Abstract
Objectives
To develop, adapt, and ensure feasibility, acceptability, and safety of the Family/Adolescent-Centered (FACE) Advance Care Planning intervention.
Patients and Methods
Two-group, randomized, controlled trial in two hospital-based outpatient clinics in Washington, D.C. and Memphis, Tennessee, from 2006 to 2008 was conducted. Participants (n = 38 dyads) included medically stable adolescents aged 14 to 21 years with HIV/AIDS and surrogates/families over age 20. Three 60- to 90-minute sessions were conducted via a semistructured family interview with a trained/certified interviewer. Intervention received: (1) Lyon Advance Care Planning Survey; (2) Respecting Choices interview; and (3) Five Wishes. Control received (1) Developmental History, (2) Health Tips, and (3) Future Plans. Feasibility was measured by percent enrollment, attendance, retention, and completeness of data. Acceptability and safety were measured by Satisfaction Questionnaire, using longitudinal regression analysis.
Results
Adolescents' mean age was 16 years; 40% were males; 92% were black; HIV transmission rate was 68% perinatal and 32% sexually acquired; 42% were asymptomatic; 29% were symptomatic; and 29% had a diagnosis of AIDS. Intervention adolescents were more likely to rate sessions positively (p = 0.002) and less likely to rate sessions negatively (p = 0.011) than controls. Guardians/surrogates were more likely to rate the sessions positively (p = 0.041) and demonstrated no difference in rating sessions negatively (p = 0.779) than controls.
Conclusions
Existing advance care planning models can be adapted for age, disease, and culture. Adolescents with HIV/AIDS were satisfied with an advance care planning approach that facilitated discussion about their end-of-life wishes with their families. Families acknowledged a life-threatening condition and were willing to initiate end-of-life conversations when their adolescents were medically stable.
Introduction
The American Academy of Pediatrics (AAP),1 the Institute of Medicine (IOM),2 and hospital-based policies3 recommend adolescents be included in end-of-life (EOL) discussions and that: (1) palliative care discussions take place when the patient is medically stable; (2) decisions be individualized; (3) decisions be shared among the adolescent, family and physician; and (4) advance care planning (ACP) become a routine, structured intervention in health care settings. Chronically ill adolescents also have expressed desire to be included in EOL conversations, and their families have asked for help.3,4 Despite these recommendations and reports, in practice, goals for shared decision-making with adolescents often fail.5–7 There is no existing model that includes adolescents in discussions about their EOL care and recognizes their capacity to provide informed consent/assent for medical treatment.8
Acceptability and feasibility of such a program will be greater when it is informed by a research base that includes the intended consumers.9,10 and when it addresses the core characteristics of effective evidence-based programs for all adolescents,11 as well as those at risk for12 or living with HIV.13–16 Evidence indicates that effective programs promote moral values, foster the development of relationships, and focus on the future by applying learning in real-life settings within the context of developing a social identity, and offer a sense of empowerment and self-care.
The Family/Adolescent Centered (FACE) Advance Care Planning intervention was based on the integration of evidence-based approaches presented in current and relevant literature. FACE was designed to provide a structured, family-centered, HIV-specific, and culturally sensitive approach to facilitating discussions about EOL care for adolescents and their families. It focused on specific essential elements of effective programs, which were theoretically grounded, structured, accurate, and targeted several systems simultaneously. 9–16 This paper describes the community-based participatory research that informed the adaptation of the FACE intervention and presents the feasibility and acceptability outcomes as measured by enrollment, attendance, retention, satisfaction, tolerability, and positive and negative emotions
Methods
Phase I: Program development
The adaptation and development of the FACE intervention began by using an iterative process to review two existing surveys and one curriculum. To ensure cultural sensitivity to our population of primarily African American HIV-positive youth, participants in Phase I were from this population but did not participate in Phase II.
Focus group: Adolescents want to be involved in shared decision-making
A focus group involving five patients with AIDS, aged 19–22 years, was convened to assess their desired level of involvement in their EOL decision-making.4 Participants reported that they want life-affirming activities, while the quality of their life is still good. They wanted sensitive primary care providers who are comfortable with discussing EOL issues in a private visit.
Survey
Based on the focus group responses, literature review, and interviews with doctors a survey was developed to find out what adolescents want with respect to involvement in their own EOL care.4 Adolescents reported they did not talk to either parents or doctors about their preferences, for fear of upsetting them. Adolescents preferred to have EOL conversations earlier in the course of a life-threatening illness.
Focus group with families about program development
Family members were recruited from a support group for bereaved parents, which was an outgrowth of a family-based adherence group.17 They met for a one-time, 2-hour audiotaped focus group to determine if they would have been interested in having a discussion with their adolescent about their EOL care. Families felt the strain of trying to talk to their adolescents about advance care planning and were afraid everyone would panic, “because you don't know which way to go and when to talk about it.” Families felt parent and adolescent facilitated communication to help with EOL decision-making would be beneficial.
Families felt that their adolescent's preferences for EOL care should be respected. One grandparent related that she had to endure medical interventions for her grandchild over her objections (e.g., tube feeding). Two caregivers revealed after experiencing the death of their child or grandchild that they prepared living wills for themselves and gave a trusted person power of attorney in the event they could not make their own medical decisions.
Families thought talking about HIV would be less stressful in a structured program, because they would get a lot of support. “It's hard to talk about and sometimes you find young people who might want to talk about it; some might not, but it's always right there in the back of their heads.” Families felt that their children would not want to burden them by telling them what they would want and not want with regard to EOL care. For those families whose children told them what they wanted, they found it helpful, albeit painful.
