Abstract
Objective
To provide an overview of the genetic epidemiology of substance use and misuse in adolescents.
Method
We present a selective review of genetically informative research strategies, their limitations and key findings examining issues related to the heritability of substance use and substance use disorders in children and adolescents.
Results
Adoption, twin and extended family designs have established there is a strong heritable component to liability to nicotine, alcohol and illicit drug dependence in adults. However, shared environmental influences are relatively stronger in youth samples and at earlier stages of substance involvement (e.g., use). There is considerable overlap in the genetic influences associated with the abuse/ dependence across drug classes while shared genetic influences also contribute to the commonly observed associations between substance use disorders and both externalizing and, to a lesser extent, internalizing psychopathology. Rapid technological advances have made the identification of specific gene variants that influence risks for substance use disorders feasible and linkage and association (including genomewide association studies) have identified promising candidate genes implicated in the development of substance use disorders.
Conclusions
Studies using genetically informative research designs, including those that examine aggregate genetic factors and those examining specific gene variants, individually and in interaction with environmental influences, offer promising avenues not only for delineating genetic effects on substance use disorders but also for understanding the unfolding of risk across development and the interaction between environmental and genetic factors in the etiology of these disorders.
Keywords: substance use disorders, adolescence, twin, genetics, gene by environment interaction
INTRODUCTION
The initial use of tobacco, alcohol and illicit drugs typically occurs during adolescence and some experience with these substances is now normative among adolescents in the United States and throughout the developed world.1 While many youth who use drugs do so only infrequently and without experiencing any apparent adverse consequences, there are at least three principal reasons why substance use should be a major concern for child and adolescent psychiatrists: First, the acute effects of intoxication may have potentially serious long-term consequences, including increasing risks of motor vehicle and other unintentional injuries.2 Second, a substantial proportion of adolescents report meeting criteria for abuse and/ or dependence on these substances, and substance abuse/ dependence are among the most prevalent psychiatric disorders in adolescents3 while the majority of adults who develop a substance use disorder report onset during adolescence.4 Finally, the age of onset of substance use is prognostic of subsequent risks for the development of abuse/ dependence and other measures of drug related harm.5-8
The goal of this review is to present an overview of the genetic epidemiology of substance use and misuse in adolescents. However, the study of genetic and environmental influences is predicated on an accurate understanding of the behavior being studied. As with all behavioral or psychiatric disorders there is considerable need both for precise definition and assessment of these behaviors9 and to place them within a broader developmental framework.10 Specific issues that need to be considered include: a) Reliance on self report. Although self–reports may underestimate the true extent of substance use, they are generally accepted as reliable and valid indicators of substance use.11 Unlike some other areas of child and adolescent psychiatry where parental or other collateral reports are recognized as highly informative, parental reports of their child’s substance use may be less reliable and valid as adolescents actively try to conceal the extent of their substance use from parents. While the use of biological samples (e.g., urine tests) may be ill suited for assessing lifetime patterns of substance use, they are recognized as useful in clinic referred samples and for assessing treatment compliance. b) Are DSM defined abuse and dependence criteria appropriate indices in children and adolescents? DSM12 criteria for substance abuse and dependence were largely developed for use in adults and there have been ongoing concerns about the extent to which they may be appropriate for adolescents or young adults. Some symptoms (e.g., withdrawal) only occur after many years of heavy drinking and, given low prevalence in adolescence samples, may have limited utility for this age group.13 Conversely, some symptoms of abuse, particularly items relating to getting into trouble with friends or family members may occur, because of parental restrictions, in adolescents who drink alcohol or use drugs only infrequently. Martin and Winters9 described such individuals as “diagnostic imposters”. Similarly, there are several dependence symptoms with high prevalence among adolescents, (particularly “tolerance” and “drinking more or longer than intended”) that identify many adolescents with relatively low levels of consumption.14 c) The extent to which normative behaviors may be indices of risk. Given that some use of tobacco, alcohol and also cannabis is normative among older teens, age of initiation and use is an important consideration: use of these substances among younger adolescents and children may be an indicator of heightened risks for the development of abuse/ dependence15,16 and related risks.17-19,20
Thus, despite numerous challenges in assessing substance use disorders in adolescents, there has been increasing recognition of the public health and scientific importance of researching these behaviors; and in recent years there have been considerable advances in our understanding of them and, in particular, the mechanisms by which genetic factors may contribute to risks for substance use and substance use disorders.
METHOD
In this paper we present a selective review of genetically informative research designs and discuss key findings relating to the heritability of substance use disorders, the comorbidity between substance use and other psychiatric disorders and gene by environmental interplay in the development of these disorders.
RESULTS
Genetically informative research approaches and their limitations
Adoption Studies
Some of the earliest findings suggesting heritable influences on substance use came from adoption studies in which offspring outcomes were compared with analogous measures in the biological and adoptive parents. Strong associations between biological parent and offspring behavior are interpreted as suggesting heritable influences, while strong associations between adoptive parent and offspring behavior suggest the role of rearing environment. While the seminal Iowa21 and Swedish22 adoption studies established strong correlations between biological parents and offspring diagnoses of alcohol and drug use disorders, they were based on adult samples.
