Abstract
Background
Because of the rarity of hilar cholangiocarcinoma, its prognostic risk factors have not been sufficiently analyzed. This retrospective study was undertaken to evaluate various pathologic risk factors which influenced survival after curative hepatic resection or transplantation.
Methods
Between 1981 and 1996, 72 patients (43 males and 29 females) with hilar cholangiocarcinoma underwent hepatic resection (34 patients) or transplantation (38 patients) with curative intent. Medical records and pathologic specimens were reviewed to examine the various prognostic risk factors. Survival was calculated by the method of Kaplan-Meier using the log rank test with adjustment for the type of operation. Survival statistics were calculated first for each kind of treatment separately, and then combined for the calculation of the final significance value.
Results
Survival rates for 1, 3, and 5 years after hepatic resection were 74%, 34%, and 9%, respectively, and those after transplantation were 60%, 32%, and 25%, respectively. Univariate analysis revealed that T-3, positive lymph nodes, positive surgical margins, and pTNM stage III and IV were statistically significant poor prognostic factors. Multivariate analysis revealed that pTNM stage 0, I, and II, negative lymph node, and negative surgical margins were statistically significant good prognostic factors.
For the patients in pTNM stage 0-II with negative surgical margins, 1-, 3-, and 5-year survivals were 80%, 73%, and 73%, respectively. For patients in pTNM stage IV-A with negative lymph nodes and surgical margins, 1-, 3-, and 5-year survivals were 66%, 37%, and 37%, respectively.
Conclusions
Satisfactory longterm survivals can be obtained by curative surgery for hilar cholangiocarcinoma either with hepatic resection or liver transplantation. Redefining pTNM stage III and IV-A is proposed to better define prognosis.
Hilar cholangiocarcinoma (Altemeier-Klatskin tumor1,2) is an uncommon malignant neoplasm, arising from the bile duct epithelium of the common hepatic duct or its first and second bifurcation. Because of the rarity of this malignancy, its prognostic risk factors have not been completely analyzed. In particular, the reliability of pTNM staging has not been evaluable in previous reports.3–13 We describe our 15-year experience with hilar cholangiocarcinoma, with particular focus on the various risk factors that influenced the survival of patients after subtotal hepatic resection, and after total hepatectomy with liver replacement.
METHODS
Patients
Between 1981 and 1996, 72 patients with hilar cholangiocarcinoma underwent either hepatic resection (Hx, n=34) or orthotopic liver transplantation (OLT, n=38) with curative intent at the University of Pittsburgh Medical Center. There were 43 males and 29 females. Ages ranged from 19 to 81 years (mean and median, 51 years). Median followup period to December 31, 1997 was 76.7±5.0 (SE) months.
Surgical procedures
Partial hepatectomy (Hx) was the procedure of choice for the patients with anatomically resectable tumors who did not have advanced cirrhosis or sclerosing cholangitis. Total hepatectomy with liver replacement was performed when tumor extension or underlying cirrhosis and/or sclerosing cholangitis precluded Hx.
Hepatic resection
All 34 patients treated with subtotal hepatectomy and extrahepatic bile duct excision also had extensive regional lymph node dissection in continuity. The biliary system was reconstructed by anastomosis(es) between the intrahepatic duct or ducts (as many as four) and a Roux-en-Y limb of jejunum. Only two patients received central resection, and the remaining 32 patients received lobectomy or more extensive resection with techniques that have been described elsewhere.14–16 The portal vein was resected with the tumor and was reconstructed in five patients, the hepatic artery was reconstructed in three patients, and both in one patient. All 3 portions of the caudate lobe were removed in 22 patients and 1 or 2 portions were resected in 12 patients.
Transplantation
These 38 patients could not be treated with subtotal hepatectomy. Twenty-seven underwent OUT either because the extent of the tumor required total hepatectomy for complete removal (n= 14) or because of concomitant advanced cirrhosis or severe sclerosing cholangitis, or both, precluded partial hepatectomy (n=13).