Critical review of revised program protocol by HIV-positive youth
In July 2004, the Adolescent Trials Network (ATN)/National Institutes of Child and Human Development agreed to review the revised protocol. The preexisting 15-member youth Community Advisory Board (CAB), aged 16 to 26, reviewed the proposed FACE protocol, discussed issues, and offered feedback.
Critical review by expert panel
In 2006, key local and national leaders in adolescent development and HIV/AIDS were consulted in a 1-day, face-to-face meeting to finalize the intervention. The panel included leaders from the religious community; a psychologist and person living with HIV/AIDS; a chaplain at the children's hospital; an adolescent medicine specialist and a leader from the American Academy of Pediatrics who was the Chair of the Section of Bioethics; an international leader from the Society for Research in Child Development; a lead developer of the Respecting Choices® interview; and an adolescent medicine physician specialist in the care of youth with HIV.
Beta-testing of the FACE curriculum
Finally, we conducted FACE intervention sessions with three volunteer dyads from the local CAB, which were audiotaped, transcribed, then reviewed by the investigators. Feedback from the volunteers was integrated into the study protocol, resulting in more refined guidelines for training and the research design and three-session FACE intervention described in Table 1.
Table 1.
Description of Family Centered Advance Care Planning (FACE) Intervention
| Session 1—Foundation | Session 1—Goals | Session 1—Process |
|---|---|---|
| Lyon Family Centered Advance Care Planning (ACP) Survey–Adolescent & Surrogate Versions©: Survey of Attitudes, Influences and Preferences. This three-part, 31-question survey engages the participant in EOL questions referring to general illness and not one specific illness. | 1. To assess the adolescents' and surrogates' values, beliefs, and life experiences with illness and EOL care; 2. To assess when adolescents and their surrogates prefer to initiate EOL discussion and planning. |
1. Trained/certified interviewer orients the family to the study and to the issues, providing information, such as the right to change your mind or the right of patients who decide to forgo life-sustaining treatments to be offered other important treatments and to not be abandoned; 2. Adolescent is surveyed separately from the guardian/surrogate in a private room; 3. Guardian/surrogate is surveyed privately with regard to what they believe their adolescent prefers and asking about what they believe influences their adolescent's thinking and attitudes about EOL care; 4. Interviewer invites both the guardian/ surrogate and adolescent into the private office and highlights the similarities in their responses, building cohesion. 5. Interviewer informs the family that participation is not based on their stage of illness, offering hope and states studies show patients are comforted by such discussions. |
| Respecting Choices® Family Centered ACP Interview | 1. To facilitate conversations and shared decision making between the adolescent and guardian/ surrogate about ACP, providing an opportunity to express fears, values, beliefs and goals with regard to death and dying; 2. To prepare the guardian/surrogate to be able to fully represent the adolescent's wishes. |
Stage 1 assesses the adolescent's understanding of his or her current medical condition, prognosis, and potential complications, as well as his or her fears, concerns, hopes and experiences. Stage 2 explores the philosophy the adolescent might have regarding planning for future medical decision-making and their understanding of the facts. Stage 3 briefly reviews the rationale for future medical decisions the adolescent would want the legal guardian or chosen surrogate to understand and act on. Stage 4 uses the Statement of Treatment Preferences survey to describe real clinical situations common to HIV that the adolescent could experience and related treatment choices that the surrogate might make. Stage 5 summarizes the value of the previous discussion, as well as the need for future discussions as situations and preferences change. Remaining questions or gaps in information regarding health condition/care/ treatment options are identified and the family is referred to the physician or resources. |
| The Five Wishes© is a legal document that helps a person express how they want to be treated if they are seriously ill and unable to speak for him/herself. It is unique among all other living will and health agent forms because it looks to all of a person's needs: medical, personal, emotional, and spiritual. | To let the family and doctors know: 1. Which person the teen wants to make health care decisions for him/her, if unable to make them; 2. The kind of medical treatment the teen wants or does not want; 3. How comfortable teen wants to be; 4. How teen wants people to treat him/her. 5. What teen wants loved ones to know. |
For adolescents under the age of 18 the Five Wishes© must be signed by their parent or legal guardian to be legally sufficient. The FACE intervention involves the invitation to include other family members and loved ones, inviting them to listen to the conversation that emerges between the adolescent and his/her guardian or surrogate. Processes, such as labeling feelings and concerns, as well as finding solutions to any identified problem, are facilitated. Appropriate referrals are made to help resolve conflicts over decision making (e.g., a hospital ethicist or their doctor) or spiritual issues (e.g., a hospital chaplain or their clergy). |
Three 60- to 90-minute sessions, scheduled 1 week apart with a 1-month window between sessions.
EOL, end of life; ACP, advance care planning; HIV, human immunodeficiency virus.
