Abstract
Bone morphogenetic proteins (BMPs) play a central role in local bone regeneration strategies, whereas the anabolic features of parathyroid hormone (PTH) are particularly appealing for the systemic treatment of generalized bone loss. The aim of the current study was to investigate whether local BMP-2-induced bone regeneration could be enhanced by systemic administration of PTH (1–34). Empty or BMP-2-loaded poly(lactic-co glycolic acid)/poly(propylene fumarate)/gelatin composites were implanted subcutaneously and in femoral defects in rats (n = 9). For the orthotopic site, empty defects were also tested. Each of the conditions was investigated in combination with daily administered subcutaneous PTH (1–34) injections in the neck. After 8 weeks of implantation, bone mineral density (BMD) and bone volume were analyzed using microcomputed tomography and histology. Ectopic bone formation and almost complete healing of the femoral defect were only seen in rats that received BMP-2-loaded composites. Additional treatment of the rats with PTH (1–34) resulted in significantly (p < 0.05) enhanced BMD and bone volume in the BMP-2 composites at both implantation sites. Despite its effect on BMD in the humerus and vertebra, PTH (1–34) treatment had no significant effect on BMD and bone volume in the empty femoral defects and the ectopically or orthotopically implanted empty composites. Histological analysis showed that the newly formed bone had a normal woven and trabecular appearance. Overall, this study suggests that intermittent administration of a low PTH dose alone has limited potential to enhance local bone regeneration in a critical-sized defect in rats. However, when combined with local BMP-2-releasing scaffolds, PTH administration significantly enhanced osteogenesis in both ectopic and orthotopic sites.
Introduction
Bone tissue engineering has the potential to provide alternative therapies for the increasing number of grafting procedures in orthopedic surgery. It strives to create the appropriate in vivo environment to form autologous bone by inducing the regeneration potential of the patient's own tissue. A promising strategy to build up such a local environment is the use of a drug delivery vehicle for local release of bioactive molecules that are essential to the bone regeneration process.
Bone morphogenetic proteins (BMPs) play a central role in most bone regenerative strategies. Several members of the BMP family are capable of initiating the complete cascade of bone formation, including the recruitment of mesenchymal stem cells and their differentiation into osteoblasts.1 These osteoinductive BMPs have generated significant interest as alternative therapy for the repair of local bone defect and spinal fusion. So far, this has resulted in the approval of two BMPs (BMP-2 and BMP-7) in collagen delivery vehicles for specific orthopedic applications in humans.2–4
In contrast to the localized actions of BMPs, parathyroid hormone (PTH) is one of the major systemic regulators of bone metabolism. It is secreted from the parathyroid glands and travels through the bloodstream to act upon bone. PTH is a double-edged sword for bone metabolism: whereas continuous PTH infusion causes the bone disease osteitis fibrosa, pulsed administration induces bone formation.5,6 The anabolic features of intermittent PTH administration have made it particularly appealing for the treatment of patients with generalized bone loss in osteoporosis, in which it was shown to increase bone mass and reduce fracture rate.7
Given the enhanced bone formation by local application of BMPs or systemic application of PTH, combination therapies may be beneficial for bone tissue engineering. PTH has shown to restore the age-related reduction in osteoinductive capacity and increased bone mineral density (BMD) of ectopic ossicles induced by BMP-2-loaded collagen delivery vehicles.8,9 However, the effects of PTH or its combination with BMP-2 on orthotopic bone regeneration in bone tissue engineering remain relatively unexplored.
The aim of this study was to investigate the effect of PTH, BMP-2, and combination treatments on ectopic and orthotopic bone formation. Implants were based on poly(lactic-co glycolic acid)/poly(propylene fumarate)/gelatin (PLGA/PPF/gelatin) microsphere/scaffold composites. These composites are biodegradable and mechanically stable scaffolds previously shown to release BMP-2 over a period of time that coincides with normal expression of osteoinductive factors during bone healing.10–12
Materials and Methods
Experimental design
A total of 54 rats were used for the experiment. The rats were divided into six groups of nine animals for each treatment (Table 1). The three different surgical treatments consisted of (1) no implant, (2) implantation of empty composite, and (3) implantation of BMP-2-loaded composite. Each of the surgical treatments was studied alone or in combination with PTH injections administered in the subcutis of the neck. The treatments were studied in both ectopic (subcutaneous) and orthotopic (critical-sized femoral defect) sites. Whereas the ectopic site shows the osteoinductive potential of the construct without interference of osteoconduction or periosteal bone formation as disturbing mechanisms in the BMP-2-induced bone formation, the femoral defect represents a clinically applicable site. The empty defect with no implant was only studied orthotopically. The implants were removed after 8 weeks of implantation for evaluation of bone formation by microcomputed tomography (μCT), dual energy X-ray absorptiometry (DEXA), and histology.