Eleven patients were treated with upper abdominal exenteration and cluster organ transplantation (OLT-CL) because of highly unfavorable conditions such as lymph node involvement, direct invasion of tumor into adjacent organs, or regional metastasis (n= 10), or because dense fibrotic reaction caused by preoperative radiation therapy made the intraoperative assessment of tumor impossible in a patient with sclerosing cholangitis (n= 1). In addition to the liver, the composite allografts included various combinations of pancreas, duodenum, and short segments of proximal jejunum. The technique of orthotopic liver transplantation, including OLT-CL, and the post-transplant regimens of immunosuppression have been described elsewhere.17–21
Adjunct therapy
Forty-four of the 72 patients received adjunct external radiation therapy with or without 5-FU sensitization before or after the operation. These regimens were highly variable because of changes in protocol during the prolonged period of case accrual.
Pathological characteristics
In the 34 patients who underwent hepatic resection, the pathologic diagnosis of hilar cholangiocarcinoma was established preoperatively in 18 (53%) by brush biopsy or previous exploratory operation. The suspected diagnosis was confirmed intraoperatively in the remaining 16. Similarly, the diagnosis was known for certain preoperatively in only 21 of the 38 patients (55%) treated with transplantation. The definitive diagnosis was established during transplantation in 10 (26%) more, but only after pathologic examination of the excised liver in the remaining 7 (18%).
Pathologic findings in the 72 patients were stratified according to pTNM classification and staging22 (Table 1), and are summarized in Table 2 and 3 as determined after all preoperative, operative, and pathologic information was complete. Fifty-one of the 72 patients (71%) had pTNM stage IV tumors. At the other end of the spectrum, 5 of the 72 patients (7%) had pTNM stage 0 or I tumors. Four of the five underwent transplantation because of sclerosing cholangitis or other disease of the native liver.
Table 1.
pTNM Staging of Hilar Cholangiocarcinoma*
| Stage | T | N | M |
|---|---|---|---|
| Stage 0 | T-is | NO | MO |
| Stage I | T1 | NO | MO |
| Stage II | T2 | NO | MO |
| Stage III | T1 | N1, N2 | MO |
| T2 | N1, N2 | MO | |
| Stage IV A | T3 | Any N | MO |
| Stage IV B | Any T | Any N | M1 |
From: Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomized trial of Tacrolimus compared to cyclosporin for hepatic transplantation. J Am Coll Surg 1996;183:117–125, with permission.
M1, distant metastasis MO, no metastasis; N1, hepatoduodenal ligament; N2, other regional; NO, no lymph node involvement; T-is, carcinoma in situ; T1, duct wall; T2, perifibromuscular connective tissue; T3, adjacent structure (liver).
Table 2.
T, N and M Classification of the 72 Patients with Hilar Cholangiocarcinoma
| Classification | Hx (n=34) |
OLT (n=27) |
OLT-CL (n=11) |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| T | is | 0 | 3 | 11 | 1 | 9 | |
| 1 | 0 | 1 | 4 | 0 | |||
| 2 | 7 | 21 | 9 | 33 | 0 | ||
| 3 | 27 | 79 | 14 | 52 | 10 | 91 | |
| N | pos. | 14 | 41 | 6 | 22 | 5 | 46 |
| M | pos. | 4 | 12 | 1 | 4 | 4 | 36 |
Hx, hepatic resection; is, in situ; OLT, orthotopic liver transplantation; OLT-CL, organ cluster transplantation; pos., positive.
Table 3.
pTNM Stages of 72 Patients with Hilar Cholangiocarcinoma
| pTNM Stage | Hx (n=34) |
OLT (n=27) |
OLT-CL (n=11) |
|||
|---|---|---|---|---|---|---|
| % | n | % | n | % | n | |
| 0 | 0 | 11 | 3 | 9 | 1 | |
| I | 0 | 4 | 1 | 0 | ||
| II | 12 | 4 | 33 | 9 | 0 | |
| III | 9 | 3 | 0 | 0 | ||
| IV-A | 67 | 23 | 48 | 13 | 55 | 6 |
| IV-B | 12 | 4 | 4 | 1 | 36 | 4 |
Hx, hepatic resection; OLT, orthotopic liver transplantation; OLT-CL, organ cluster transplantation.