FACE Intervention
Session 1
The Lyon Family Centered Advance Care Planning Survey© was developed by including elements from an approved adaptation of the AARP18 survey about factors influencing EOL decision-making. Also included are items from the earlier version of the adolescent survey designed to assess if adolescents wish to participate in shared decision making about EOL care, and if so when and with whom.4
Session 2
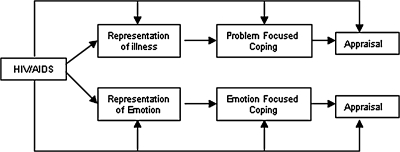
The Respecting Choices® Family Centered-HIV Specific ACP Interview®, was adapted from the adult version of this disease-specific patient-centered ACP interview developed by Briggs and Hammes for patients with end-stage chronic illness.19–22 The Respecting Choices interview consists of ACP facilitation skills embedded in the nationally recognized ACP facilitator curriculum19 with a decision aide tool (the Statement of Treatment Preference form) to assist in clarifying goals for life-sustaining treatment. Specific interview questions are based on Leventhal's self-regulation model (SRM),23–25 which is widely used to study health behaviors in a range of chronic diseases26 (Fig. 1). Leventhal posits an illness representation is a set of thoughts (whether medically accurate or not) that a person holds about a health problem based on five dimensions: identity, cause, time line, consequences, and cure/control.23
FIG. 1.
Model of Leventhal's theory of self-regulation.
Only minor wording changes were necessary to create the FACE interview version used for this study.
Session 3
The Five Wishes©27 is an advance directive that helps individuals document medical decisions if they are seriously ill or unable to speak for themselves. The Five Wishes was used with adolescents as a tool for documenting their preferences for EOL medical decisions with their family.
Phase II: Pilot study/feasibility/acceptability
Phase II was conducted from 2007–2008 in two hospital-based outpatient adolescent HIV-specialty clinics in Washington, D.C. and Memphis, Tennessee. The adapted and developed curriculum and measures were reviewed and Institutional Review Board (IRB) approved, respectively, at each site and assent/consent was obtained per institutional guidelines. Inclusion criteria for adolescents were: HIV-positive, aged 14–21, and knew own HIV status. Surrogates were the legal guardian for those under age 18 or chosen surrogate (21 years or older) for those aged 18 and older. Exclusion criteria were severe depression, in foster care, severe developmental delays, psychosis, and dementia. Additional family members could participate but their data were not used in the analysis.
Facilitators were certified through the Respecting Choices competency criteria based on a submitted video demonstrating fidelity in implementing Session 2, the Respecting Choices® Family Centered-HIV Specific ACP Interview. To ensure subsequent protocol implementation, standardization, and fidelity, the principal investigator monitored audio/videotapes of Session 2. Training also was provided for the highly structured Sessions 1 and 3, but formal competency criteria were not established as the administration was straightforward. Also, by Session 3, time to complete the Five Wishes advance directive, the families were well prepared as a result of Session 2.
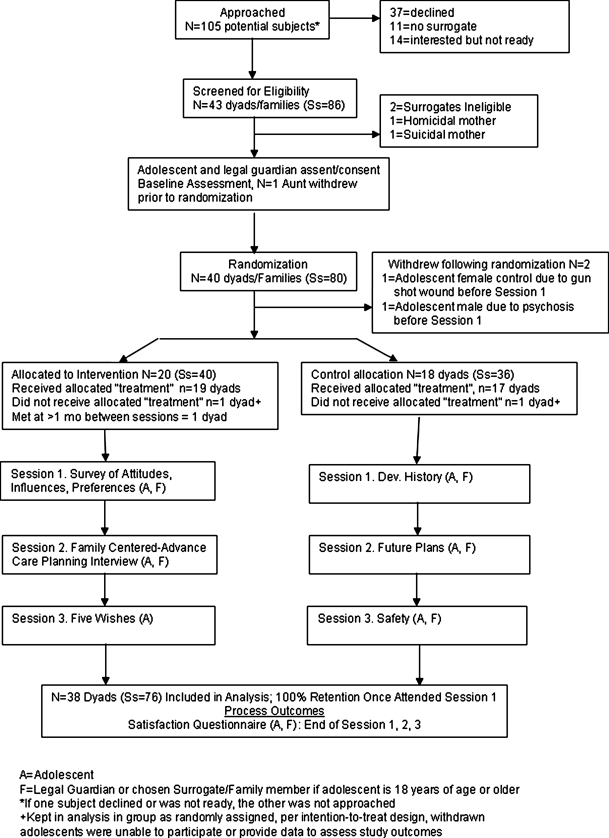
The pilot study was implemented as a randomized controlled clinical trial whereby participants were computer randomized to either the FACE intervention (Fig. 2) or the Healthy Living control condition, using an intention-to-treat design. The developed FACE intervention and the Healthy Living control included three 60- to 90-minute adapted and standardized sessions. Sessions were scheduled 1 week apart and were followed immediately by a 30-minute postsession feedback assessment conducted by a trained research assistant, not the session facilitators.
FIG. 2.
Protocol schema.
The Healthy Living control condition also has three 60- to 90-minute sessions. To control for the Hawthorn effect, the purpose of the control condition was to control for time and attention. The control group was exposed to the following: Session 1 required the adolescent and family complete a developmental history (Barkley) survey28; Session 2 consisted of a discussion on health and safety, such as wearing a helmet, exercise (Bright Futures) 29; and Session 3 consisted of a school and career planning interview (Adolescent Employment Readiness Center).30
Each of the three intervention and three control sessions were immediately followed by a Satisfaction Questionnaire administered by a research assistant.
Measures
Demographic data, enrollment, attendance, retention, and completeness of data were gathered through medical chart review and interviews with participants. The Satisfaction Questionnaire was developed during Phase I based on the Science in a Fishbowl workshop at the 2003 National Institutes of Health/National Institutes of Mental Health (NIH/NIMH) Annual Conference on Families Affected and Infected with HIV/AIDS in Washington, D.C. The principal investigator and two colleagues presented the concept, surrounded in the first circle by community participants and in the outer circle by research scientists.