The adoption design has important limitations: adoption studies have typically relied on official records to characterize biological parent psychopathology (e.g., arrest, hospitalization) and are likely to identify only severe cases. Related to this, biological parents who give their children up for adoption are not representative of the general population of biological parents. Additionally, due to both adoption agency screening and self-selection by adoptive parents, the number of adopted individuals raised in high-risk environments is likely to be limited: compared with the general population, adoptive parents are likely to be older, more affluent and less likely to show high rates of psychopathology. Furthermore, while the link between biological parents and offspring are presumed to exclude shared environment, exposure to maternal interuterine environment as well as age at adoption can result in sharing of environmental factors between biological mothers and their offspring.23
Twin studies
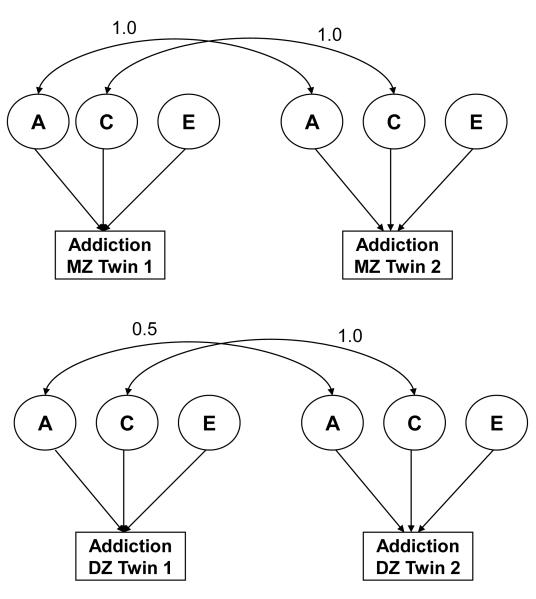
Although there have been several studies of twins reared apart,24 because of the rarity of such pairs most research examines twins reared together. These studies compare concordance rates between members of monozygotic/identical and dizygotic/fraternal twins for a trait or disorder, with a higher degree of similarity in MZ (who share 100% of their genes) than in DZ twins (who, on average share 50% of their segregating genes) being interpreted as providing evidence for heritable influences on the behavior being studied. As shown in Figure 1, the correlation between a pair of twins can be expressed as a path diagram, where rectangles represent observed behavior and circles represent latent sources of variation: additive genetic (A), shared environmental (C) and non-shared environmental (E). Heritability is the proportion of total variance in behavior that is attributed to additive (and, in the case of broad heritability, non-additive, when they play a role) genetic factors. Several assumptions and potential limitations underlying the analysis of twin data that need to be considered25, including: a) whether the equal environment assumption (which posits that the correlation for C is 1.0 in both MZ and DZ twin pairs) is justified. Despite concerns, general support for this assumption has been reported across a range of psychiatric phenotypes.26 b) The assumption of random mating, the violation of which can inflate estimates of shared environment; (c) If some of the genetic variation in outcome is due to non-additive genetic effects (dominance or epistasis), the importance of shared family environmental influences may be underestimated. d) Genetic effects and gene by shared environment effects are confounded. Nonetheless, it is possible to explicitly model this gene-environment interplay by obtaining estimates of heritability that are conditioned on, or vary as a continuous function of, environmental exposure.
Figure 1.
A standard ACE model is depicted in a pair of monozygotic (MZ) and dizygotic (DZ) twins. Note: The rectangles represent observed behavior (phenotype) such as addiction while the circles represent latent/unmeasured contributors to variance in that behavior. A includes all segregating genes of the same ancestral origin. Heritability is A/(A+C+E). C includes those environments that make members of a twin pair similar to each other (e.g. prenatal environment, neighborhood, similar perceptions of parenting). E includes those environments that are unique to each twin (e.g. traumatic life event, differing perceptions of parenting) and measurement error.
The Children of Twins Design
An extension of the classical twin study is the children-of-twins (COT) design which compares outcomes in offspring of twins. Using data on children of affected and unaffected MZ and DZ pairs, four groups can be defined: (1) high genetic risk and high environmental risk (parent, MZ or DZ, is affected), (2) high genetic risk but reduced environmental risk (parent is unaffected but parent’s MZ twin is affected), (3) intermediate genetic risk but reduced environmental risk (parent is unaffected but parent’s DZ twin is affected), and (4) children at low genetic and low environmental risk (parent and co-twin are both unaffected). The COT design allows for detection of genetic transmission that could in principle account for associations between an apparent environmental risk-factor and offspring outcomes27. It can also be used to detect the environmental consequences of parental substance use disorders that may or may not depend upon offspring genetic vulnerability or be masked by genetic non-additivity and therefore remain undetected in the traditional twin study design.
One of the relatively few published studies utilizing this design reported that, while offspring of MZ and DZ twins with a history of alcohol dependence were significantly more likely to exhibit alcohol abuse/ dependence than were offspring of nonalcoholic fathers, offspring of an unaffected (i.e., no history of abuse or dependence) MZ twin whose co-twin had alcohol dependence were no more likely to exhibit alcohol abuse or dependence than were offspring of nonalcoholic twins whose co-twin was also unaffected. This pattern of results suggests that a low risk environment (no exposure to paternal alcohol dependence) can ameliorate the influence of high risk genetic background.28
In addition to COT, extended twin designs that incorporate information on spouses of twins (i.e. to test for the assumption of random mating, or whether spousal correlations for behaviors like smoking and drinking is attributable to shared genes and environments) as well as parents and non-twin siblings have been employed 29. Using, primarily, the classical twin design and its multivariate extensions, a host of studies have examined the sources of individual differences in alcohol, tobacco and illicit drug involvement.
Evidence for heritable influences on substance initiation and use
While it is well established that about 50-70% of the variation in alcohol use disorders is due to heritable factors,30 initiation of alcohol use is largely influenced by shared environmental influences, with a review by Hopfer et al31 indicating that in the region of 50-70% of variation in alcohol initiation could be attributed to shared environmental factors.
There is less evidence for the role of shared environmental influences on adolescent smoking. Several twin studies report that the heritability of smoking ranges from 25%32 to 80%,33 with higher heritability estimates for current smoking and regular or daily smoking. In contrast, Koopmans et al34 found no evidence for heritable influences of smoking in adolescents aged 12-14 years – heritability increased to 27% in women aged 15-16 years and to 30-60% at ages 17-25 years. To some extent, these discrepancies are attributable to variability in definitions of smoking. Likewise, modest genetic and prominent shared environmental factors have been noted as contributors to variation in initiation of cannabis and other illicit drugs.35
Evidence for heritable influences on substance use disorders
Studies of alcohol, nicotine and illicit drug use disorders during adolescence are relatively uncommon as a majority of at-risk individuals meet criteria for abuse/dependence during early adulthood. Nonetheless, studies of one or more abuse/dependence symptoms during adolescence find strong evidence for the role of heritable influences (50% and greater).36,37 While the role of shared environment appears to be non-significant in adult studies of substance use disorders, it remains an important contributor to variation in adolescent substance use problems.38
The relationship between genetics of use and genetics of abuse/dependence?
Genes influencing substance initiation may also impact transitions to substance use disorders. Recognizing this conditional nature of substance use disorders, several genetic studies have modeled the genetic and environmental overlap across substance use and later abuse/dependence. Overwhelmingly, genetic influences on earlier stages of substance use have been found to be heavily correlated with genes influencing continued use and abuse/dependence – however, the source and extent of covariation differs across drugs. For instance,nearly 80-90% of the heritable influences on DSM-IV alcohol abuse/dependence overlap with indices of alcohol consumption.39,40. However, in adolescent samples, the overlap between use and escalation (binge drinking, getting drunk) was moderate 41. Furthermore, particularly for alcohol, shared environment also facilitates escalation of use. A study of Finnish adolescent twins42 examining the genetic overlap across alcohol use, frequency of consumption and problem drinking found that while drinking frequency and problem drinking shared over 50% of their genetic influences, the link between initiation and problem drinking was largely due to shared environment.
