Abstract
It has been difficult to establish whether we are limited to the heart muscle cells we are born with or if cardiomyocytes are generated also later in life. We have taken advantage of the integration of 14C, generated by nuclear bomb tests during the Cold War, into DNA to establish the age of cardiomyocytes in humans. We report that cardiomyocytes renew, with a gradual decrease from 1% turning over annually at the age of 20 to 0.3% at the age of 75. Less than 50% of cardiomyocytes are exchanged during a normal lifespan. The capacity to generate cardiomyocytes in the adult human heart suggests that it may be rational to work towards the development of therapeutic strategies aiming to stimulate this process in cardiac pathologies.
Myocardial damage often results in chronic heart failure due to loss and insufficient regeneration of cardiomyocytes. This has prompted efforts to devise cardiomyocyte replacement therapies by cell transplantation or by the promotion of endogenous regenerative processes. The development of cell transplantation strategies is advancing fast and some are currently being evaluated in clinical trials (1, 2). Stimulating endogenous regenerative processes is attractive as it potentially could provide a non-invasive therapy and circumvent the immunosuppression required for allografts. However, it is unclear whether such regenerative strategies are realistic as it has been difficult to establish whether cardiomyocytes can be generated after the perinatal period in humans.
Stem/progenitor cells with the potential to generate cardiomyocytes in vitro remain in the adult rodent and human myocardium (3, 4). Moreover, mature cardiomyocytes have been suggested to be able to reenter the cell cycle and duplicate (5). However, studies over several decades in rodents using labeled nucleotide analogues have lead to conflicting results ranging from no to substantial generation of cardiomyocytes postnatally (6). A recent genetic labeling study, which enabled detection of cardiomyocyte generation by stem/progenitor cells (but not by cardiomyocyte duplication), demonstrated cardiomyocyte renewal after myocardial injury, but not during one year in the healthy mouse (7).
It is possible that humans, who live much longer than rodents, may have a different requirement for cardiomyocyte replacement. Cell turnover has been difficult to study in humans since the use of labeled nucleotide analogues and other strategies commonly used in experimental animals cannot readily be adapted for studies in humans due to safety concerns. The limited functional recovery after loss of myocardium and the fact that primary cardiac tumours are very rare, indicate limited proliferation within the adult human heart (8). Several studies have described the presence of molecular markers associated with mitosis in the human myocardium (5), but this provides limited information as it is difficult to deduce the future fate of a potentially dividing cell in terms of differentiation and long-term survival.
We have measured 14C from nuclear bomb tests in genomic DNA of human myocardial cells, which allows retrospective birth dating (9-11). 14C levels in the atmosphere remained relatively stable until the Cold War when above ground nuclear bomb tests caused a dramatic increase (12, 13). Even though the detonations were conducted at a limited number of locations, the elevated 14C levels in the atmosphere rapidly equalized around the globe as 14CO2. After the Test-Ban Treaty in 1963, the 14C levels have dropped exponentially, not primarily because of radioactive decay (half-life 5730 years), but by diffusion from the atmosphere (14). Newly created atmospheric 14C reacts with oxygen to form 14CO2, which is incorporated by plants through photosynthesis. By eating plants, and animals that live off plants, the 14C concentration in the human body mirrors that in the atmosphere at any given point in time (15-18). Since DNA is stable after a cell has gone through its last cell division, the 14C level in DNA serves as a date mark for when a cell was born and can be used to retrospectively birth date cells in humans (9-11).