Table 1.
Experimental Groups and Group Sizes
| Scaffold (orthotopic/ectopic) | No treatment | PTH (1–34) treatment |
|---|---|---|
| Unfilled defect (control)a | n = 9 | n = 9 |
| Empty implant | n = 9 | n = 9 |
| BMP-2-loaded implant | n = 9 | n = 9 |
Only applicable for orthotopic location.
BMP, bone morphogenetic proteins; PTH, parathyroid hormone.
Materials
PLGA (Medisorb®; Lakeshore Biomaterials) with a 50:50 lactic-to-glycolic acid ratio and a weight-average molecular weight (Mw) of 23 kDa was used for microsphere preparation. PPF with a Mw of 3100 and a polydispersity index of 2.7 was synthesized by using a two-step reaction process as previously described.13 Recombinant human BMP-2 (Medtronic Sofamor Danek) was concentrated by centrifuging at 5000 g in a Centricon-10 filter unit (Amicon) and reconstituted to the appropriate concentrations in an aqueous buffer consisting of 5 mM glutamate, 5 NaCl, 0.5% sucrose, 2.5% glycine, and 0.01% polysorbate 80; pH 4.5 (all Sigma-Aldrich). The receptor binding 34-amino acid N-terminal fragment of the PTH molecule [PTH (1–34); Bachem] was diluted in an aqueous buffer for injection consisting of 0.9% NaCl, 2% heat-inactivated serum (obtained from Sprague-Dawley rats), and 1 mM HCl. N-vinylpyrrolidinone (NVP; Acros), bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO; Ciba Specialty Chemicals), gelatin (300 Bloom number, derived from acid-cured porcine skin; Sigma-Aldrich), glutaraldehyde (Sigma-Aldrich), and glycine (Sigma-Aldrich) were used as received.
BMP-2 delivery vehicle
The implant consisted of PLGA microspheres incorporated into a solid PPF rod that was surrounded by a cylindrical gelatin hydrogel. The fabrication procedure of the PLGA microsphere/PPF/gelatin composites was described previously.11,12 The BMP-2-loaded or unloaded PLGA microspheres were fabricated using a water-in-oil-in-water (W1-O-W2) double-emulsion solvent extraction technique.14 On the basis of an starting amount of 1.3 μg BMP-2/mg PLGA and a previously achieved entrapment efficiency of 85%, the loading of the microspheres was estimated at 1.1 μg BMP-2/mg PLGA.12
The cylindrical microsphere/PPF scaffolds were then created by photocrosslinking PPF with NVP under UV light using BAPO as a photoinitiator.15 PLGA microspheres (55 wt%) were combined with a PPF/NVP paste (45 wt%) and injected into a glass mold to create a solid cylindrical composite with a diameter of 1.6 mm and a length of 5.5 mm.11,12 On the basis of an average weight of 10.3 ± 0.8 mg/cylinder, BMP-2 loading in the microsphere/PPF rods was estimated at 6.5 ± 0.5 μg/cylinder. The gelatin hydrogels were fabricated by chemically crosslinking a 10 wt% aqueous gelatin solution with 0.004 wt% glutaraldehyde in tubular molds (1.6 mm inner diameter and 3.5 mm outer diameter) for a period of 1 h.11,15–17 The crosslinked hydrogels were placed in a 100 mM glycine solution for 1 h to block the residual aldehyde groups. The microsphere/PPF rods and gelatin hydrogels were sterilized separately in 70% alcohol, washed in phosphate-buffered saline, and stored at −20°C. The microsphere/PPF rods were inserted into the tubular gelatin hydrogels just before implantation.