These shared environmental factors appear to be less prominent in the transitions for smoking initiation/regular smoking to nicotine dependence, with correlated genetic vulnerabilities serving as the primary source of covariation across these stages of smoking. In an adult sample, 60 -74% % of the liability to nicotine dependence was shared with earlier stages of smoking33,43. In contrast, Heath et al44 reported minimal overlap between genetic influences on persistent smoking and those on initiation, leading to speculation that the etiology of persistent smoking (i.e. inability to quit) may be distinct from nicotine dependence.
Relative to alcohol, Fowler and colleagues reported significantly greater covariance between use and misuse of cigarettes and cannabis 41. Perhaps the most convincing support for genetic overlap across stages of substance use arises from the study of cannabis and other illicit drugs, where results from adult samples show evidence for substantial genetic overlap (50-80%).45,46 Gillespie et al46 reported that cannabis availability accounted for 92-96% of the shared environmental (and only a modest 2-3% of the genetic) influences on cannabis initiation and abuse. The study, thus, presented two key conclusions – (a) that cannabis availability is heritable (18%), which is presumably due to individual predispositions to environmental exposure (or gene-environment correlation) and (b) that drug availability may represent a majority of shared environmental variance (C) in twin studies of substance use.
The consistent finding of genetic overlap between stages of substance use has important implications for genetic studies of adolescent cohorts. The moderate to high extent to which genetic factors are shared across stages of substance involvement suggest that a shared predisposition plays a role in trajectories of substance use and misuse. Thus, genetic factors identified in adolescents who use a substance occasionally may well be involved in later stages of dependence.
Comorbidity
Comorbidity, the occurrence of multiple psychiatric conditions in one individual, is highly prevalent and has strong implications for prognosis and course of both treated and untreated conditions.47,48 Numerous studies have documented that use and misuse of different substances is highly correlated and that substance use and abuse/dependence are highly associated with externalizing psychopathology and, to a lesser extent, internalizing psychopathology. Multivariate twin studies can be used to model the extent to which the same genetic factors influence vulnerability across substances and psychopathology.
Comorbidity between use of different substances
Evidence from twin studies supports the role of correlated genetic influences on use of alcohol and other drugs, as well as smoking, although to a lesser extent. For instance, Young et al36 report genetic correlations of 0.15-0.3 between alcohol, tobacco and cannabis use. Similarly, Koopmans et al49 reported that the genetic correlation between use of alcohol and tobacco is negligible during adolescence but is unmasked during early adulthood. Despite the reduced effect of shared genetic influences on tobacco use, Han et al50 found evidence for a heritable (48%) common predisposition to alcohol, tobacco and drug use during adolescence.
While the genetic overlap between smoking and other forms of adolescent substance use appears to be tenuous, these links appear to be sensitive to the measures of smoking used and the comorbid drug under study. One study reported that as heritability of smoking increased, the genetic correlation between drinking and smoking also increased34. Smoking and cannabis use also share common genetic factors.51 Intriguingly, two studies have reported that cannabis initiation could be linked to smoking progression, independent of its relationship with smoking initiation 52,53.
Finally, it is noteworthy that illicit drugs themselves share genetic influences to a considerable extent. One study of an adult sample reported that a single genetic factor was responsible for nearly all of the heritable influences on illicit substance use – the exception was opiate and sedative use, for which 70% and 50% of the genetic influences, respectively, were drug specific.54
Comorbidity between abuse/ dependence on different substances
There is robust evidence for shared genetic influences on alcohol, nicotine and drug dependence. Two studies focusing exclusively on the genetic and environmental underpinnings of illicit drug abuse/ dependence in males reported somewhat divergent results: Kendler et al55 found no evidence for substance specific genetic influences on cannabis, sedatives, stimulants, cocaine opiates and hallucinogens. In contrast, Tsuang et al56 reported specific genetic influences on opiate abuse, although most genetic influences were shared across these substances. Expanding this work to include licit as well as illicit drug dependence in both males and females, Kendler et al57 concluded that there are two genetic factors (one predisposing largely to licit drug dependence and one to illicit drug dependence) underlying dependence on these drugs, although there was also evidence of quite large specific genetic influences on both nicotine and caffeine.
The extent to which these conclusions, which are based on adult samples, may generalize to adolescent samples or to earlier stages of substance use remains unclear. In adolescents, problem use of alcohol, tobacco and cannabis is influenced by correlated genetic, but not shared environmental, factors.36 Also, Rhee et al58 demonstrated that alcohol and drug dependence may be manifestations of a single common and heritable liability in adolescents. These authors examined multiple conceptualizations of comorbidity and determined that, in adolescents, underlying alcohol and drug dependence was a common liability and that these disorders were alternate forms of that liability. Together, these results suggest substantial overlap of both genetic and environmental factors associated with the development of abuse/ dependence across a range of licit and illicit drugs (the licit and illicit drug factors identified by Kendler et al57 were highly correlated; r = .82). In contrast, utilizing a children-of-twins design, Volk et al59 reported that after accounting for the offspring correlation between alcohol and nicotine dependence, there was evidence for the specificity of genetic transmission of vulnerability to alcohol and nicotine dependence.
Comorbidity with other psychiatric disorders
There is growing evidence of substantial genetic overlap of alcohol and drug use disorders with both externalizing60-62 and internalizing disorders.63 For example, in a study of adolescent twins Button et al64 reported that 35% of the covariance between conduct disorder and drug use disorders could be attributed to common genetic sources and 46% to shared environmental influences. Using COT data, Knopik et al65 demonstrated that intergenerational links between maternal alcohol dependence and offspring ADHD may be mediated by pleiotropic genetic effects, again supporting the important role of genetic influences on both within individual comorbidity and familial aggregation.
In contrast, multiple studies of adolescents suggest that associations between alcohol use and externalizing problems during adolescence are largely attributable to shared environmental influences.66,67 While parental alcohol consumption and dependence have long been recognized as predicting both substance use and externalizing disorders in offspring, twin studies imply that parental alcohol use may link offspring alcohol involvement to other psychopathology via familial environmental mechanisms.