We first carbon dated left ventricle myocardial cells, including cardiomyocytes and other cell types, to determine the extent of postnatal DNA synthesis in the human heart. DNA was extracted and 14C levels measured by accelerator mass spectrometry (see table S1 and S2 for 14C values and associated data). The cellular birth dates can be inferred from determining at what time the sample’s 14C level corresponded to the atmospheric levels (Fig. 1A). 14C levels from all individuals born around or after the nuclear bomb tests corresponded to atmospheric levels several years after the subjects’ birth (Fig. 1B), indicating substantial postnatal DNA synthesis. Analysis of individuals born before the period of nuclear bomb tests allows for sensitive detection of any turnover after 1955, due to the dramatic increase in 14C levels. By analyzing individuals born at different time points prior to 1955 it is possible to establish up to which age DNA synthesis occurs, or whether it continues beyond that age. In all studied cases, born up to 22 years before the onset of the nuclear bomb tests, 14C concentrations were elevated compared to the pre-nuclear bomb test levels (Fig. 1C). Thus, DNA is synthesized many years after birth, indicating that cells in the human heart do renew into adulthood.
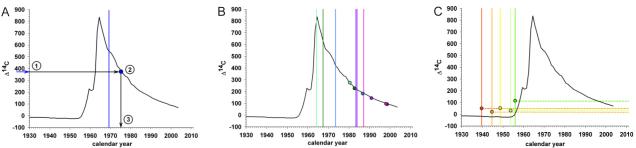
Figure 1. Cell turnover in the heart.
(A) Schematic figure demonstrating the strategy to establish cell age by 14C dating. The black curve in all graphs shows the atmospheric levels of 14C over the last decades since 1930 (data from (14)). The vertical bar indicates the date of birth of the individual. The measured 14C concentration (1) is related to the atmospheric 14C level by using the established atmospheric 14C bomb curve (2). The average birth-date of the population can be inferred by determining where the data point intersects the x-axis (3). 14C levels in DNA of cells from the left ventricle myocardium in individuals born after (B) or before (C) the nuclear bomb tests correspond to time points substantially after the time of birth, indicating postnatal cell turnover. The vertical bar indicates the date of birth of each individual and the similarly colored dots represent the 14C data for the same individual. For individuals born before the increase in 14C levels, it is not possible to directly infer an age as the measured level can be a result of incorporation during the rising and/or falling part of the atmospheric curve, thus the level is indicated by a dotted horizontal line.
Since cardiomyocytes comprise only about 20% of all cells within the human myocardium (19), it is not possible to infer from this data whether there is postnatal renewal of cardiomyocytes, or whether cell turnover in the myocardium is limited to other cell populations. We therefore set out to specifically birth date cardiomyocytes. Many cardiomyocytes are binucleated and it is difficult to distinguish a binucleated cell from two aggregating mononucleated cells (of which one could be a non-cardiomyocyte) in the flow cytometer. Hence, rather than separating myocardial cells based on cell surface or cytoplasmic markers, we developed a strategy to isolate cardiomyocyte nuclei by flow cytometry.
We found that the well characterized cardiomyocyte specific proteins cardiac troponin I (cTroponin I, also known as TNNI3) and cardiac troponin T (cTroponin T, also known as TNNT2) (for review see (20)) have evolutionarily conserved nuclear localization signals and are partly localized in the nuclei of cardiomyocytes (fig. S1 and S2). Antibodies to cTroponin I and T identify the same subpopulation of nuclei in the myocardium (Fig. 2, A to D) and retrospective birth dating of nuclei isolated with antibodies against either epitope gave similar results (table S1). Western blot and qRT-PCR analysis of sorted nuclei demonstrated a high enrichment of cTroponin I and T in the positive fraction and a depletion in the negative, validating the efficiency of the strategy (Fig. 2, E to H and data not shown). We assessed the potential transfer of cTroponin I and T during tissue processing by mixing cardiac tissue with another tissue devoid of these proteins, and found that there was negligible transfer of cTroponin I or T to non-cardiomyocyte nuclei during tissue dissociation, nuclear preparation or flow cytometric sorting (fig. S2).