Animals and procedures
The bone-forming capacity of the scaffolds was tested in both ectopic and orthotopic models in 12-week-old Harlan Sprague Dawley rats (weight 324 ± 12 g). The experiments were performed according to established protocols approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. The rats were anesthetized with an intramuscular injection of a ketamine/xylazine mixture (45/10 mg/kg) and the surgical sites were shaved and disinfected with a 30% betadine solution. The right femur was exposed through a lateral approach and was circumferentially stripped from soft tissue and periosteum. A predrilled, high-density polyethylene plate (23 × 4 × 4 mm, custom-made) was fixed along the anterior cortex of the femur using four threaded Kirschner wires (1 mm diameter; Zimmer). After plate fixation, a 5-mm mid-diaphyseal, full-thickness defect was created using a surgical burr and irrigated with physiologic saline to remove bone debris and bone marrow. After insertion of the implant in the defect, the wound was closed in layers using resorbable sutures (Vicryl 1/0; Ethicon, Inc.) and nonresorbable nylon skin sutures. In addition to the orthotopic implant, each rat received an ectopic implant in a subcutaneous pocket at the lower left limb.
Postoperative analgesia was provided by intramuscular injections of buprenorphine (0.1 mg/kg) for the duration of 72 h and acetaminophen (880 mg/L added to the water bottle) for the duration of 1 week.
PTH (1–34) treatment was started on the third postoperative day. PTH injections were administered in the subcutis of the neck at a dosage of 10 μg/kg/day for 5 days a week till the end of the 8 week follow-up period. In addition to PTH injections, the rats received fluorochrome markers calceine green (10 mg/kg; Sigma-Aldrich) and alizarin red (10 mg/kg; Sigma-Aldrich) after 4 and 6 weeks, respectively. After 8 weeks, the animals were sacrificed by an overdose of pentobarbital and the vessels were perfused with Microfil® (Flow Tech) to study vessel formation at the implantation site using previously described methods.11 Following a midline laparatomy, the aorta and vena cava were canulated to clear the lower extremities from blood with heparinized saline. The vessels were subsequently infused until the toes were clearly colored blue by the Microfil compound. Since there were no significant differences between the treatment groups, these data are not shown. After polymerization of the compound, the implants were removed and fixed in phosphate-buffered saline containing 1.5% glutaraldehyde. The vertebral column and humerus were also harvested to analyze the effects of PTH treatment on overall bone density. Before μCT and DEXA scans, the two K-wires closest to the defect were removed from the femur.
BMD measurements
BMD measurements were performed by DEXA using a PIXImus densitometer (software version 1.44.005; Lunar Corporation). Before the measurements, the PIXImus densitometer was calibrated using a hydroxyapatite phantom that was provided by the manufacturer. The excised implants, humerus, and vertebral column were scanned by the machine and the regions of interest were identified for the analysis of the BMD.
μCT analysis
The implants were scanned on a custom-build μCT system at 0.49° angular increments (providing 721 views around 360°) using 18 keV.18 The projections were reconstructed using a modified Feldkamp cone beam tomographic reconstruction algorithm into a three-dimensional image consisting of 20 μm cubic voxels with a radiopacity represented by a 16-bit grayscale value. The bone formation in the implants was analyzed using image analysis software Analyze 6.0 (Biomedical Imaging Resource; Mayo Clinic, Rochester, MN). Due to the difficulty to distinguish the newly formed bone from the original femur, a 6-mm section of the femur was used for bone quantifications. The reconstructions and volume quantification of the ectopic and orthotopic bone were obtained using standardized thresholds.
Histology
After μCT and BMD measurements, the subcutaneous implants and femurs were dehydrated in a graded series of alcohol and embedded in, respectively, glycol-methylmethacrylate and methylmethacrylate for histological evaluation. The subcutaneous samples were longitudinally sectioned using a rotary microtome (Reichert-Jung Supercut 2050 microtome; Leica Microsystems) and the femurs using a sawing microtome (Leica SP1600; Leica Microsystems). The sections were stained with trichrome Goldner, hematoxylin/eosin, and/or methylene blue/basic fuchsine, and evaluated for general tissue response and bone formation. Additional unstained sections were evaluated for incorporation of fluorochrome labels using a fluorescence microscope (E600 Nikon) with a double-filter block (dichroic mirror 505 and 590 nm).