Smoking and externalizing problems also co-occur. Of considerable interest, the comorbidity between smoking, ADHD, conduct problems and other dimensions of delinquency has been partly attributed to maternal smoking during pregnancy (SDP). Multiple studies show increased rates of smoking and externalizing psychopathology in offspring of mothers with a history of SDP, however the extent to which the effects of SDP on offspring outcomes are genetic or environmental remain inconclusive. However, as SDP is a good indicator of maternal nicotine dependence and smoking persistence, it is often difficult to disentangle the independent effects of SDP on offspring smoking from familial transmission of vulnerability68. Insight into this confound may be offered by a recent study comparing related mother-offspring pairs with offspring conceived by assisted reproductive technologies, such as oocyte donations (i.e. where the mother is genetically unrelated to the offspring). This study reported that the link between ADHD and SDP was more noticeable in related pairs, thus arguing against an environmental/causal role of SDP69
Not surprisingly, genetic influences on adolescent illicit drug use have been linked to a general predisposition to impulsivity, disinhibition and correspondingly, to externalizing behaviors.70 In adult samples drug dependence shares genetic influences with antisocial personality disorder and conduct disorder – for instance, Kendler et al 71 found that 64-86% of the genetic variation in these disorders was attributable to a common underlying factor.
There have been fewer studies examining the genetic comorbidity between substance involvement and internalizing disorders. For instance, shared genes only explain a modest proportion of the covariance between smoking and depressive symptoms during adolescence,72 however, the impact of shared genetic vulnerability gains prominence during adulthood.73,74 There also appears to be a genetic link between cannabis misuse and vulnerability to major depression in adult samples. Twins who used cannabis were more likely to also report depression and suicidality compared with co-twins who never used cannabis.75 However, Fu and colleagues argue that genetic covariation between depression and cannabis abuse/dependence is attributable to comorbid antisocial personality disorder, albeit, perhaps to a greater extent in men.76
Endophenotypes
There is increasing interest in the concept of endophenotypes - defined by Gottesman and Gould77 as a measurable index of liability to a phenotype that is often assumed to be more proximal to the biological underpinnings of the behavior. Endophenotypes are not only associated with disease (or behavior) but are transmitted in families (i.e. heritable) of affected individuals and while they may co-segregate with disease, they are never a consequence of it. A wide variety of endophenotypes have been identified as indices of externalizing behavior. P300, for instance, reflects human cognitive ability to respond to ‘oddball’ stimuli. In a study of adolescent twins, reduced P300 was noted in the unaffected co-twins of twins who developed alcohol dependence during early adulthood.78 Other commonly used endophenotypes for the study of substance use (and other psychopathology) include behavioral sensitivity (for alcohol, measured using sway scores of subjective high assessment scales)79 and EEG activity (e.g. beta wave patterns)80.
Genomics
With rapid technological advances, there is increasing interest in moving from delineation of latent (aggregate) genetic influences to the identification of specific gene variants that may confer risk for the development of substance use disorders. The earliest genomic efforts utilized a linkage approach (see Figure 2 (panel A)). Studies applying this method identified regions on chromosome 3 and 9 for adolescent drug dependence vulnerability81 and for cannabis dependence symptoms. 82 The advantage of linkage analysis is that it allows for a parametric or non-parametric scan of the entire genome, which can, if well-powered, lead to discovery of novel genetic regions. However, these methods are often low resolution (a centiMorgan is equivalent to 1,000,000 bases of DNA, and linkage regions often span 10-50 cM). An alternative, hypothesis-focused technique is candidate gene analysis which characterizes a gene of putative biological importance with single nucleotide polymorphisms (SNPs) and compares the prevalence of the risk allele in those who are affected and unaffected (and can be related or unrelated, see panels B and C of Figure 2). SNPs are single base pair allelic variations that naturally occur across individuals. For instance, rs279871, is a SNP in intron 7 of the gamma-aminobutyric acid receptor A (GABRA2) gene. Dick and colleagues83 demonstrated that individuals with one or more copies of the A allele were more likely to report illicit drug dependence independent of the age of onset of drug dependence. However, an increase in risk for alcohol dependence, by genotype, was only noted in those with ages of onset of alcohol dependence in their mid-twenties. Thus, the association between this polymorphism and alcohol (but not drug) dependence appeared to be absent during adolescence. Further, in an adolescent sample, Corley and colleagues84 found several genes, including GABRA2, CNR1 and CHRNA2 to be associated with adolescent antisocial drug dependence.
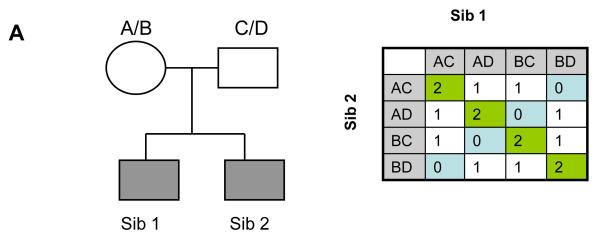
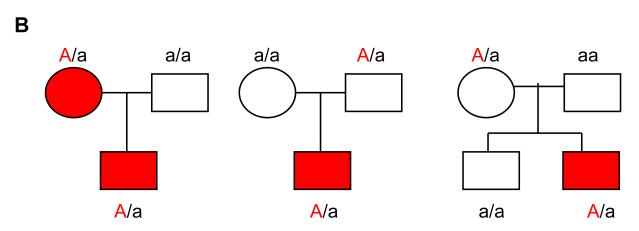
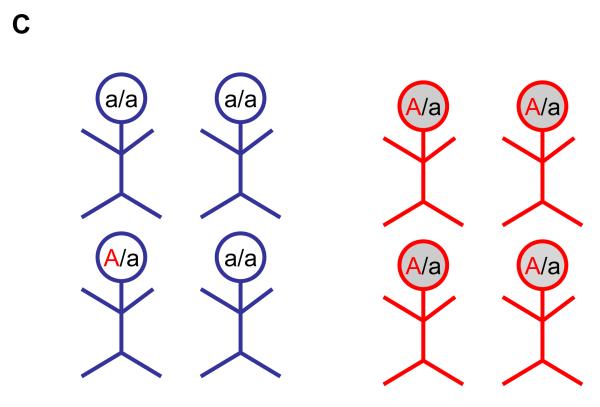
Figure 2.