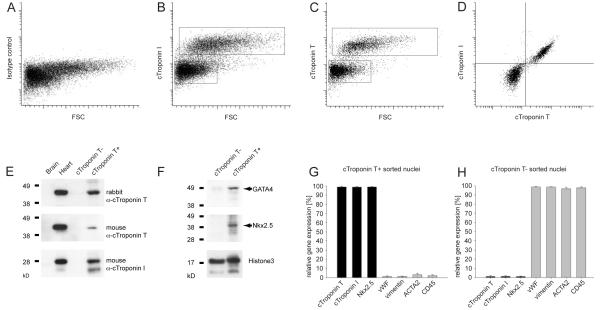
Figure 2. Isolation of cardiomyocyte nuclei.
(A-C) Flow cytometric analysis of cardiomyocyte nuclei from the left ventricle of the human heart with an isotype control antibody or antibodies to the cardiomyocyte specific antigens cTroponin I or T. Boxes denote the boundaries for the positive and negative sort populations. (D) cTroponin I and T are present in the same subpopulation of heart cell nuclei. (E) Western blot analysis of flow cytometry-isolated nuclei demonstrates close to all detectable cTroponin T (analyzed with two different antibodies) and I protein in the cTroponin T-positive fraction. Brain and heart tissue was used as negative and positive controls, respectively. (F) The cardiac troponin T-positive population is enriched for the cardiomyocyte-specific transcription factors Nkx2.5 and GATA4. Both fractions contain similar amounts of the nuclear protein histone 3 (loading control). (G) Gene expression analysis of flow cytometry-isolated nuclei shows a high expression level of cardiomyocyte specific genes in the cTroponin T-positive fraction (cTroponin I and T, Nkx2.5), while marker genes for endothelial cells (vWF), fibroblasts (vimentin), smooth muscle (ACTA2) and leukocytes (CD45) are highly expressed in the cTroponin T-negative fraction (H). Bars in (G, H) show the average from 3 independent experiments (+−SD).
We assessed the specificity of the isolation procedure with known cardiomyocyte-specific markers and markers of non-cardiomyocytes present in the myocardium. There was a high enrichment of nuclei containing the known cardiomyocyte-specific nuclear markers Nkx2.5 and GATA4 in the cTroponin positive fraction, with little contamination of nuclei expressing markers for fibroblasts, smooth muscle cells, endothelial cells or hematopoietic cells (Fig. 2, F to H and data not shown). Conversely, cardiomyocyte markers were depleted in the cTroponin negative fraction (Fig. 2, F to H and data not shown), indicating that close to all cardiomyocytes were isolated in the positive fraction. Sorting whole cells with antibodies to a non-nuclear cardiomyocyte specific epitope confirmed that nuclear cTroponin I and T are specific to cardiomyocytes, but resulted in lower purity compared to sorting nuclei (fig. S3). Flow cytometric reanalysis of all sorted samples demonstrated a DNA content corrected cardiomyocyte purity of 96±1.8% (mean ±SD, table S1 and fig. S4). Thus, flow cytometry with antibodies against cTroponin I or T allows specific isolation of cardiomyocyte and non-cardiomyocyte nuclei.
We extracted DNA from cardiomyocyte nuclei (5±2×107, mean±SD, table S1) and measured the 14C concentration in genomic DNA. By analyzing the 14C concentration also in unsorted myocardial nuclei (>108), we were able to mathematically compensate for any contamination in the cardiomyocyte fraction in the individual cases, reducing the risk that contamination with a cell population with a different turnover rate would skew the result for cardiomyocytes. All individuals born before the onset of the nuclear bomb tests had 14C levels in cardiomyocyte genomic DNA that was higher than the pre-bomb atmospheric levels, demonstrating DNA synthesis after 1955 (Fig. 3A). Similarly, all individuals born near or after the time of the nuclear bomb tests had 14C concentrations in cardiomyocyte DNA corresponding to several years after their birth, establishing postnatal cardiomyocyte DNA synthesis (Fig. 3B).