Despite the curative intent of the operations, histopathologic examination of the surgical margins revealed malignant cells in 14 of the 34 patients (41%) in Hx group, 6 of the 27 OLT recipients (22%), and 1 of the 11 in OLT-CL group (9%). Five of the 34 patients in Hx group and 27 of the 38 patients in transplant group had concomitant cirrhosis of the liver. In addition, one patient in Hx group had liver fluke, one patient in OLT group had Caroli s disease, and two patients in OLT-CL group had choledochal cysts that had been resected previously.
Statistical analysis
The results were summarized with followup to December 31, 1997. Cumulative overall patient survival rates were calculated by the method of Kaplan-Meier using the log rank test with adjustment for the type of operation (Hx, OLT, and OLT-CL). The observed and expected number of deaths were calculated first for each type of operation separately, and then combined for the calculation of the final log-rank statistic.23
Potential risk factors examined by univariate analysis are shown in Table 4. The stepwise Cox proportional hazard regression model was used to assess the relative prognostic influence on patient survival.
Table 4.
Univariate Analysis of Prognostic Risk Factors
| Risk Factor | n |
Cumulative survival no. (mo+SE) |
|||
|---|---|---|---|---|---|
| Hx | Tx | Hx | Tx | p Value | |
| Gender | |||||
| Male | 18 | 25 | |||
| Female | 16 | 13 | 21.3 + 4.5 | 12.7 + 4.9 | >0.61 |
| Age(y) | |||||
| ≤60 | 19 | 33 | 31.6 + 6.7 | 14.3 + 3.1 | >0.30 |
| >60 | 15 | 5 | 16.6 + 7.8 | 4.3 + 4.6 | |
| Associated diseases | |||||
| Yes | 6 | 30 | 16.6 + 6.0 | 14.3 + 3.5 | >0.73 |
| No | 28 | 8 | 24.2 + 6.7 | 5.0 + 3.0 | |
| Tumor | |||||
| Tis. 1.2 | 7 | 14 | 38.8 + 3.8 | 99.3 + 81.3 | < 0.008 |
| 3 | 27 | 24 | 18.0 + 2.4 | 12.3 + 3.6 | |
| Lymph node | |||||
| Negative | 20 | 27 | 24.3 + 10.4 | 17.7 + 5.3 | < 0.025 |
| Positive | 14 | 11 | 19.1 +6.3 | 9.9 + 4.6 | |
| Metastasis | |||||
| Yes | 4 | 5 | 0.8 + 1.2 | 26.4 + 9.6 | >0.17 |
| No | 30 | 33 | 24.3 + 5.3 | 12.6 + 3.4 | |
| pTNM stage | |||||
| 0, I, II | 4 | 14 | 39.4 + 5.9 | 99.3 + 81.3 | < 0.007 |
| III IV | 30 | 24 | 19.1 + 4.6 | 12.3 + 3.6 | |
| Margins | |||||
| Negative | 20 | 31 | 24.2 + 10.3 | 18.9 + 7.5 | < 0.024 |
| Positive | 14 | 7 | 19.1 + 5.0 | 4.3 + 0.4 | |
| Adjuvant therapy | |||||
| Yes | 26 | 18 | 24.3 + 5.1 | 16.7 + 5.1 | < 0.043 |
| No | 8 | 20 | 0.9 + 0.41 | 12.3 + 3.0 | |
| Type of operation | |||||
| Hx | 34 | 23.4 + 3.8 | > 0.81 | ||
| Tx | 38 | 12.7 + 3.4 | |||
Survival analysis was performed with the method of Kaplan-Meter with stratification by type of operation (eg, resection or transplantation). Each statistic analysis was computed separately for both types or operation, after which the values of observed and expected number or deaths in each strata were combined with the final log-rank statistic. All p values shown were obtained by this procedure.