Brainstorming the pros and cons of the protocol concept and CAB feedback yielded 13 items (See Appendix A) of the Satisfaction Questionnaire, which was designed to assess acceptability of the intervention. The Satisfaction Questionnaire poses statements such as “it was useful,” “it was helpful,” “I was satisfied,” and “it was worthwhile,” etc. Responses were on a five-point Likert scale from strongly disagree to strongly agree. Participants' report of their satisfaction with the sessions, whether it was worthwhile, helpful, or useful, directly contributed to the assessment of the intervention's acceptability. The majority of items measured emotional reactions to each session, as a primary concern of the scientists and community participants was that talking about death and dying might be unsafe, i.e., result in emotional distress.
Data analysis
Statistical analyses were done by Stata 10.0 (StataCorp, College Station, TX). Frequency distributions characterized demographic data, percent enrollment, attendance, retention, and completeness of data. Longitudinal regression models evaluated changes over time in safety outcomes, operationally defined as positive or negative emotions immediately after Sessions 1, 2, and 3. Each model included study group to compare the effect of the intervention and control status and as necessary the interaction of group by time in order to take account of any time differences in effects of intervention. Results were considered statistically significant if the two-tailed α (type I error) levels was less than 0.05.
Results
Phase II pilot study
Of 105 potentially eligible subjects, 37 (35%) declined to participate (Fig. 2). An additional 14 (13%) were interested but not ready and 11 (10%) interested adolescents aged 18 or over could not identify a surrogate and were thereby ineligible. Eighty-six potential subjects (43 dyads) were screened for eligibility, of whom 2 surrogates (2 dyads) were ineligible. One family withdrew from the study after the baseline assessment but before randomization. The remaining 80 subjects (40 dyads) were randomized, meeting target enrollment based on preliminary power analysis. Prior to Session 1, one adolescent became psychotic resulting in withdrawal; another was shot in the community, was incapacitated, and withdrew. Thus, 40 intervention subjects (20 dyads) and 36 control subjects (18 dyads) resulted in a total of 76 study participants. One intervention dyad and one control dyad did not receive the allocated condition due to misinterpretation of the assignment based on the information provided postrandomization. (The problem was corrected). Consistent with the intent-to-treat design, data were kept in the analysis, as if each received the correct assignment. Errors resulted from confusion in the computer screen layout after randomization that, once discovered, was corrected. Adolescents' baseline characteristics in each condition are provided in Table 2, demonstrating successful randomization.
Table 2.
Baseline Characteristics for Intervention and Control Adolescents with HIV/AIDS (n = 38) Testing for Effects of Randomization
| Intervention n = 20 | Controls n = 18 | p value | |
|---|---|---|---|
| Age (in years) | p = 0.838 NSa | ||
| Mean (SD) | 16.65 (2.11) | 16.58 (2.38) | |
| Range | 14–21 | 14–20 | |
| Gender | p = 1.0 NSb | ||
| Males | 8 (40%) | 7 (39%) | |
| Females | 12 (60%) | 11 (61%) | |
| Transgender (M > F) | 0 (0%) | 0 (0%) | |
| Race/Ethnicity | p = 1.0 NSb | ||
| Black/African American | 17 (94%) | 18 (90%) | |
| White/Caucasian | 1 (6%) | 1 (5%) | |
| American Indian/Alaskan | 0 (0%) | 1 (5%) | |
| Mode of HIV Transmission | p = 0.489 NSb | ||
| Perinatal infection | 15 (75%) | 11 (61%) | |
| Behavioral infection | 5 (25%) | 7 (39%) | |
| CDC Classification | p = 0.061 NSb | ||
| A 1-3 (asymptomatic) | 5 (26%) | 11 (61%) | |
| B 1-3 (symptomatic) | 6 (32%) | 5 (28%) | |
| C 1-3c (AIDS) | 8 (42%) | 2 (11%) | |
| Education | p = 0.673 NSb | ||
| No high school diploma/in high school | 12 (60%) | 10 (56%) | |
| HS or GED equivalent | 4 (20%) | 2 (11%) | |
| Some college/no bachelors | 4 (20%) | 2 (11%) | |
| Income | p = 0.805 NSb | ||
| ≤ federal poverty line | 7 (35%) | 6 (33%) | |
| 100%–200% of federal poverty line | 1 (5%) | 3 (17%) | |
| 201%–300% of federal poverty line | 4 (20%) | 4 (22%) | |
| > 300 of federal poverty line | 6 (30%) | 3 (17%) | |
| Unknown | 2 (10%) | 2 (11%) | |
| Housing Status | p = 1.00 NSb | ||
| Permanently housed | 18 (90%) | 17 (94%) | |
| Unstable living arrangement | 2 (10%) | 1 (6%) | |
| Sexual orientation | p = 0.893 NSb | ||
| Heterosexual | 17 (85%) | 15 (83%) | |
| Homosexual | 1 (5%) | 1 (5%) | |
| Bisexual | 2 (10%) | 1 (5%) | |
| Don't know | 0 (0%) | 1 (5%) | |
| Marital Status | p = 1.0 NSb | ||
| Single | 19 (95%) | 17 (94%) | |
| Married/living together | 1 (5%) | 1 (5%) |
t test.
Fisher's exact test. No statistically significant difference between groups, showing success of randomization.
No patient had category C1.
Centers for Disease Control and Prevention: 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 1992;41:1–17.
NS, no statistically significant difference; SD, standard deviation.