Three methods for the analysis of genomic data are shown. Note: Panel A. The principle of identity-by-descent (IBD) which underlies Affected Sibling Pair (ASP) linkage analysis is shown. A genetic marker has four allelic forms (A,B,C,D) – in the pedigree, both parents are heterozygous (Mom: A/B; Dad: C/D). A pair of affected offspring (ASP, Sib 1 and 2, shaded to show affection status) are equally likely to be AC, AD, BC and BD. As shown in the table, 16 potential genotype combinations are expected in an ASP – by chance, 25%, for instance, are expected to shared both alleles IBD (IBD=2, along the diagonal). However, if a marker is close enough to a disease mutation to be transmitted more often than by chance to affected offspring, IBD=2 should exceed 25% in a pool of ASPs. This is evidence that the linkage region harbors susceptibility loci.
IBD (0)=blue cells; IBD (1)= white cells; IBD (2)= green cells
H(0): IBD (0) = 25%; IBD (1) = 50%; IBD (2) = 25%
H(A): IBD (0) < 25%; IBD (1) > 50%; IBD (2) > 25%
H(A) is evidence for linkage.
Panel B. A transmission disequilibrium test (TDT) which is the simplest form of testing for genotype-phenotype association in trios (parents and an affected offspring, shown in red) is shown. Note: A single nucleotide polymorphism with 2 alleles (A and a) where A (coded in red) confers vulnerability to disease is transmitted more often from the heterozygous (A/a) parent to the affected offspring than expected by chance alone. Extensions exist, where in addition to transmission, genotypes of siblings discordant for affection status, are also included (far left in panel B)
Panel C. The premise for an unrelated case-control association study is shown. Note: In this design that draws from a genetically homogeneous population of unrelated individuals (blue), affected individuals (shaded red circles) are assumed to be more likely to carry the risk allele (A, coded in red) than unaffected individuals (blue).
While candidate gene studies allow the investigator to focus on a specific gene with high resolution, they preclude the possibility of gene discovery. Genomewide association studies, where SNPs are used to map the entire genome with considerable density, combine the resolution of gene association studies with the exploratory capabilities of linkage. For SUDs, such studies are fairly recent and while there are, currently, no published reports in adolescent populations, several large studies of adult samples have identified possible genetic influences on smoking85, alcohol dependence86 and most recently, cannabis dependence87.
There are caveats to genomic studies of adolescents. First, twin studies suggest that genetic influences on SUDs gain prominence during early adulthood while shared environmental factors are more influential during adolescence. Second, due to the developmental course of SUDs, onsets during adolescence are uncommon, particularly in the general population. This dramatically reduces statistical power and influences the methodology for genomic studies.
The role of genes in comorbidity
While genomic findings for individual psychoactive substances are limited, the identification of genes that explain comorbidity across SUDs and with other externalizing psychopathology has shown some promise. Studies of alcohol, nicotine and drug dependence have found significant associations with SNPs in GABRA2, DRD2/ANKK1 and CNR1. Perhaps the most compelling of these, GABRA2, has also been shown to be associated with several aspects of externalizing behavior, including conduct disorder83 and impulsivity. Thus, GABRA2 may directly influence liability to behavioral undercontrol, which in turn, may increase an adolescent’s likelihood of experimenting with and sustained use of psychoactive substances.
Gene by environment interaction
Thus far, our discussion has focused on the importance of genetic and environmental factors, independent of each other. However, there has been increasing interest in and recognition of the importance of gene-environment interplay in the etiology of substance use and other psychiatric disorders.88 There are two related mechanisms by which genes work in concert with environmental exposures – gene-environment correlation and gene-environment interaction. GE correlation refers to genetic predisposition that influences our likelihood of being exposed to a certain environment. For instance, heritable influences have been found to influence deviant peer affiliations suggesting that one’s own vulnerability to substance use is partly responsible for exposure to deviant peer groups. On the other hand, gene-environment interaction refers to moderation of genetic predisposition as a consequence of environmental exposure – for example, studies of adolescent Finnish twins indicated that in less stable neighborhoods there was greater evidence of genetic influence.89 Conversely, in more supervised and restricted environments, there was less opportunity to express genetic predispositions and greater influence of environmental effects.89,90 These analyses suggest that less restrictive environments provide greater opportunities for the expression of genetic predispositions, although again it appears that these effects may be age specific: the findings described above were reported for a sample of 16-18 year olds but using a very similar approach these authors were unable to replicate findings of significant moderation of genetic effects by socioregional factors in a younger sample of 12 to 14 year old Finnish twins.91 Likewise, low levels of parental monitoring92 as well as increased affiliations with substance using peers,93 have been found to augment the importance of genetic influences of substance use.
Measured genotype x environment
As heritability can be moderated by changing environmental exposure, genotype may only influence behavior in certain environmental milieux. Some of the earliest, most influential – and arguably most controversial – work demonstrating the importance of measured gene by environment interaction was reported by Caspi et al94 who found that a functional polymorphism in the promoter region of the serotonin transporter (5-HTT/SLC6A4) gene moderated the influence of stressful life events on depression. While these results have been subject to multiple attempts at replication with mixed results (see reviews by Merikangas95 and Caspi96), they have been highly influential in alerting the field to the possibility and promise of gene by environment interactions in the etiology of psychiatric and substance use disorders. In the study of environmental moderation of genotypic risk for substance involvement, Dick et al97 recently explored the potential moderating influence of parental monitoring on the association between GABRA2 and a broad measure of externalizing behaviors in a longitudinal sample of adolescents. Although their analyses did not focus on alcohol or other drug use, their finding that the association of GABRA2 with externalizing trajectories diminished with high levels of parental monitoring, clearly parallel the findings above.
A further example of possible gene by environment interactions centers on the etiology of schizophreniform and related psychotic disorders, where cannabis use assumes the role of environmental exposure. Controversially, it has been proposed that adolescent cannabis use may increase risks of psychosis related disorders,98 although only a small percentage of those using cannabis develop psychosis. Longitudinal analyses suggest a possible explanation for this apparent contradiction: adolescent onset cannabis use was strongly associated (OR=10.9) with schizophreniform disorder among individuals characterized by the Val/ Val genotype of COMT while among individuals with one or two copies of the Met allele, adolescent onset cannabis use was not significantly associated with increased risks of schizophreniform disorder.99 While this broad pattern of findings was reported across multiple measures of “psychosis”, there have been no published replications in other general population samples. Nonetheless, parallel findings from cannabis challenge100 and experience sampling101 methodologies support the hypothesis that carriers of the Val/Val genotype may be particularly susceptible to the psychosis inducing effects of THC.