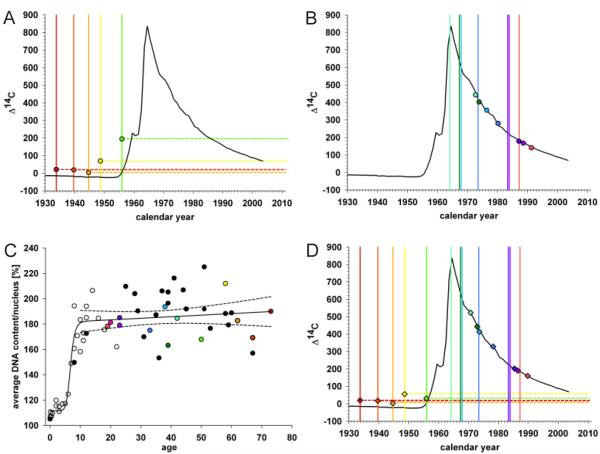
Figure 3. Cardiomyocyte turnover in adulthood.
(A) The 14C levels in cardiomyocyte DNA from individuals born before the time of the atmospheric radiocarbon increase correspond to time points after the birth of all individuals. The vertical bar indicates year of birth, with the correspondingly colored data point indicating the delta 14C value. (B) 14C levels in cardiomyocyte DNA from individuals born after the time of the nuclear bomb test. (C) Average DNA content (2n=100%) per cardiomyocyte nucleus from individuals (without severe heart enlargement, see figure S5) of different ages. Ploidy was measured by flow cytometry. Colored data points identify individuals analyzed for 14C (n=13). Black data points are from individuals only analyzed with regard to ploidy level (n=23) and white data points are taken from Adler et al. (n=26) (24, 26). The dashed lines indicate the 95% confidence interval for the regression curve. (D) 14C values corrected for the physiologically occurring polyploidization of cardiomyocytes during childhood for individuals born before and after the bomb spike, calculated based on the individual average DNA content per cardiomyocyte nucleus. The 14C content is not affected in individuals where the polyploidization occurred before the increase in atmospheric levels.
There is no increase in the number of cardiomyocytes after the postnatal period but rather a slow continuous decrease with age (21). About 25% of cardiomyocytes are binucleated in humans at birth, and this proportion stays constant throughout life (22). Thus, the postnatal cardiomyocyte DNA synthesis detected by 14C analysis cannot be explained by an increase in cardiomyocyte number or binucleation. However, the heart grows during childhood, as the increasing demand of contractile capacity is met by hypertrophy of cardiomyocytes. Almost all cardiomyocyte nuclei are diploid at the time of birth, but the DNA of most nuclei is duplicated to become tetraploid in childhood when the cells undergo hypertrophy (Fig. 3C, fig. S5, (23-25)). After the age of ten there is no further increase in cardiomyocyte nuclei DNA content (R=0.135, p=0.384, Fig. 3C). The DNA synthesis associated with polyploidization of cardiomyocyte DNA results in incorporation of 14C concentrations corresponding to the atmospheric levels during childhood.
Three of the individuals born before the nuclear bomb tests were more than 10 years old at the onset of the increase in atmospheric 14C. That their 14C concentration in cardiomyocyte DNA was above the pre-nuclear bomb test levels (Fig. 3A) cannot be explained by DNA synthesis associated with polyploidization, but indicates cardiomyocyte renewal after 1955. Moreover, in the individuals born after the nuclear bomb tests the difference between the birth date of the person and the date corresponding to the 14C level in cardiomyocyte DNA increased with the age of the individual (fig. S6, table S1), demonstrating that cardiomyocyte DNA synthesis is not restricted to a limited period in childhood but continues in adulthood.