Table 3 compares projected and actual outcomes. The study was able to enroll 98% of eligibles, achieve 100% compliance (attendance at all 3 sessions), 95% retention of enrollees, and 100% data completion rates, and achieve 92% satisfaction, all of which exceeded the projected cutpoints of 50%, 80%, 85%, 90%, and 90%, respectively.
Table 3.
Feasibility and Acceptability Criteria of Family Centered Advance Care Planning (FACE) Intervention (n = 76; 38 dyads/families): A Comparison of Projected and Actual Outcomes
| Criteria | Projected outcomes | Actual |
|---|---|---|
| Enrollment of eligible families | >50% | 98% |
| 40/41 | ||
| Attendance at all three sessions | >80% | 93% |
| 38/41 | ||
| Retention | >85% | 93% |
| 38/41 | ||
| Completeness of data satisfaction—Question 6 of Satisfaction Questionnaire “I felt satisfied” (5-point Likert scale from strongly disagree to strongly agree) Percentage reporting agree or strongly agree | >90% | 100% |
| >90% | 92% |
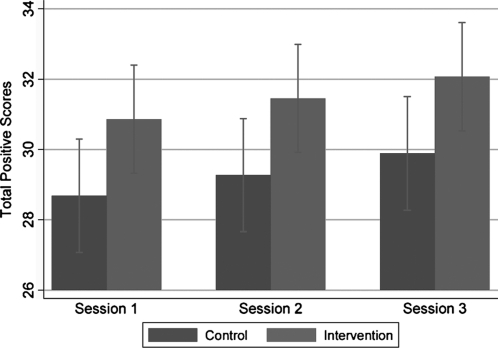
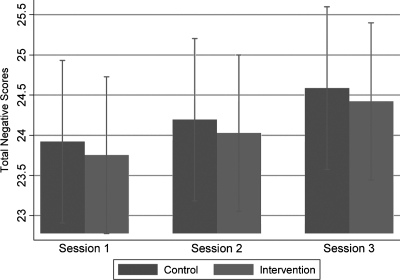
Figures 3 and 4 and 5 and 6 evaluate and compare the Satisfaction Questionnaire scores immediately after Sessions 1, 2, and 3 between the intervention and control group for guardians/surrogates and adolescents, respectively. Figure 3 illustrates intervention guardians/surrogates were more likely than controls to rate all the sessions positively (p = 0.041). Guardians/surrogates in both the intervention and control conditions rated Session 3 higher in total positive score compared to Session 1 (p = 0.012). There was no evidence that the differential between the intervention and controls differed by time (p = 0.215). Figure 4 found little evidence of a difference in overall ratings of negative emotion between guardians/surrogates in the intervention and control group (p = 0.779), nor evidence of such a difference between sessions (p = 0.175).
FIG. 3.
Comparison of results regarding positive emotions on Satisfaction Questionnaire Scores in guardians/surrogates after Sessions 1, 2, and 3.
FIG. 4.
Comparison of results regarding negative emotions on Satisfaction Questionnaire Scores in guardians/surrogates after Sessions 1, 2, and 3.
FIG. 5.
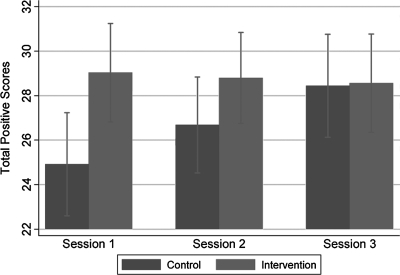
Comparison of results regarding positive emotions on Satisfaction Questionnaire Scores in adolescents after Sessions 1, 2, and 3.
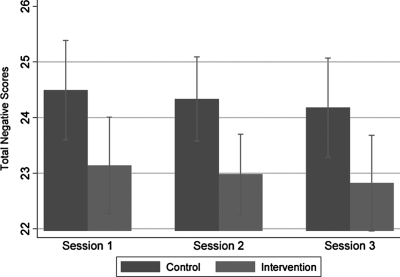
FIG. 6.
Comparison of results regarding negative emotions on Satisfaction Questionnaire Scores in adolescents after Sessions 1, 2, and 3.
Figure 5 illustrates that intervention adolescents were significantly more likely than controls to rate sessions positively in Sessions 1 and 2 but not Session 3 as indicated by a statistically significant interaction between Session and condition (p = 0.001). Figure 6 illustrates that adolescents in the intervention reported lower total negative scores overall than the control group (p = 0.011). There was no evidence of an interaction between Session and condition (p = 0.517), i.e., negative ratings were consistently low across sessions. No safety measures, such as an additional Problem Solving Session, referral to psychotherapist, ethicist, or chaplain were needed.
Discussion
The FACE intervention is the first structured and individualized model for adolescents living with HIV to meet AAP1 and IOm2 guidelines as well as clinical recommendations.31–36 FACE was conducted when the patient was medically stable; respected individual differences, acknowledging those who prefer to have their doctor or family make these decisions for them, or who are interested but not ready; promoted shared decision-making; and was conducted by certified/trained facilitators utilizing a structured curriculum.