Environmental modification of gene expression
Epigenetics refers to heritable and de novo changes in gene expression that do not involve changes in DNA sequence102. Thus, individuals with the same genotype may demonstrate variations in gene expression in response to exogenous (e.g. prolonged exposure to stress) or endogenous (e.g. elevated cortisol levels in response to stress) environments. Such modification often occurs early in development103 and mechanisms inducing epigenetic modification include gene methylation, chromatin remodeling and imprinting. Does epigenetic modification contribute to the etiology of adolescent SUDs and if so, is it a cause or consequence of SUDs? A host of epidemiological studies have shown the important role of childhood traumas, parental neglect and other early adversity on elevated risk for adolescent SUDs – to what extent these environmental risk factors induce change in expression of addiction-related genes is unknown. Furthermore, such epigenetic mechanisms may be one of several pathways from drug experimentation to persistent use and dependence. What is better understood is drug-induced neuronal plasticity - for instance, repeated cocaine administration has been found to relate to modification of neuronal plasticity and increased preference for cocaine in mice104. Studies in humans are largely limited by availability of tissue with gene expression congruent with activity in the central nervous system. Epigenetic modification may be gene and tissue-specific and if so, such effects will be challenging to capture within the human paradigm.
DISCUSSION
Conclusions & Future Directions
The goal of this review was to provide an overview of the genetic epidemiology of substance involvement during adolescence. We note here that there is a distinguished body of literature surrounding the behavior genetics methods described here – a review of this105 and extensive, historical reviews of some of the issues discussed here may be found elsewhere106,107. However, results reviewed here (and elsewhere), from a substantial number of adoption, twin and extended family studies, have demonstrated moderate to strong genetic components to the liability to develop substance use disorders. One apparent limitation of this review and of the existing literature is that there have been few genetically informative research studies of use or abuse/ dependence on substances other than tobacco, alcohol and cannabis. This most likely reflects the relatively low base rate of use or abuse/ dependence on “hard” drugs such as cocaine or heroin among adolescents and the resultant reduction in statistical power for studying these outcomes in family based studies. Nonetheless, the extent to which the findings discussed above generalize to the use of other substances is unknown. Perhaps what is most needed in the studies of latent genetic influences on adolescent substance use is a refined investigation of how environmental risk and protective influences modify biological vulnerability. Of note, the role of stressful life events, such as childhood sexual or physical abuse on exacerbating genetic risk for subsequent development of substance use disorders, may have important implications.
Future directions
While rapid technological advances have made the search for specific gene variants influencing substance use disorders feasible, genomewide association studies of adolescent samples are lacking. Furthermore, the extent to which specific genes, individually or in concert with environmental factors, influence substance use at various developmental milestones or continuously, across the lifespan, need to be identified. How these genomic and environmental factors moderate efficacy of interventions related to adolescent substance use are increasingly under investigation and there is no doubt that a refined understanding of the genetic architecture of adolescent substance use and misuse will be of considerable clinical utility. To this end, one possible future avenue could involve the identification of genes whose measured effects could be incorporated into twin models to begin to ‘explain away’ the extent to which latent genetic factors influence SUDs. From a genomic perspective, the era of ‘next-generation sequencing’ has arrived108 – whether deep sequencing genomic regions of interest will reveal new polymorphisms that may have considerable impact on the etiology of adolescent SUDs remains to be seen. Finally, the extent to which prolonged exposure to psychoactive substances during adolescence leads to modifications in gene expression may be an area of considerable interest. Neuroimaging studies have already begun to investigate the effects of adolescent substance use on the adolescent brain, in some instances, taking specific genes into account as well – this area will likely witness rapid development109,110. It is hoped, with considerable optimism, that adequately powered, genetically informative studies of adolescent substance use will soon illuminate the underpinnings of the complex architecture of adolescent substance involvement and its various comorbidities.
Acknowledgments
Preparation of this manuscript was supported by grants DA18267 (ML), DA18660 (ML), DA25886 (AA), DA23668, AA17688 (AH) and AA11998(AH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Lynskey, Agrawal, and Heath report no biomedical financial interests or potential conflicts of interest.
Reference List
- (1).Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hingson RW, Assailly JP, Williams AF. Underage drinking: frequency, consequences, and interventions. Traffic Inj Prev. 2004;5:228–236. doi: 10.1080/15389580490465256. [DOI] [PubMed] [Google Scholar]
- (3).Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. J Am Acad Child Adolesc Psychiatry. 2005;44:972–986. doi: 10.1097/01.chi.0000172552.41596.6f. [DOI] [PubMed] [Google Scholar]
- (4).Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- (6).Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcohol Clin Exp Res. 1999;23:513–522. [PubMed] [Google Scholar]
- (7).Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. JAMA. 2000;284:1527–1533. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- (8).Lynskey MT, Bucholz KK, Madden PAF, Heath AC. Early onset alcohol use behaviors and subsequent alcohol-related driving risks: A twin study. Journal of Studies on Alcohol and Drugs. 2007;68:798–804. doi: 10.15288/jsad.2007.68.798. [DOI] [PubMed] [Google Scholar]
- (9).Martin CS, Winters KC. Diagnosis and assessment of alcohol use disorders among adolescents. Alcohol Health Res World. 1998;22:95–105. [PMC free article] [PubMed] [Google Scholar]
- (10).Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: a developmental framework. Pediatrics. 2008;121(Suppl 4):S235–S251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- (11).Lintonen T, Ahlstrom S, Metso L. The reliability of self-reported drinking in adolescence. Alcohol Alcohol. 2004;39:362–368. doi: 10.1093/alcalc/agh071. [DOI] [PubMed] [Google Scholar]
- (12).American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- (13).Martin CS, Kaczynski NA, Maisto SA, Bukstein OM, Moss HB. Patterns of DSM-IV alcohol abuse and dependence symptoms in adolescent drinkers. J Stud Alcohol. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- (14).Chung T, Martin CS. What were they thinking? Adolescents’ interpretations of DSM-IV alcohol dependence symptom queries and implications for diagnostic validity. Drug Alcohol Depend. 2005;80:191–200. doi: 10.1016/j.drugalcdep.2005.03.023. [DOI] [PubMed] [Google Scholar]