Hx. hepatic resection; Tx. transplantation (orthotopic liver transplantation+organ cluster transplantation).
Values of p<0.05 were considered significant.
Because the precise timing of tumor recurrence could not be determined in the majority of patients, tumor-free survivals could not be calculated.
RESULTS
Perioperative mortality
Five of the 34 patients (14.7%) in Hx group, 6 of the 27 patients (22.2%) in OLT group and 2 of the 11 patients (18.2%) in OLT-CL group died of various complications within three months after surgery. Overall perioperative mortality was 18%, with no statistically significant difference between the three types of operation.
Survival rates
One- to 5-year cumulative survival rates for the 34 patients after Hx were 73.5%, 50.0%, 33.9%, 13.6%, and 9.1%, respectively. Survival at these milestones for the 27 patients after OLT were 59.3%, 40.7%, 36.2%, 36.2%, and 36.2%, respectively, and for the 11 patients after OLT-CL it was 54.6%, 27.3%, 9.1%, 9.1%, and 9.1%, respectively (Fig. 1). The differences in survival among the three groups of patients were not significant (p>0.37).
Figure 1.

Survivals after hepatic resection (Hx), orthotopic liver transplantation (OLT), and organ cluster transplantation (OLT-CL).
The effect of tumor stage
Survival of the patients with T-is, T-1, or T-2 tumors tended to be higher than those with T-3 tumors after Hx (p<0.096). After transplantation (OLT+OLT-CL), patients in the T-is, T-1, and T-2 categories had significantly better survival (p<0.038) than those listed as T-3. When the survivals of all 72 patients were pooled, survival for the 51 patients with T-3 tumors was significantly lower than for those with T-is, T-1, and T-2 tumors (Fig. 2). The unadjusted p value was <0.001 and the adjusted p value (p*) according to the types of operation23 was <0.008.
Figure 2.

Survival of patients with T3 tumors was significantly lower than for those with T-is, 1, and 2.
Survival according to the lymph node (N) status also is shown separately in Figure 3. Although the survival of the patients without lymph node involvement (N-0) was higher than those with positive nodes (N-1), the differences did not reach statistical significance within either the resection group (p<0.082) or the transplantation group (p<0.16). However, when the two groups were pooled (n=72), survival with N-O was significantly higher than with N-1 (Fig. 3) (p<0.023, p*<0.025).
Figure 3.
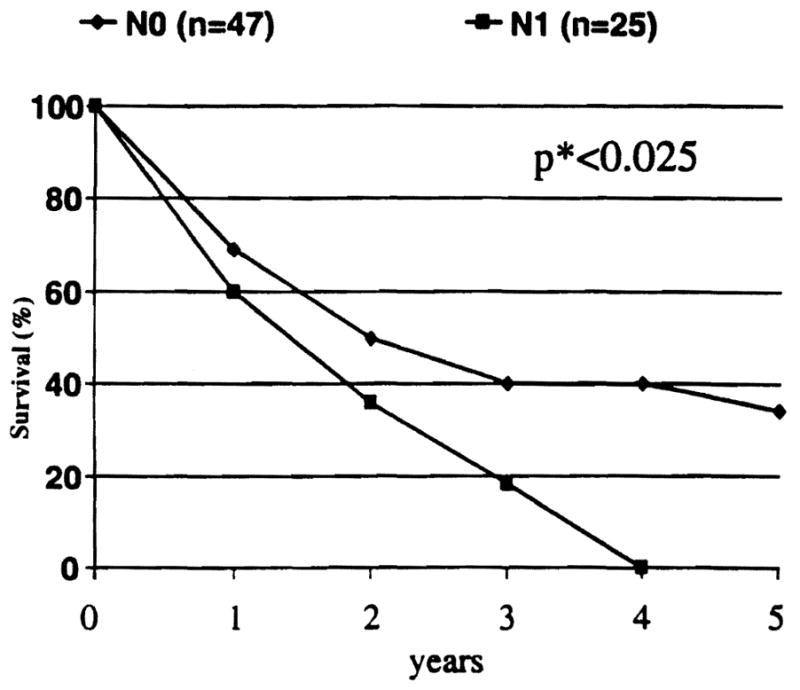
Survival of patients with positive lymph nodes (N1) was significantly lower than for those with negative lymph nodes (NO).