A contribution of this study is the involvement of the targeted consumers, adolescents with HIV/AIDS and their families, at an informative stage prior to intervention development. This process of community consultation, combined with attending to core characteristics of successful interventions, yielded remarkably high rates of retention, overcoming previously noted barriers to palliative care research.37
FACE demonstrated feasibility, enrolling the targeted sample size and yielding a high rate of satisfaction for African American families, who generally have been underrepresented in EOL research (NIH State of the Science Conference Statement on Improving End-of-Life Care, December 6–8, 2004). The adaptation of the sessions to be culturally sensitive through a process of community review38–43 was successful. African Americans historically have experienced discrimination by health care institutions44 and may interpret discussion of do-not-resuscitate (DNR) orders as euthanasia or an attempt to deny beneficial care,45 which may account for the low levels of hospice care or use of DNRs among the 41 children who died during Pediatric AIDS Clinical Trials.46 By involving consumers, key stakeholders, and experts, FACE overcame the barriers that have been identified in adult African Americans who want more life-sustaining treatments, yet are less likely to have discussed life-sustaining treatments with their physicians and families.18,47
Communication and decision making are key factors affecting dying children and their families.48 Research in this area has been inhibited by the anxiety-provoking and sad nature of the topic.49 Consistent with previous research, the results of this study demonstrate the positive contribution of conversations about EOL care, providing a model for therapeutic benefit to families who want help with ACP.50,51
Contrary to our hypotheses, controls had higher negative ratings than intervention adolescents. Qualitative feedback suggests conflict emerged for controls, e.g., one youth revealed he had a housekeeper while attending college; his mother objected. Another did not realize how much education becoming an architect required.
This study had limitations. Focus group results were not formally analyzed. Only those families ready to discuss ACP agreed to participate potentially creating selection bias; however, our intention was to respect families' choices by working only with those who expressed interest and readiness. Sustainability of FACE is unknown, as is the value of these earlier conversations for respecting choices when dying. FACE took place in clinics that serve a predominantly African American population residing in urban areas and may not generalize. We do not know anything about families who declined participation or those who could not identify a surrogate. Research subjects, interviewers, and research assistants were not blinded to condition, potentially introducing bias.
As with adult studies,18–22 future research should explore the adaptation of the FACE intervention with adolescents suffering from other life-limiting conditions and from other cultural groups. The World Health Organization52 advocates palliative care as an essential component of HIV from the point of diagnosis to the end of life and into bereavement. Our findings provide support for the continued development of an ongoing process, initiated early and maintained throughout an illness, to help adolescents and their families face EOL decision-making together.
Appendix
Acknowledgments
This study was funded by grant 5R34MH072541-03 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
Abstract with preliminary data was presented at the Society of Adolescent Medicine Annual conference, 2007.
The authors would like to thank the families who participated in this study who generously gave of their time and the research assistants who carefully implemented the protocol with fidelity. A special thanks to Ellin Kao, M.P.H. who also helped with the preparation of this manuscript.
Author Disclosure Statement
Linda Briggs receives royalties from selected Respecting Choices educational materials.
References
- 1.American Academy of Pediatrics. Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics. 2000;106:351–357. [PubMed] [Google Scholar]
- 2.Field MJ, editor; Behrman RE, editor. When Children Die: Improving Palliative and End-of-Life Care for Children and their Families. Washington, D.C.: Institute of Medicine, National Academy Press; 2002. [PubMed] [Google Scholar]
- 3.Hinds PS.Drew D.Oakes LL.Fouladi M.Spunt SL.Church C.Furman WL.End-of-life care preferences of pediatric patients with cancer J Clin Oncol 2005239055–9057.16314610 [Google Scholar]
- 4.Lyon ME. McCabe MA. Patel K. D'Angelo LJ. What do adolescents want? An exploratory study regarding end-of-life decision-making. http://journals.elsevierhealth.com/periodicals/jah. [Feb 11;2009 ];J Adolesc Health. 2004 35:529. doi: 10.1016/j.jadohealth.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Walsh-Kelly CM. Lang KR. Chevako J. Blank EL. Korom N. Kirk K. Gray A. Advance directives in a pediatric emergency department. Pediatrics. 1999;103:826–830. doi: 10.1542/peds.103.4.826. [DOI] [PubMed] [Google Scholar]
- 6.Solomon MZ. Sellers DE. Heller KS. Dokken DL. Levetown M. Rushton C. Truog RD. Fleischman AR. New and lingering controversies in pediatric end-of-life care. Pediatrics. 2005;116:872–883. doi: 10.1542/peds.2004-0905. [DOI] [PubMed] [Google Scholar]