Polyploidization of cardiomyocyte DNA occurs in a stereotypical manner during a rather short period in childhood (Fig. 3C, (23-25)), making it possible to calculate its impact on 14C values in each individual (see supporting online text and (26)). By subtracting the childhood polyploidization-associated 14C incorporation from the measured value in each case, we could estimate polyploidization-independent 14C values. In all cases, the polyploidization-independent 14C values corresponded to time points after birth for each individual (Fig. 3D), indicating cardiomyocyte renewal. Analysis of 14C concentrations in DNA from only diploid or only polyploid cardiomyocyte nuclei demonstrated similar degrees of 14C integration after childhood in both compartments, providing direct evidence for cardiomyocyte renewal independently of polyploidization and indicating similar turnover of diploid and polyploid cells (see supporting online text, fig. S7 and table S3). In the five oldest individuals, who all were born before or at the onset of the nuclear bomb tests, the 14C values were lower than contemporary values (Fig. 3D), establishing that not all cardiomyocytes had been exchanged after 1955 but that a substantial fraction remains from early in life even in the elderly.
Increased cardiac workload in pathological situations often results in cardiomyocyte hypertrophy and heart enlargement, and can at late stages result in polyploidization in adulthood (fig. S5, 24). A few of the included subjects had cardiac pathology (table S2). We cannot exclude the possibility that adult polyploidization may contribute to some 14C integration in the cases with pathology. However, none of the included subjects had severe heart enlargement nor a pathological cardiomyocyte ploidy profile and there was no significant difference in 14C integration in cardiomyocyte DNA in the subjects with cardiac pathology compared to healthy individuals (table S1, Fig. 3C and fig. S5). Moreover, mathematical modeling of the kinetics of DNA synthesis and 14C integration showed that the measured 14C concentrations in cardiomyocyte DNA could not be a result of polyploidization during adulthood (see supporting online text).
Several studies of sex-mismatched transplant recipients have indicated fusion of human cardiomyocytes with other cells (27). However, fusion appears to mainly occur transiently after transplantation and even in the acute phase the fusion rate is too low to explain the 14C data (fig. S8). DNA damage and repair is very limited in differentiated cells (28) and is, at least in neurons, well below the detection limit of the employed method (10, 11). Although cell fusion and DNA repair may affect 14C levels in cardiomyocyte DNA, available data suggest that the magnitude of these processes make them negligible in the current context and that the 14C data we report here (after compensation for polyploidization) likely accurately reflects cell renewal.
Mathematical modeling of 14C data from individuals born both before and after the nuclear bomb tests, which provides slightly different and complementary information, as well as of subjects of different age within these groups can provide an integrated view on cell turnover (9). We used an analytical model that includes polyploidization in childhood to assess which one of many scenarios for cell birth and death best describes the data. Times at which cells are born, ploidize and die are tracked. The atmospheric 14C values corresponding to DNA synthesis events are integrated to yield a calculated 14C level, based on each subject’s birth date, age at death and DNA content. The calculated 14C levels were fitted to the purity-corrected values to find the best renewal rates for each scenario (see supporting online text for a comprehensive description of the modeling). We first calculated what the annual turnover rate would be in each individual if the rate was constant throughout life. This indicated annual turnover rates of 0.2-2% (Fig. 4A). However, there was a clear negative correlation to age (R=−0.85; p=0.005), establishing that the turnover rate declines with age. The strong negative correlation to age also indicates that there is limited interindividual variation in the cardiomyocyte turnover rate and its decrease with age.
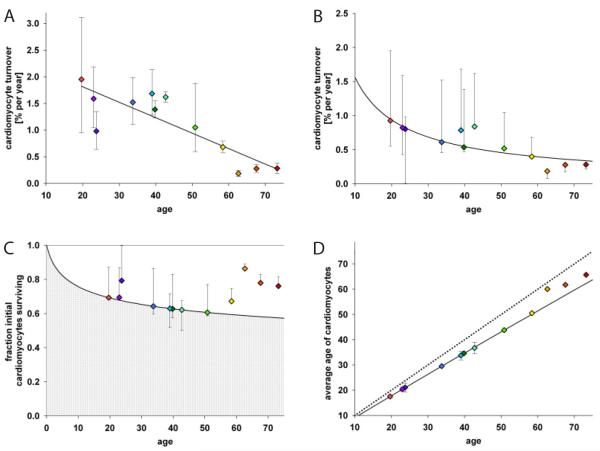
Figure 4. Dynamics of cardiomyocyte turnover.