- (15).Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- (16).Hanna EZ, Grant BF. Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcohol Clin Exp Res. 1999;23:513–522. [PubMed] [Google Scholar]
- (17).Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. JAMA. 2000;284:1527–1533. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- (18).Lynskey MT, Bucholz KK, Madden PA, Heath AC. Early-onset alcohol-use behaviors and subsequent alcohol-related driving risks in young women: a twin study. J Stud Alcohol Drugs. 2007;68:798–804. doi: 10.15288/jsad.2007.68.798. [DOI] [PubMed] [Google Scholar]
- (19).Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. JAMA. 2000;284:1527–1533. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- (20).Hall W, Lynskey M. Is cannabis a gateway drug: Testing hypotheses about the relationship between cannabis use and use of other illicit drugs. Drug and Alcohol Review. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- (21).Cadoret RJ, Troughton E, O’Gorman TW, Heywood E. An adoption study of genetic and environmental factors in drug abuse. Arch Gen Psychiatry. 1986;43:1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- (22).Cloninger CR, Bohman M, Sigvardsson S, von Knorring AL. Psychopathology in adopted-out children of alcoholics. The Stockholm Adoption Study. Recent Dev Alcohol. 1985;3:37–51. doi: 10.1007/978-1-4615-7715-7_4. 37-51. [DOI] [PubMed] [Google Scholar]
- (23).Bouchard TJ, Jr., Lykken DT, McGue M, Segal NL, Tellegen A. Sources of human psychological differences: the Minnesota Study of Twins Reared Apart. Science. 1990;250:223–228. doi: 10.1126/science.2218526. [DOI] [PubMed] [Google Scholar]
- (24).Grove WM, Eckert ED, Heston L, Bouchard TJ, Jr., Segal N, Lykken DT. Heritability of substance abuse and antisocial behavior: a study of monozygotic twins reared apart. Biol Psychiatry. 1990;27:1293–1304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- (25).Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Kluwer Academic Publishers; Netherlands: 1992. [Google Scholar]
- (26).Kendler KS, Gardner CO., Jr. Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- (27).Heath AC, Todorov AA, Nelson EC, Madden PA, Bucholz KK, Martin NG. Gene-environment interaction effects on behavioral variation and risk of complex disorders: the example of alcoholism and other psychiatric disorders. Twin Res. 2002;5:30–37. doi: 10.1375/1369052022875. [DOI] [PubMed] [Google Scholar]
- (28).Jacob T, Waterman B, Heath A, et al. Genetic and environmental effects on offspringalcoholism: new insights using an offspring-of-twins design. Arch Gen Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- (29).Maes HH, Neale MC, Kendler KS, Martin NG, Heath AC, Eaves LJ. Genetic andCultural Transmission of Smoking Initiation: An Extended Twin Kinship Model. Behav Genet. 2006 doi: 10.1007/s10519-006-9085-4. [DOI] [PubMed] [Google Scholar]
- (30).Heath AC, Bucholz KK, Madden PA, et al. Genetic and environmental contributionsto alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- (31).Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. J Am Acad Child Adolesc Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- (32).Rende R, Slomkowski C, McCaffery J, Lloyd-Richardson EE, Niaura R. A twin-sibling study of tobacco use in adolescence: etiology of individual differences and extreme scores. Nicotine Tob Res. 2005;7:413–419. doi: 10.1080/14622200500125609. [DOI] [PubMed] [Google Scholar]
- (33).Maes HH, Sullivan PF, Bulik CM, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- (34).Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics ofsmoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- (35).McGue M, Elkins I, Iacono WG. Genetic and environmental influences onadolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- (36).Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- (37).Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- (38).Rose RJ. A developmental behavior-genetic perspective on alcoholism risk. Alcohol Health Res World. 1998;22:131–143. [PMC free article] [PubMed] [Google Scholar]
- (39).Kendler KS, Myers J, Dick D, Prescott CA. The Relationship Between GeneticInfluences on Alcohol Dependence and on Patterns of Alcohol Consumption. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Grant JD, Agrawal A, Bucholz KK, et al. Alcohol consumption indices of geneticrisk for alcohol dependence. Biol Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Fowler TK, Lifford K, Shelton K, et al. Exploring the relationship between geneticand environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behav Genet. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. Apopulation-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- (44).Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- (45).Agrawal A, Neale M, Jacobson K, Prescott CA, Kendler KS. Illicit drug use andabuse/dependence: modeling of two-stage variables using the CCC approach. Addict Behav. 2005;30:1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- (46).Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stagemodel from cannabis availability, cannabis initiation and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues andresearch strategies. J Child Psychol Psychiatry. 1991;32:1063–1080. doi: 10.1111/j.1469-7610.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- (48).Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- (49).Koopmans JR, van Doornen LJ, Boomsma DI. Association between alcohol use andsmoking in adolescent and young adult twins: a bivariate genetic analysis. Alcohol Clin Exp Res. 1997;21:537–546. [PubMed] [Google Scholar]
- (50).Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance usein adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- (51).Agrawal A, Silberg JL, Lynskey MT, Maes HH, Eaves LJ. Mechanisms underlyingthe lifetime co-occurrence of tobacco and cannabis use in adolescent and young adult twins. Drug Alcohol Depend. 2010;108:49–55. doi: 10.1016/j.drugalcdep.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Huizink AC, Levalahti E, Korhonen T, et al. Tobacco, cannabis, and other illicit druguse among finnish adolescent twins: causal relationship or correlated liabilities? J Stud Alcohol Drugs. 2010;71:5–14. doi: 10.15288/jsad.2010.71.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Neale MC, Harvey E, Maes HH, et al. Extensions to the Modeling of Initiation and Progression: Applications to Substance Use and Abuse. Behav Genet. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- (54).Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am J Med Genet. 2000;96:665–670. [PubMed] [Google Scholar]
- (55).Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- (56).Tsuang MT, Lyons MJ, Meyer JM, et al. Co-occurence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- (57).Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence 1. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- (58).Rhee SH, Hewitt JK, Young SE, et al. Comorbidity between alcohol dependence and illicit drug dependence in adolescents with antisocial behavior and matched controls. Drug Alcohol Depend. 2006 doi: 10.1016/j.drugalcdep.2005.12.003. [DOI] [PubMed] [Google Scholar]
- (59).Volk HE, Scherrer JF, Bucholz KK, et al. Evidence for specificity of transmission of alcohol and nicotine dependence in an offspring of twins design. Drug Alcohol Depend. 2007;87:225–232. doi: 10.1016/j.drugalcdep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- (60).Haber JR, Jacob T, Heath AC. Paternal alcoholism and offspring conduct disorder: evidence for the ‘common genes’ hypothesis. Twin Res Hum Genet. 2005;8:120–131. doi: 10.1375/1832427053738782. [DOI] [PubMed] [Google Scholar]