- 7.Hinds PS. Burghen EA. Pritchard M. Conducting end-of-life studies in pediatric oncology. West J Nurs Res. 2007;29:448–465. doi: 10.1177/0193945906295533. [DOI] [PubMed] [Google Scholar]
- 8.Weithorn LA. Campbell SB. The competency of children, adolescents to make informed treatment decisions. Child Dev. 1982;53:1589–1598. [PubMed] [Google Scholar]
- 9.Rich M. Ginsburg KR. The reason and rhyme of qualitative research: Why, when, and how to use qualitative methods in the study of adolescent health. J Adolesc Health. 1999;25:371–378. doi: 10.1016/s1054-139x(99)00068-3. [DOI] [PubMed] [Google Scholar]
- 10.Evans C. Lambert H. Implementing community interventions for HIV prevention: Insights from project ethnography. Soc Sci Med. 2008;66:467–478. doi: 10.1016/j.socscimed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Evans DL, editor; Foa EB, editor; Gur RE, editor; Hendin H, editor; O'Biren CP, editor; Seligman ME, editor; Walsh T, editor. Treating, Preventing Adolescent Mental Health Disorders: What we know, What We Don't Know: A Research Agenda for Improving the Mental Health of Our Youth. New York: Oxford University Press; 2005. [Google Scholar]
- 12.DiClemente RJ. Crosby RA. Preventing HIV infections in adolescents: What works for uninfected teens. In: Lyon ME, editor; D'Angelo LE, editor. Teenagers HIV and AIDS: Insights from Youths Living with the Virus. Westport, CT: Praeger Publishers; 2006. pp. 143–161. [Google Scholar]
- 13.Galbraith JS. Stanton B. Boekeloo B. King W. Desmond S. Howard D. Black MM. Carey JW. Exploring implementation and fidelity of evidence-based behavioral interventions for HIV prevention: Lessons learned from the focus on kids diffusion case study. Health Educ Behav. 2008;0 doi: 10.1177/1090198108315366. 1090198108315366v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram BL. Flannery D. Elkavich A. Rotheram-Borus MJ. Common processes in evidence-based adolescent HIV prevention. AIDS Behav. 2008;12:374–383. doi: 10.1007/s10461-008-9369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malow RM. Kershaw T. Sipsma H. Rosenberg R. Devieux JG. HIV preventive interventions for adolescents: A look back and ahead. Curr HIV/AIDS Rep. 2007;4:173–180. doi: 10.1007/s11904-007-0025-6. [DOI] [PubMed] [Google Scholar]
- 16.Tevendale HD. Lightfoot M. Programs that work: Prevention for positives. In: Lyon ME, editor; D'Angelo LJ, editor. Teenagers HIV and AIDS: Insights from Youths Living with the Virus. Westport, CT: Praeger Publishers; 2006. pp. 105–126. [Google Scholar]
- 17.Lyon M. Trexler C. Akpan-Townsend C. Pao M. Selden K. Fletcher J. Addlestone IC. D'Angelo LJ. A family group approach to increasing adherence to therapy in HIV infected youth: Results of a pilot project. AIDS Patient Care STDs. 2003;17:299–308. doi: 10.1089/108729103322108175. [DOI] [PubMed] [Google Scholar]
- 18.AARP. Report prepared by Rachelle Cummins MA. AARP North Carolina End of Life Care Survey: African American Members. 2003. hhtp://research.aarp.org. [Feb 11;2009 ]. hhtp://research.aarp.org
- 19.Hammes BJ. Briggs L. Respecting Choices®: Advance Care Planning Facilitator Manual—Revised. La Crosse, WI: Gundersen Lutheran Medical Foundation; 2007. [Google Scholar]
- 20.Romer AL. Hammes BJ. Communication, trust, and making choices: Advance care planning four years on. J Palliat Med. 2004;7:335–340. doi: 10.1089/109662104773709495. [DOI] [PubMed] [Google Scholar]
- 21.Briggs LA. Kirchhoff KT. Hammes BJ. Song MK. Colvin ER. Patient-centered advance care planning in special patient populations: A pilot study. J Prof Nurs. 2004;20:47–58. doi: 10.1016/j.profnurs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Song MK. Kirchhoff KT. Douglas J. Ward S. Hammes B. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Med Care. 2005;43:1049–1053. doi: 10.1097/01.mlr.0000178192.10283.b4. [DOI] [PubMed] [Google Scholar]
- 23.Leventhal HH. Nerenz DR. Steele DJ. Illness representations and coping with health threats. In: Baum A, editor; Taylor SE, editor; Singer JE, editor. Handbook of Psychology and Health, Vol. IV: Social Psychological Aspects of Health. New Jersey: Lawrence Erlbaum Associates, Inc; 984. pp. 219–252. [Google Scholar]
- 24.Leventhal H. Diefenbach M. The Active Side of Illness Cognition. In: Skelton JA, editor; Croyle RT, editor. Mental Representation in Health and Illness. New York: Springer Verlag; 1991. pp. 247–272. [Google Scholar]
- 25.Leventhal H. Benyamini Y. Shafer C. Lay beliefs about health and illness. In: Ayers S, editor. Cambridge Handbook of Psychology, Health and Medicine. Cambridge: Cambridge University Press; 2007. pp. 124–128. [Google Scholar]
- 26.French DP. Weinman J. Current issues and new directions in psychology and health: “Assessing illness perceptions: Beyond the IPQ.”. Psychol Health. 2008;23:5–9. doi: 10.1080/08870440701616714. [DOI] [PubMed] [Google Scholar]
- 27.Towey J. Aging with Dignity a service program developed through a grant from The Robert Wood Johnson Foundation. The Five Wishes. 1997. www.agingwithdignity.org/ [Feb 11;2009 ]. www.agingwithdignity.org/
- 28.Barkley RA. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. New York: The Guilford Press; 1993. [Google Scholar]