When the individual factors of tumor depth (T), lymph node involvement (N), and metastatic spread (M) are combined for pTNM staging,21 survival of patients in the resection cohort with pTNM stage III and IV tended to be lower than in stages I–II (p< 0.096). This inverse correlation was statistically significant in the transplantation cohort (p<0.038). When the 72 cases were pooled, the survival of the 21 patients with pTNM stages 0–II was significantly higher than those of 51 patients with stages III and IV (Fig. 4) (p<0.002, p*<0.007).
Figure 4.
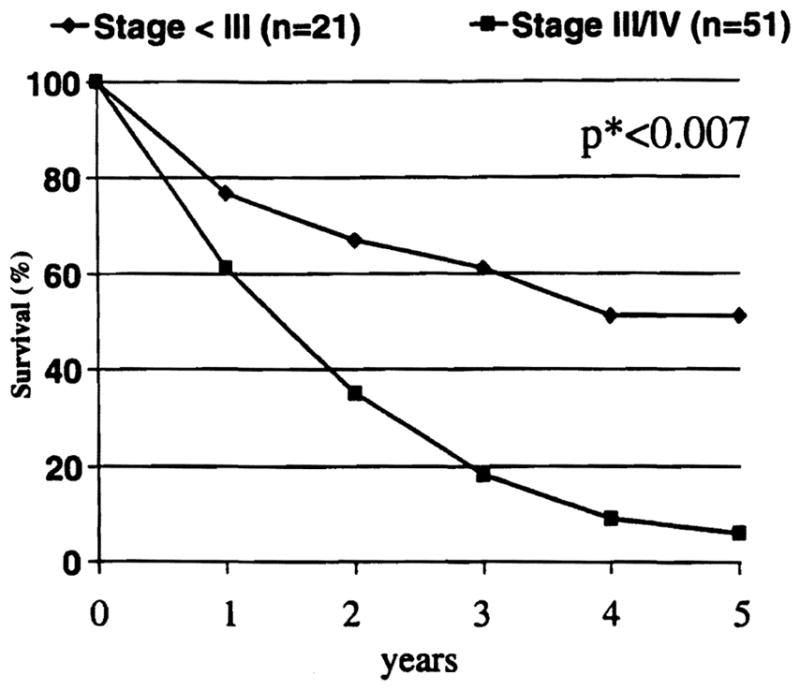
Survival of patients with stage III and IV were significantly lower than for those with less than stage III tumors.
Positive surgical margins
After hepatic resection with curative intent, survival of patients with positive microscopic surgical margins was similar to those with negative margins (p>0.26). After transplantation with curative intent, however, positive surgical margins were associated with a significantly reduced survival (p<0.011). When the resection and transplantation cohorts were combined, the adverse effect of a positive margin was significant (unadjusted p-value <0.015; adjusted p-value [p*]<0.024) (Fig. 5).
Figure 5.

Survival of patients with negative microscopic margins was significantly better than for those with positive margins.
Additional observations
Although the total of nine patients with regional metastasis (local peritoneum or omentum in five, intrahepatic metastasis in two, pancreas in one, and Roux-en-y jejunum in one) at the time of operation (Table 2) was too small for adequate statistical analysis, it was noteworthy that 4 stage IV-B patients survived 353 days, 803 days, 987 days and 2,885 days after OLT-CL. Another patient is alive with tumor recurrence more than 2 years after hepatic resection. The remaining four patients died within 3 months after surgery from various complications. Other factors, such as gender, age, concomitant liver diseases, and types of operation did not influence survival.