Statistical analysis
All data are given as means ± standard deviations for n = 9. Independent t-tests were performed to analyze the BMD and bone volumes of the ectopic implants. The BMD and bone volumes of the orthotopic implants were statistically compared using one-way analysis of variance with Bonferroni's post hoc tests. The effect of PTH treatment on overall BMD was analyzed using two-way analysis of variance. All tests were performed by SPSS (version 13.0; SPSS, Inc.), and the level of significance was set at p < 0.05.
Results
Animal
Six animals (one in empty defect group, two in empty defect/PTH group, one in empty composite group, and two in the empty composite/PTH group) developed deep infections of their femoral defects during the study. Their BMD and bone volume measurements were excluded from further analysis. The other animals remained in good health and did not show any signs of complications.
Bone mineral density
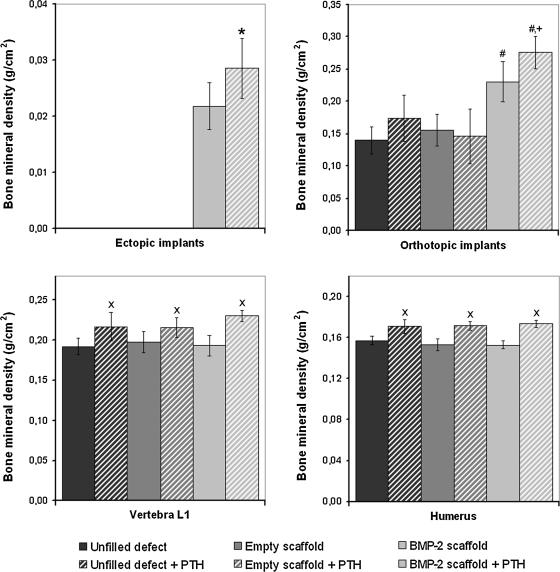
After 8 weeks of implantation, all implants were easily identified and retrieved for further analysis. At the ectopic site, bone formation was only seen in BMP-2-loaded scaffolds. The ectopic BMP-2 implants in the PTH-treated animals had a significantly (p < 0.05) higher BMD than the BMP-2 implants in the nontreated animals (Fig. 1). At the orthotopic site, the BMP-2-loaded scaffolds had a significantly higher BMD than the unfilled defects or defects filled with empty scaffolds (p < 0.02). Further, BMD of the BMP-2-containing scaffolds in these femoral defects was significantly higher in the animals treated with PTH injections (p < 0.04). PTH administration had no effect on the BMD of empty defects or defects treated with empty scaffolds. The BMD of the right humerus and vertebra L1 of the PTH-treated rats was significantly higher than that of the nontreated rats (p < 0.05).
FIG. 1.
Bone mineral density of the ectopic implants, orthotopic implants, vertebra L1, and humerus after 8 weeks of follow-up. The symbols indicate significant differences relative to (*) ectopic bone morphogenetic proteins (BMP)-2-loaded implants, (#) unfilled and empty scaffold filled orthotopic defects, (+) all other groups, or (x) nontreated vertebrae or humeri (p < 0.05). PTH, parathyroid hormone.
Bone volume
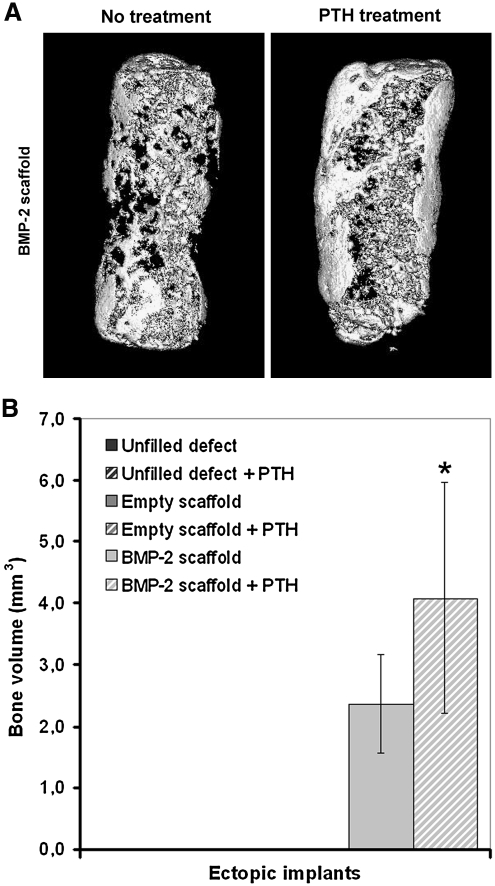
The effects of BMP-2 released from the scaffold and of PTH treatment on the ectopic and orthotopic volumes of the newly formed bone were studied by μCT imaging at 20 μm resolution. No radiological signs of bone formation were seen in the ectopically implanted empty scaffolds. Ectopic bone formation was seen in the μCT reconstructions of BMP-2-containing implants. Part of the ectopic implant surface was covered by a compact shell of bone, and trabecular bone was seen inside the pores of the scaffold (Fig. 2A). Quantification of the bone volume showed that PTH had a significant effect on ectopic bone formation in these scaffolds (p = 0.02, Fig. 2B).