- 29.American Academy of Pediatrics: Bright Futures. http://brightfutures.aap.org/web/healthCareProfessionalstoolsAndResources.asp. [Jan;2005 ]. http://brightfutures.aap.org/web/healthCareProfessionalstoolsAndResources.asp
- 30.Wolf-Branigin M. Schuyler V. White P. Improving quality of life and career attitudes of youth with disabilities: Experiences from the Adolescent Employment Readiness Center. Research on Social Work Practice. 2007;17:324–334. [Google Scholar]
- 31.McCabe MA. Rushton CH. Glover J. Murray MG. Leikin S. Implications of the Patient Self-Determination Act: Guidelines for involving adolescent in medial decision making. J Adolesc Health. 1996;19:319–324. doi: 10.1016/S1054-139X(96)00160-7. [DOI] [PubMed] [Google Scholar]
- 32.Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. JAMA. 2005;294:359–365. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 33.Contro N. Larson J. Scofield S. Sources B. Cohen H. Family perspectives on the quality of pediatric palliative care. Arch Pediatric Adolesc Med. 2002;156:14–19. doi: 10.1001/archpedi.156.1.14. [DOI] [PubMed] [Google Scholar]
- 34.Feudtner C. Perspectives on quality at the end of life. Arch Pediatr Adolesc Med. 2004;158:415–417. doi: 10.1001/archpedi.158.5.415. [DOI] [PubMed] [Google Scholar]
- 35.Hutton N. Pediatric palliative care. Arch Pediatr Adolesc Med. 2002;156:9–10. doi: 10.1001/archpedi.156.1.9. [DOI] [PubMed] [Google Scholar]
- 36.Widger K. Davies D. Drouin DJ. Beaune L. Daoust L. Farran RP. Humbert N. Nalewajek F. Rattray M. Rugg M. Bishop M. Pediatric patients receiving palliative care in Canada. Arch Pediatr & Adolesc Med. 2007;161:597–602. doi: 10.1001/archpedi.161.6.597. [DOI] [PubMed] [Google Scholar]
- 37.Davies B. Sehring SA. Partridge C. Cooper BA. Hughes A. Philp JC. Amidi-Nouri A. Kramer RF. Barriers to palliative care for children: Perceptions of pediatric health providers. Pediatrics. 2008;121:282–288. doi: 10.1542/peds.2006-3153. [DOI] [PubMed] [Google Scholar]
- 38.American Psychological Association. Guidelines on multicultural education, training, research, practice, and organizational change for psychologists. Am Psychol. 2003;58:377–402. doi: 10.1037/0003-066x.58.5.377. [DOI] [PubMed] [Google Scholar]
- 39.Crawley L. Marshall P. Lo B. The End-of-Life Consensus Panel. Strategies for culturally effective end-of-life care. Ann Intern Med. 2002;136:673–679. doi: 10.7326/0003-4819-136-9-200205070-00010. [DOI] [PubMed] [Google Scholar]
- 40.Krauss BJ. Goldsamt L. Bula E. Sember R. The white researcher in the multicultural community: Lessons in HIV prevention education learned in the field. J Health Educ. 1997;28(suppl):S67–71. [Google Scholar]
- 41.Lyon M. Woodward K. Nonstigmatizing ways to engage HIV-positive African-American teens in mental health and support services: A commentary. J Natl Med Assoc. 2003;95:196–200. [PMC free article] [PubMed] [Google Scholar]
- 42.McCormick A. McKay MM. Wilson M. McKinney L. Paikoff R. Bell C. Baptiste D. Coleman D. Gillming G. Madison S. Scott R. Involving families in an urban HIV preventive intervention: How community collaboration addresses barriers to participation. AIDS Educ Prev. 2000;12:299–307. [PubMed] [Google Scholar]
- 43.McNeil JI. A model for cultural competency in the HIV management of African American patients. J Natl Med Assoc. 2003;95:3S–7S. [PMC free article] [PubMed] [Google Scholar]
- 44.Smith DB. Health Care Divided: Race, Healing a Nation. Ann Arbor, MI: The University of Michigan Press; 2002. pp. 24–27. [Google Scholar]
- 45.Emanuel EJ. Fairclough DL. Emanuel L. Attitudes and desires related to euthanasia and physician-assisted suicide among terminally ill patients and their caregivers. JAMA. 2000;284:2460–2468. doi: 10.1001/jama.284.19.2460. [DOI] [PubMed] [Google Scholar]
- 46.Lyon ME. Williams PL. Woods ER. Hutton N. Butler AM. Sibinga E. Brady MT. Oleske JM for the Pediatric AIDS Clinical Trials Group. Do not resuscitate orders and/or hospice care, psychological health and quality of life among children/adolescents with AIDS. J Palliat Med. 2008;11:459–469. doi: 10.1089/jpm.2007.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haas JS. Weissman JS. Cleary PD. Goldberg J. Gatsonis C. Seage GR., III Fowler FJ., Jr Massagli MP. Makadon HJ. Epstein AM. Discussion of preferences for life-sustaining care by persons with AIDS. Arch Intern Med. 1993;153:1241–1248. [PubMed] [Google Scholar]
- 48.Hinds PS. Schum L. Baker JN. Wolfe J. Key factors affecting dying children and their families. J Palliat Med. 2006;8:S70–S78. doi: 10.1089/jpm.2005.8.s-70. [DOI] [PubMed] [Google Scholar]
- 49.Kane JR. Pediatric palliative care moving forward: Empathy, competence, quality, and the need for systematic change. J Palliat Med. 2006;9:847–849. doi: 10.1089/jpm.2006.9.847. [DOI] [PubMed] [Google Scholar]
- 50.Pessin H. Galietta M. Nelson CJ. Brescia R. Rosenfeld B. Brietbart W. Burden and benefit of psychosocial research at the end of life. J Palliat Med. 2008;11:627–632. doi: 10.1089/jpm.2007.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wharton RH. Levine KR. Buka S. Emanuel L. Advance care planning for children with special health care needs: A survey of parental attitudes. Pediatrics. 1996;97:682–687. [PubMed] [Google Scholar]
- 52.World Health Organization. Improving HIV management in sub-Saharan Africa: How much palliative care is needed? AIDS Care. 2007;19:1304–1306. doi: 10.1080/09540120701402863. [DOI] [PubMed] [Google Scholar]