Although overall survival of the patients who received adjunct therapy was significantly higher than those who did not receive it, the difference became insignificant when the patients who died within 3 months were excluded from the analysis. The results of univariate analysis are summarized in Table 5. Multivariate analysis revealed the following to be good prognostic factors: 1) negative margins, 2) negative lymph nodes, and 3) T classification of T-2 or less.
Table 5.
Characteristics of 5-year survivors
| Patient no. | Age (y) | Gender | Operation | pTNM Stage | Adjuvant therapy | Result |
|---|---|---|---|---|---|---|
| 1 | 40 | Male | OLT-CL | IV-B(T3, NO, M1) | None | Alive, free of tumor, >8 y |
| 2 | 43 | Male | OLT | I(T1, NO, MO) | Preop. radiation | Alive, free of tumor, >15 y |
| 3 | 24 | Female | OLT | II (T2, NO, MO) | Preop. radiation | Alive, free of tumor, > 11 y |
| 4 | 38 | Male | OLT | II (T2, NO, MO) | Postop. radiation | Died with tumor, 8 y, 3 mo |
| 5 | 51 | Male | OLT | II (T2, NO, MO) | Preop. radiation | Alive, free of tumor, >7 y |
| 6 | 47 | Female | OLT | II (T2, NO, MO) | Preop. radiation | Alive, free of tumor, >7 y |
| 7 | 35 | Male | OLT | II (T2, NO, MO) | Postop. radiation | Alive, free of tumor, >7 y |
| 8 | 52 | Male | OLT | II (T2, NO, MO) | None | Alive, free of tumor, >6 y |
| 9 | 44 | Female | Hx (RTS) | IV-A (T3, NO, MO) | Postop. radiation | Died with tumor, 8 y, 9 mo |
| 10 | 32 | Female | Hx (RTS) | II (T2, NO, MO) | Postop. radiation | Alive, free of tumor, >5 y |
Hx, hepatic resection; OLT, orthotopic liver transplantation; OLT-CL, cluster liver transplantation; Preop., preoperative; Postop., postoperative; RTS, right trisegmentectomy.
Tumor recurrence
Forty of the 72 patients died with tumor recurrence. In 28, recurrence was confirmed by biopsy or necropsy, and in the remaining 12, recurrence was clinically determined by various imaging techniques. The exact timing of tumor recurrence was often difficult to determine because of its cryptic nature. However, the recurrence was diagnosed histologically or clinically within 3 years in 37 of the 40 patients. The most common site of tumor recurrence was around the hepatic hilum, followed by the liver, lung, bone, and skin. Recurrence was first confirmed histologically in one patient 5 years after OLT and in another 7 years after Hx.
Five-year survivors
Ten patients have survived more than 5 years after surgery. The characteristics of these patients are summarized in Table 5. Interestingly, 8 of the 10 patients were treated with transplantation.
DISCUSSION
The poor longterm survival after treatment of hilar cholangiocarcinoma has been well documented. The 5-year survival rate after surgery with curative intent ranges in literature reports from 5% to 20%.3–13 This has been explained in part by a high perioperative mortality due to hepatic failure and sepsis, particularly when a major hepatic resection is combined with excision of the extrahepatic bile duct. Preoperative intubation of the obstructed biliary tract, antibiotic therapy, and embolization of the portal vein branch may reduce the perioperative mortality.24,25
The principal reasons for the poor results is the biologic behavior of the tumor, and its location in a strategically critical area. A tumor-free surgical margin is essential for a reasonable chance of 5-year survival.4,6,8–12,26 To achieve this, hepatic resection has been combined with pancreatoduodenectomy,27 or other extended procedures when the tumor has invaded the liver or peripheral region.12,28,29
Other more radical options have been liver transplantation plus regional lymphadenectomy4 and organ cluster transplantation.19,20 In addition to securing tumor-free margins, liver transplantation or its more drastic variations may offer the only hope when extensive hilar cholangiocarcinoma is associated with advanced cirrhosis of the liver, sclerosing cholangitis, or both. Orthotopic liver transplantation with pancreatodudenectomy has been performed with less mortality and morbidity than the formal organ cluster transplantation.28,29
The role of liver transplantation in the treatment of hilar cholangiocarcinoma has been controversial because of the inefficient use of organs in the face of organ shortage.4,8–11,20 Categorical denial of liver transplantation for cholangiocarcinoma30 is not justifiable on medical grounds with the 36% 5-year survival after OLT reported herein (Fig. 1). When and if the organ supply is relieved by perfection of xenotransplantation, liver or cluster transplantation could become the preferred strategy. Meanwhile, the best way to improve outcomes and avoid organ allograft waste will be by accurate staging, an objective that often has been difficult to accomplish.
