Abstract
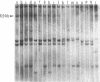
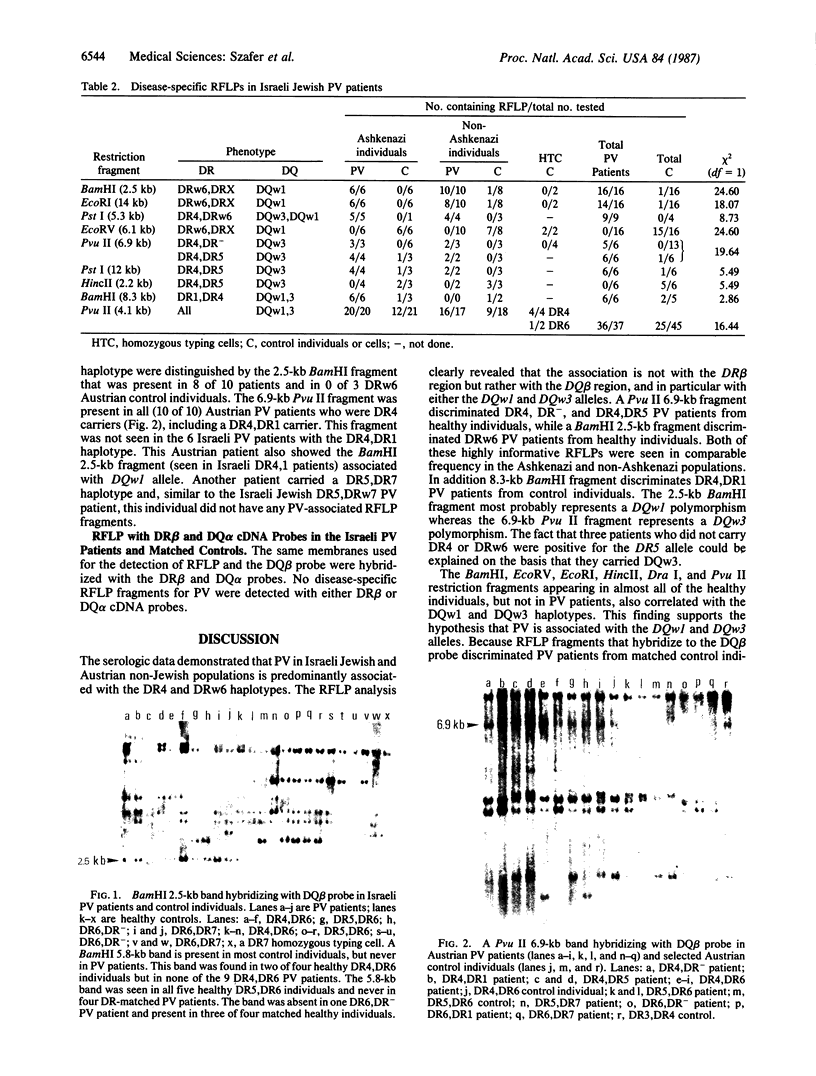
Pemphigus vulgaris in Israeli Ashkenazi and non-Ashkenazi Jews and in Austrian non-Jewish patients is strongly associated with the DR4 and DRw6 alleles of the HLA-D region class II genes. Restriction fragment length polymorphism analysis was undertaken with DQ beta, DQ alpha, and DR beta cDNA probes. Hybridization with the DQ beta probe identifies Pvu II, BamHI, and EcoRV fragments that absolutely discriminate pemphigus vulgaris patients from healthy DR-, DQ-, and ethnic-matched controls. In contrast the DQ alpha and DR beta probes failed to identify disease-specific restriction fragment length polymorphism fragments. These studies indicate that DQw1 and DQw3 polymorphisms carried by pemphigus vulgaris patients may be directly involved in predisposition to the disease or may be tightly linked to the susceptibility gene itself. To our knowledge, this is the first example of an HLA restriction fragment length polymorphism that is highly associated with susceptibility to autoimmune disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Korman A. J., Roux-Dosseto M., Bono R., Strominger J. L. cDNA clone for the heavy chain of the human B cell alloantigen DC1: strong sequence homology to the HLA-DR heavy chain. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6337–6341. doi: 10.1073/pnas.79.20.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. I., Estess P., St John T., Saiki R., Watling D. L., Erlich H. A., McDevitt H. O. DNA sequence and characterization of human class II major histocompatibility complex beta chains from the DR1 haplotype. Proc Natl Acad Sci U S A. 1985 May;82(10):3405–3409. doi: 10.1073/pnas.82.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J., Rassenti L., Smoot S., Smith K., Newby C., Hohlfeld R., Toyka K., McDevitt H., Steinman L. HLA-DQ beta-chain polymorphism linked to myasthenia gravis. Lancet. 1986 May 10;1(8489):1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- Brautbar C., Moscovitz M., Livshits T., Haim S., Hacham-Zadeh S., Cohen H. A., Sharon R., Nelken D., Cohen T. HLA-DRw4 in pemphigus vulgaris patients in Israel. Tissue Antigens. 1980 Sep;16(3):238–243. doi: 10.1111/j.1399-0039.1980.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Brautbar C., Zlotogora J., Laufer N., Cohen T., Albert E. D. Do identical HLA-DR3 genes convey susceptibility to celiac disease and insulin dependent diabetes mellitus? Tissue Antigens. 