(A) Individual data fitting assuming a constant turnover (see supporting online text) reveals an almost linear decline of cardiomyocyte turnover with age (R=−0.85; p=0.005). A constant turnover hypothesis might therefore not represent the turnover dynamics accurately. (B) Global fitting of all data points (see supporting online text, SSE=9.4×103) shows an age-dependent decline of cardiomyocyte turnover. (C) Fraction of cardiomyocytes remaining from birth is depicted as gray area, and the white area is the contribution of new cells. Estimation is from the best global fitting. (D) Cardiomyocyte age estimates from the best global fitting. The dotted line represents the no cell turnover scenario, where the average age of cardiomyocytes equals the age of the individual. The black line shows the best global fitting. Colored diamonds indicate computed data points from 14C-dated subjects. Error bars in all graphs are calculated for each subject individually showing the interval of possible values fitted with the respective mathematical scenario.
We next tested a series of different models allowing turnover rates to change with age. The best fit was found with an inverse-linear declining turnover rate (Fig. 4B), where younger cardiomyocytes were more likely than old to be replaced (see supporting online text). This model predicts that cardiomyocytes are renewed at a rate of approximately 1% per year at the age of 20 and 0.3% at the age of 75 (Fig. 4B). With this turnover rate, the majority of cardiomyocytes will never be exchanged during a normal lifespan (Fig. 4C). At the age of 50, 60% of the cardiomyocytes remain from the time around birth and 40% have been generated later (Fig. 4C). The age of cardiomyocytes is in average 6 years younger than the individual (Fig. 4D). The 14C data indicate a substantially higher renewal rate for non-cardiomyocytes, with a median annual turnover of 18% and a mean age of 4.0 years (see supporting online text). Our data does not allow us to identify whether new cardiomyocytes derive from cardiomyocyte duplication or from a stem/progenitor pool, as both would result in similar 14C integration in DNA.
Analysis of cell proliferation in the human myocardium has previously indicated a cardiomyocyte proliferation rate that could result in the exchange of all cardiomyocytes within 5 years (29), but the 14C concentrations in DNA exclude such a high mitotic renewal rate. We asked whether cardiomyocytes may be heterogeneous, with an identifiable subpopulation turning over relatively fast and the rest not turning over at all. This scenario is incompatible with the data, and it is most likely that the vast majority of cardiomyocytes have a similar probability to be exchanged at a given age (see supporting online text).
The limited functional recovery in humans after myocardial injury clearly demonstrates failing regeneration of cardiomyocytes. The renewal of cardiomyocytes indicated by the continuous integration of 14C, suggests that the development of pharmacological strategies to stimulate this process may be a rational alternative or complement to cell transplantation strategies for cardiomyocyte replacement.
Supplementary Material
Acknowledgments
We thank Richard Lee, Kirsty Spalding and members of the Frisén lab for valuable discussions, M. Toro and K. Hamrin for help with flow cytometry. Paula Reimer for assistance with radiocarbon interpretation, Robert Cassidy for technical advice, M. Stahlberg and T. Bergman for help with HPLC and D. Kurdyla and P. Zermeno for producing graphite. This study was supported by grants from the Swedish Heart-Lung Foundation, the Swedish Research Council, Knut och Alice Wallenbergs Stiftelse, Human Frontiers Science Program, the Swedish Cancer Society, the Foundation for Strategic Research, the Karolinska Institutet, the Juvenile Diabetes Research Foundation, NIH/NCRR (RR13461), European Commission FP7 CardioCell and the Tobias Foundation. This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under contract DE-AC52-(07NA27344). R.D.B. and F. B.-H. were supported by fellowship from the Canadian Institutes of Health Research, and F.B.-H. was also supported by a fellowship from the Christopher and Dana Reeve Foundation.