Tumor depth (the T in the pTNM) correlated well with survival in our study and those by others.4,5,7,9–13 Lymph node involvement was an ominous prognostic finding in our patients as has been reported by others.4,11,12 However, the inability to accurately identify lymph node involvement, even in the regional nodes, has made this aspect of staging unreliable.5–7,9,10
Multifactorial pTNM staging has correlated in a general way with prognosis.4,11–13 However, it should be emphasized how critical is the subanalysis of the factors contributing to the pTNM stages III (T1, T2, N1, N2, MO) and IV-A (T3, Any N, MO). For example, in our study of 72 patients, there were 24 with T3, NO, MO (pTNM stage IV-A). The 1- to 5-year survival rates of these patients with tumor-free lymph nodes were 65%, 35%, 28%, 28%, and 28% if the surgical margins were free of tumor. These results were better than those of patients with positive nodes in the ostensibly more favorable pTNM stage III and in patients of the same stage IV-B with positive lymph nodes.
Leaving the pTNM staging aside, there were 28 patients with the highly favorable combination of negative surgical margins and negative lymph nodes. The 1-, 3-, and 5-year survival of these patients was 68%, 50%, and 50%, respectively. As with the final assessment of the lymph nodes, however, the involvement of the margins often was learned too late to influence surgical decisions. Although the operations in all 72 cases were performed with curative intent, the surgical margins were found by histologic examination to be positive for malignant cells in 41% of the patients treated with Hx, 22% after OLT, and 9% after OLT-CL.
It is noteworthy that the rate of negative margins increased with the extent of surgical extirpation. However, because the mortality and morbidity of OLT-CL has been excessive,20 we have preferred recently to use the less radical procedure of orthotopic liver transplantation in combination with pancreatoduodenectomy for selected patients with hilar cholangiocarcinoma.
There was no 5-year survival with lymph node involvement in our 72 patients (Table 5), although there have been several such cases reported in the literature.7,11,12 Extensive nodal dissection combined with adjuvant therapy may prove to be beneficial.8 However, in our series, adjunct radiation therapy did not significantly prolong survival when we excluded the patients who died within 3 months after surgery.
CONCLUSIONS
Negative surgical margins, noninvolvement of lymph nodes, and tumor depth of T-2 or less were statistically significant good prognostic factors. A 5-year survival of 50% can be achieved by hepatic resection and orthotopic liver transplantation for hilar cholangiocarcinoma when lymph nodes and surgical margins are free of tumor, in the absence of distant metastases. Categorical denial of liver transplantation for hilar cholangiocarcinoma is unjustified. We suggest redefining pTNM stage III and IV-A to better reflect prognosis.