1984 Jan;23(1):58–60. doi: 10.1111/j.1399-0039.1984.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Cohen D., Le Gall I., Marcadet A., Font M. P., Lalouel J. M., Dausset J. Clusters of HLA class II beta restriction fragments describe allelic series. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7870–7874. doi: 10.1073/pnas.81.24.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N., Brautbar C., Font M. P., Dausset J., Cohen D. HLA-DR2-associated Dw subtypes correlate with RFLP clusters: most DR2 IDDM patients belong to one of these clusters. Immunogenetics. 1986;23(2):84–89. doi: 10.1007/BF00377966. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Festenstein H., Awad J., Hitman G. A., Cutbush S., Groves A. V., Cassell P., Ollier W., Sachs J. A. New HLA DNA polymorphisms associated with autoimmune diseases. Nature. 1986 Jul 3;322(6074):64–67. doi: 10.1038/322064a0. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Austin R. K., Kelleher D., Nepom G. T., Kagnoff M. F. An HLA-D region restriction fragment length polymorphism associated with celiac disease. J Exp Med. 1986 Jul 1;164(1):333–338. doi: 10.1084/jem.164.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand L., Rivat-Perran L., Huttin C., Dausset J. HLA-and Gm-linked genes affecting the degradation rate of antigens (sheep red blood cells) endocytized by macrophages. Hum Immunol. 1982 Feb;4(1):1–13. doi: 10.1016/0198-8859(82)90045-3. [DOI] [PubMed] [Google Scholar]
- Matsuki K., Juji T., Tokunaga K., Naohara T., Satake M., Honda Y. Human histocompatibility leukocyte antigen (HLA) haplotype frequencies estimated from the data on HLA class I, II, and III antigens in 111 Japanese narcoleptics. J Clin Invest. 1985 Dec;76(6):2078–2083. doi: 10.1172/JCI112211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom B. S., Palmer J., Kim S. J., Hansen J. A., Holbeck S. L., Nepom G. T. Specific genomic markers for the HLA-DQ subregion discriminate between DR4+ insulin-dependent diabetes mellitus and DR4+ seropositive juvenile rheumatoid arthritis. J Exp Med. 1986 Jul 1;164(1):345–350. doi: 10.1084/jem.164.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Thorsby E., Berle E., Nousiainen H. HLA-D region molecules restrict proliferative T cell responses to antigen. Immunol Rev. 1982;66:39–56. doi: 10.1111/j.1600-065x.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]