Footnotes
Supporting Online Material www.sciencemag.org Materials and Methods Figs. S1-S8 Tables S1–S3 Supporting online text
References and notes
- 1.Passier R, van Laake LW, Mummery CL. Nature. 2008 May 15;453:322. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Murry CE. Nat Biotechnol. 2005 Jul;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Puig S, Wang Z, Chien KR. Cell Stem Cell. 2008 Apr 10;2:320. doi: 10.1016/j.stem.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Wu SM, Chien KR, Mummery C. Cell. 2008 Feb 22;132:537. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anversa P, Nadal-Ginard B. Nature. 2002 Jan 10;415:240. doi: 10.1038/415240a. [DOI] [PubMed] [Google Scholar]
- 6.Soonpaa MH, Field LJ. Circulation research. 1998 Jul 13;83:15. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh PC, et al. Nat Med. 2007 Sep;13:970. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butany J, et al. Lancet Oncol. 2005 Apr;6:219. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 9.Spalding KL, et al. Nature. 2008 Jun 5;453:783. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj RD, et al. Proc Natl Acad Sci U S A. 2006 Aug 15;103:12564. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spalding K, Bhardwaj RD, Buchholz B, Druid H, Frisén J. Cell. 2005;122:133. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 12.De Vries H. Science. 1958 Aug 1;128:250. doi: 10.1126/science.128.3318.250. [DOI] [PubMed] [Google Scholar]
- 13.Nydal R, Lovseth K. Nature. 1965 Jun 5;206:1029. doi: 10.1038/2061029a0. [DOI] [PubMed] [Google Scholar]
- 14.Levin I, Kromer B. Radiocarbon. 2004;46:1261. [Google Scholar]
- 15.Spalding KL, Buchholz BA, Bergman L-E, Druid H, Frisén J. Nature. 2005;437:333. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 16.Libby WF, Berger R, Mead JF, Alexander GV, Ross JF. Science. 1964 Nov 27;146:1170. doi: 10.1126/science.146.3648.1170. [DOI] [PubMed] [Google Scholar]
- 17.Harkness DD. Nature. 1972 Dec 1;240:302. doi: 10.1038/240302a0. [DOI] [PubMed] [Google Scholar]
- 18.Wild EM, et al. Nucl. Instr. and Meth. in Physics Res. 2000;172:944. [Google Scholar]
- 19.Rubart M, Field LJ. Annu Rev Physiol. 2006;68:29. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 20.Parmacek MS, Solaro RJ. Progress in cardiovascular diseases. 2004 Nov-Dec;47:159. doi: 10.1016/j.pcad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Olivetti G, Melissari M, Capasso JM, Anversa P. Circulation research. 1991 Jun;68:1560. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 22.Olivetti G, et al. Journal of molecular and cellular cardiology. 1996 Jul;28:1463. doi: 10.1006/jmcc.1996.0137. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky V, Sarkisov DS, Arefyeva AM, Panova NW, Gvasava IG. Virchows Arch. 1994;424:429. doi: 10.1007/BF00190566. [DOI] [PubMed] [Google Scholar]
- 24.Adler CP. In: The development and regenerative potential of cardiac muscle. Oberpriller JO, Oberpriller JC, Mauro A, editors. Harwood academic publishers; New York: 1991. pp. 227–252. [Google Scholar]
- 25.Pfitzer P. Current Topics in Pathology. 1971;54:125. [Google Scholar]
- 26.Materials and methods are availabe as supporting material on Science Online.
- 27.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Circulation research. 2002 Apr 5;90:634. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 28.Nouspikel T, Hanawalt PC. DNA Repair. 2002;1:59. doi: 10.1016/s1568-7864(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 29.Beltrami AP, et al. N Engl J Med. 2001 Jun 7;344:1750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.