- (61).Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- (62).Slutske WS, Heath AC, Dinwiddie SH, et al. Modeling genetic and environmental influences in the etiology of conduct disorder: a study of 2,682 adult twin pairs. J Abnorm Psychol. 1997;106:266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- (63).Saraceno L, Munafo M, Heron J, Craddock N, van den Bree MB. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: a review. Addiction. 2009;104:1100–1121. doi: 10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- (64).Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin Res Hum Genet. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- (65).Knopik VS, Heath AC, Jacob T, et al. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006:1–11. doi: 10.1017/S0033291706007884. 1-11. [DOI] [PubMed] [Google Scholar]
- (66).Knopik VS, Jacob T, Haber JR, Swenson LP, Howell DN. Paternal alcoholism and offspring ADHD problems: a children of twins design. Twin Res Hum Genet. 2009;12:53–62. doi: 10.1375/twin.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin Exp Res. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- (68).Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Thapar AF, Rice F, Hay D, et al. Prenatal Smoking Might Not Cause Attention-Deficit/Hyperactivity Disorder: Evidence from a Novel Design. Biol Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- (71).Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- (72).McCaffery JM, Niaura R, Swan GE, Carmelli D. A study of depressive symptoms and smoking behavior in adult male twins from the NHLBI twin study. Nicotine Tob Res. 2003;5:77–83. doi: 10.1080/14622200307259. [DOI] [PubMed] [Google Scholar]
- (73).Avenevoli S, Steinberg L. The continuity of depression across the adolescent transition. Adv Child Dev Behav. 2001;28:139–173. doi: 10.1016/s0065-2407(02)80064-7. [DOI] [PubMed] [Google Scholar]
- (74).Johnson EO, Chase GA, Breslau N. Persistence of cigarette smoking: familial liability and the role of nicotine dependence. Addiction. 2002;97:1063–1070. doi: 10.1046/j.1360-0443.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- (75).Lynskey MT, Glowinski AL, Todorov AA, et al. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry. 2004;61:1026–1032. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- (76).Fu Q, Heath AC, Bucholz KK, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- (77).Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- (78).Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41:841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- (79).Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- (80).Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- (81).Stallings MC, Corley RP, Hewitt JK, et al. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alcohol Depend. 2003;70:295–307. doi: 10.1016/s0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- (82).Hopfer CJ, Lessem JM, Hartman CA, et al. A genome-wide scan for loci influencing adolescent cannabis dependence symptoms: Evidence for linkage on chromosomes 3 and 9. Drug Alcohol Depend. 2007;89:34–41. doi: 10.1016/j.drugalcdep.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Dick DM, Bierut LJ, Hinrichs AL, et al. The role of GABRA2 in risk for conduct disorder and alcohol and drug dependence across different developmental stages. Behav Genet. 2006;36:577–590. doi: 10.1007/s10519-005-9041-8. [DOI] [PubMed] [Google Scholar]
- (84).Corley RP, Zeiger JS, Crowley T, et al. Association of candidate genes with antisocial drug dependence in adolescents. Drug Alcohol Depend. 2008;96:90–98. doi: 10.1016/j.drugalcdep.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Treutlein J, Cichon S, Ridinger M, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Agrawal A, Lynskey M, Hinrichs A, et al. A Genomewide Association Study of DSM-IV Cannabis Dependence. Addict Biol. 2010 doi: 10.1111/j.1369-1600.2010.00255.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Moffitt TE, Caspi A, Rutter M. Strategy for Investigating Interactions Between Measured Genes and Measured Environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- (89).Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: socioregional moderation of alcohol use. J Abnorm Psychol. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- (90).Rose RJ, Dick DM, And RJ Viken, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25:637–643. [PubMed] [Google Scholar]
- (91).Dick DM, Bernard M, Aliev F, et al. The role of socioregional factors in moderating genetic influences on early adolescent behavior problems and alcohol use. Alcohol Clin Exp Res. 2009;33:1739–1748. doi: 10.1111/j.1530-0277.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Dick DM, Pagan JL, Viken R, et al. Changing environmental influences on substance use across development. Twin Res Hum Genet. 2007;10:315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Agrawal A, Balasubramanian S, Smith EK, Madden PAF, Bucholz KK, Heath AC, Lynskey MT. Peer substance involvement modifies genetic influences on regular substance involvement in young women [online ahead of print June 21, 2010] Addiction. doi: 10.1111/j.1360-0443.2010.02993.x. DOI: 10.1111/j.1360-0443.2010.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- (95).Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Dick DM, Latendresse SJ, Lansford JE, et al. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Arch Gen Psychiatry. 2009;66:649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Degenhardt L, Hall WD, Lynskey M, et al. Should burden of disease estimates include cannabis use as a risk factor for psychosis? PLoS Med. 2009;6:e1000133. doi: 10.1371/journal.pmed.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Caspi A, Moffitt TE, Cannon M, et al. Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- (100).Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. doi: 10.1038/sj.npp.1301197. [DOI] [PubMed] [Google Scholar]
- (101).Henquet C, Rosa A, Delespaul P, et al. COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of ‘switching on’ hallucinations in the flow of daily life. Acta Psychiatr Scand. 2009;119:156–160. doi: 10.1111/j.1600-0447.2008.01265.x. [DOI] [PubMed] [Google Scholar]
- (102).Renthal W, Nestler EJ. Chromatin regulation in drug addiction and depression. Dialogues Clin Neurosci. 2009;11:257–268. doi: 10.31887/DCNS.2009.11.3/wrenthal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. 182-212. [DOI] [PubMed] [Google Scholar]
- (104).Maze I, Covington HE, III, Dietz DM, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Kluwer Academic Publishers; Netherlands: 1992. [Google Scholar]
- (106).Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. Fifth Edition Worth Publishers; New York: 2008. [Google Scholar]
- (107).Kim Y-K. Handbook of Behavior Genetics. First Edition Springer Science; New York: 2009. [Google Scholar]
- (108).Avramopoulos D. Genetics of psychiatric disorders methods: molecular approaches. Psychiatr Clin North Am. 2010 March;33(1):1–13. doi: 10.1016/j.psc.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Casey BJ, Soliman F, Bath KG, Glatt CE. Imaging genetics and development: challenges and promises. Hum Brain Mapp. 2010 June;31(6):838–51. doi: 10.1002/hbm.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Casey BJ, Jones RM, Levita L, et al. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010 April;52(3):225–35. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]