. It is also known that the lesion is well established by 48 hr and that the mortality rate is consistent (2, 3). Furthermore, the possibility that the various fractions administered in these studies contained uridine (2, 3, 9), and that this was responsible for the reversal of the hepatic failure no longer exists. This report does, however, provide direct histopathological data, demonstrating that rats poisoned with D-GAL and then treated at 48 hr with HSS and 300,000 subtraction resulted in a significant increase in the DNA synthetic activity and in the percentage of labeled nuclei when compared to rats receiving saline alone (Table 4, autoradiographic data). The evidence reported here, combined with that of other authors (5–9), therefore, strongly suggest that increased survivorship in this model is related to an effect of these fractions on hepatic regeneration.
Each of the preparations studied in the present experiments was completely free of recognizable hormones such as insulin, glucagon and EGF. In fact, none of these hormones was detectable by radioimmunoassay, and the fractions have physiochemical characteristics which differ from each of these hormones. Specifically, insulin and glucagon are soluble in alcohol, while HSS is precipitated in alcohol. EGF is soluble in acid solution while HSS is precipitated.
This report presents further evidence to support the hypothesis that the liver is the source of a growth factors) able to artificially promote hepatic DNA synthesis, hepatocyte proliferation and the restitution of a histologically injured liver. Furthermore, the identification of such a growth factor for the liver may represent a significant advance for the management of clinical fulminant hepatic failure.
References
- 1.Okuda K. Fulminant hepatic failure: a review. In: Picazo J, editor. Glucagon in gastroenterology and hepatology. Pharmacological, clinical and therapeutic implications. Boston, Massachusetts: MTP Press; 1982. [Google Scholar]
- 2.Medline A, Schaffner F, Popper H. Ultrastructural features in galactosamine induced hepatitis. Exp Molec Pathol. 1970;12:201–211. doi: 10.1016/0014-4800(70)90050-x. [DOI] [PubMed] [Google Scholar]
- 3.Scharnbeck H, Schaffner F, Keppler D, et al. Ultrastructural studies on the effect of choline orotate on galactosamine induced hepatic injury in rats. Exp Molec Pathol. 1972;16:33–46. doi: 10.1016/0014-4800(72)90018-4. [DOI] [PubMed] [Google Scholar]
- 4.Koff RS, Gordon G, Sabesin SM. D-galactosamine hepatitis. I. Hepatocellular injury and fatty liver following a single dose. Proc Soc Exp Biol Med. 1971;137:696–701. [Google Scholar]
- 5.Sommer BG, Sutherland DER, Matas AJ, et al. Hepatocellular transplantation for the treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979;11:578–584. [PubMed] [Google Scholar]
- 6.Makowka L, Rotstein LE, Falk RE, et al. Reversal of toxic and anoxic induced hepatic failure by syngeneic allogenic and xenogenic hepatocyte transplantation. Surgery. 1980;88:244–253. [PubMed] [Google Scholar]
- 7.Makowka L, Falk RE, Rotstein LE, et al. Reversal of experimental acute hepatic failure in the rat. J Surg Res. 1980;29:479–487. doi: 10.1016/0022-4804(80)90016-5. [DOI] [PubMed] [Google Scholar]
- 8.Makowka L, Falk RE, Rotstein LE, et al. Cellular transplantation in the treatment of experimental hepatic failure. Science. 1980;210:901–918. doi: 10.1126/science.7001630. [DOI] [PubMed] [Google Scholar]
- 9.Makowka L. PhD Thesis. University of Toronto; Toronto, Ontario, Canada: 1982. Studies into the reversal of experimental acute hepatic failure in the rat by hepatocytes transplantation. [Google Scholar]
- 10.Miyazaki M, Makowka L, Falke RE, et al. Reversal of lethal, chemotherapeutically induced acute hepatic necrosis in rats by regenerating liver cytosol. Surgery. 1983;94:142–144. [PubMed] [Google Scholar]
- 11.O’Neill PL, Baumbartner D, Lewis WI, et al. Cell-free supernatant from hepatocyte cultures improves survival of rats with chemically induced acute liver failure. J Surg Res. 1982;32:347–359. doi: 10.1016/0022-4804(82)90112-3. [DOI] [PubMed] [Google Scholar]
- 12.Francavilla A, Ove P, Van Thiel DH, et al. Induction of hepatocyte stimulating activity by T3 and appearance of the activity despite inhibition of DNA synthesis by adriamycin. Horm Metab Res. 1984;16:237–242. doi: 10.1055/s-2007-1014755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 14.LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulatory substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaBrecque DR, Bachur MS. Hepatic stimulatory substance: physiochemical characteristics and specificity. Am J Physiol. 1982;242:G281–G288. doi: 10.1152/ajpgi.1982.242.3.G281. [DOI] [PubMed] [Google Scholar]
- 16.Burton K. Determination of DNA concentrations with diphenylamine. Meth Enzymol. 1968;12B:163–166. [Google Scholar]
- 17.Seglen PO. Preparation of isolated rat liver cells. Meth Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 18.Jirtle RL, Michalopoulos G, McLaine JR, et al. Transplantation system for determining the clonogenic survival of parenchymal hepatocytes exposed to ionizing radiation. Cancer Res. 1981;41:3512–3518. [PubMed] [Google Scholar]
- 19.Hinegardner RT. An improved fluorometric assay for DNA. Ann Biochem. 1971;39:197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- 20.Michalopoulos G, Cianciulli HD, Novotny AR, et al. Liver regeneration studies with rat hepatocytes in a serum-free medium. Biomed Res. 1981;2:217–221. [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Lilienfeld AM, Lilienfeld DE. Foundations of epidemiology. 2. New York: Oxford University Press; 1980. pp. 343–346. [Google Scholar]
- 23.Francavilla A, Ove P, Polimeno L, et al. Epidermal growth factor and proliferation in hepatocytes in primary culture isolated at different times after partial hepatectomy. Cancer Res. 1986;46:1318–1323. [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;147:179–199. [PMC free article] [PubMed] [Google Scholar]
- 25.Francavilla A, Porter KA, Benichou J, et al. Liver regeneration in dogs. Morphologic and chemical changes. J Surg Res. 1978;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farivar M, Wands JR, Isselbacher KJ, et al. Effect of pancreatic hormones on fulminant murine hepatitis. N Engl J Med. 1976;195:1517–1520. doi: 10.1056/NEJM197612302952706. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Shimizu M, Kosaka M. Nationwide statistics of severe hepatitis (fulminant hepatitis) (in Japanese) Sashin Igaku. 1979;34:2285–2288. [Google Scholar]
- 28.Starzl TE, Terblanch J, Porter KA, et al. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;1:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