Acknowledgments
Supported in put by research grants from the Veterans Administration and Project Grant No. DK-29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Akemeier WA, Gall EA, Zinninger MM, Hoxworth PL. Sclerosing carcinoma of the major intrahepatic bile ducts. Arch Surg. 1957;75:459–461. doi: 10.1001/archsurg.1957.01280150140015. [DOI] [PubMed] [Google Scholar]
- 2.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 3.Tompkins RK, Thomas D, Wile A, Longmire WP. Prognostic factors in the bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447–457. doi: 10.1097/00000658-198110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringe B, Wittekind C, Bechstein WO, et al. The role of liver transplantation in hepatobiliary malignancy. A retrospective analysis of 95 patients with particular regard to tumor stage and recurrence. Ann Surg. 1989;209:88–89. doi: 10.1097/00000658-198901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadjis NS, Blenkharn JI, Alexander N, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107:597–604. [PubMed] [Google Scholar]
- 6.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugiura Y, Nakamura S, Iida S, et al. Extensive resection of the bile ducts combined with liver resection for cancer of the main hepatic duct: a cooperative study of the Keio bile duct cancer study group. Surgery. 1994;115:445–451. [PubMed] [Google Scholar]
- 8.Schoenthaler R, Phillips TL, Castro J, et al. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco Experience. Ann Surg. 1994;219:367–374. doi: 10.1097/00000658-199403000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. doi: 10.1097/00000658-199604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: A spectrum of intrahepatic, perihilar, and distal tumors. Ann durg. 1996;224:463–475. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single center experience. Ann Surg. 1996;224:628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klempnauer J, Ridder GJ, Werner M, et al. What constitutes long term survival after surgery for hilar cholangiocarcinoma? Cancer. 1997;79:27–34. [PubMed] [Google Scholar]
- 13.Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas: A study of 62 cases. Ann Surg. 1998;227:70–79. doi: 10.1097/00000658-199801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Bell RH, Bean RW, Putnam CW. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet. 1975;141:429–437. [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Koep LJ, Weil R, et al. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–213. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Iwatsuki S, Shaw BW, et al. Left hepatic trisegmentectomy. Surg Gynecol Obstet. 1982;155:21–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwatsuki S, Starzl TE, Todo S, et al. Experience in 1,000 liver transplants under cyclosporine-steroid therapy: a surgical report. Transplant Proc. 1988;20:498–504. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alessiani M, Tzakis A, Todo S, et al. Assessment of 5-year experience with abdominal organ cluster transplantation. J Am Coll Surg. 1995;180:1–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomized trial of Tacrolimus compared to cyclosporin for hepatic transplantation. J Am Coll Surg. 1996;183:117–125. [PMC free article] [PubMed] [Google Scholar]
- 22.Sobin LH, Wittekind CH, editors. TNM Classification of Malignant Tumors. 5. New York: Wiley and Sons, Inc; 1997. pp. 81–83. [Google Scholar]
- 23.Fisher LD, Van Belle G. A methodology for the health sciences. In: Barnett V, Bradley RA, Fisher NI, et al., editors. Biostatistics. New York: J. Wiley and Sons, Inc; 1993. pp. 807–808. [Google Scholar]
- 24.Nagino M, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–681. doi: 10.1016/s0039-6060(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki S, Makuuchi M, Miyagawa S, Kakazu T. Radical operation after portal embolization for tumor or hilar bile duct. J Am Coll Surg. 1994;178:480–486. [PubMed] [Google Scholar]
- 26.Blumgart LH. Liver resection: liver and biliary tumors. In: Blumgart LH, editor. Surgery of the Liver and Biliary Tract. 2. Edinburgh: Churchill Livingstone; 1988. pp. 1251–1280. [Google Scholar]
- 27.Tsukada K, Yoshida K, Aono T, et al. Major hepatectomy and pancreato-duodenectomv for advanced carcinoma of the biliary tract. Br J Surg. 1994;81:108–110. doi: 10.1002/bjs.1800810139. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus P, Blumhardt F. Extended bile duct resection 0 a new oncological approach to the treatment of central bile duct carcinomas? Langenbecks Arch Chir. 1994;379:213–218. doi: 10.1007/BF00195875. [DOI] [PubMed] [Google Scholar]
- 29.Cherqui D, Alan R, Piedbois P, et al. Combined liver transplantation and pancreatoduodenectomv for irrescctable hilar bile duct carcinoma. Br J Surg. 1995;82:397–398. doi: 10.1002/bjs.1800820339. [DOI] [PubMed] [Google Scholar]
- 30.Schulak JA, Ferguson R, Hanto DW, et al. Liver transplantation in Ohio. Surgery. 1997;122:842–849. doi: 10.1016/s0039-6060(97)90096-9. [DOI] [PubMed] [Google Scholar]