FIG. 2.
Three-dimensional microcomputed tomography reconstructions (A) and bone volumes (B) of subcutaneously formed bone after 8 weeks of implantation in rats. The asterisk (*) indicates significant difference relative to BMP-2-loaded implants (p = 0.02).
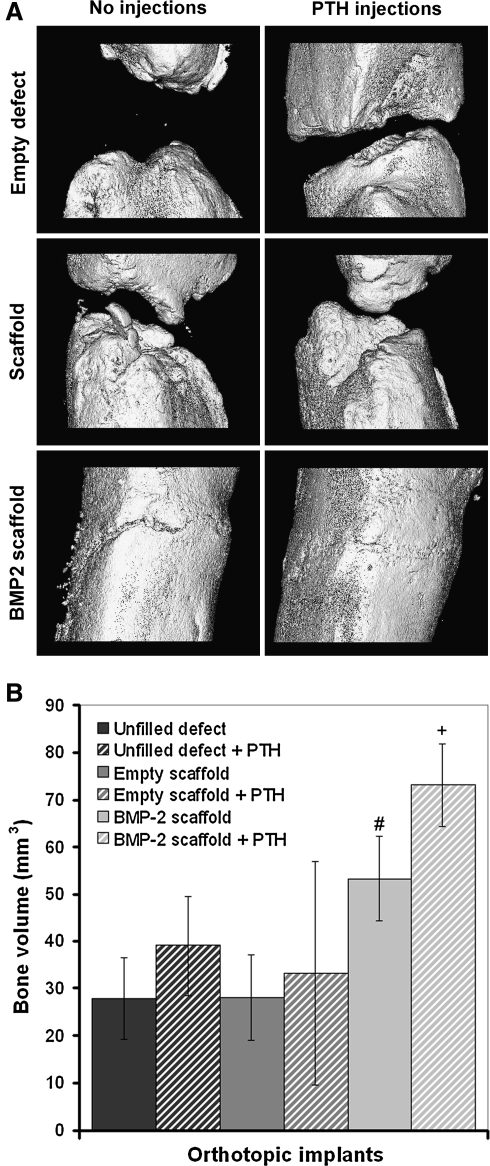
All orthotopic sites showed some newly formed bone around the defect edges (Fig. 3A). Analysis of connections between the proximal and distal part of the femur showed that none of the unfilled defects contained an osseous connection between the two femur parts. Trabecular connections between the two parts were observed in 2/8 and 2/7 of the untreated or PTH-treated defects filled with empty scaffolds, respectively, whereas almost full cortical regeneration was seen in all of the defects containing the BMP-2-loaded implants. The defects filled with BMP-2-loaded implants contained significantly more bone than the untreated unfilled defects and defects filled with empty scaffolds (p = 0.04). The bone volume in the defects of animals treated with both BMP-2 scaffolds and PTH injections was significantly higher than all other groups (p = 0.02). No significant differences were found between the untreated and PTH-treated unfilled defects or defects filled with empty scaffolds.
FIG. 3.
Three-dimensional microcomputed tomography reconstructions (A) and bone volumes (B) of newly formed bone in the 5-mm femoral defects after 8 weeks of follow-up. The symbols indicate significant differences relative to (#) unfilled defects, defects filled with empty scaffolds, or defects filled with empty scaffolds treated with parathyroid hormone (PTH) injections and (+) all defects (p < 0.05).
Histology
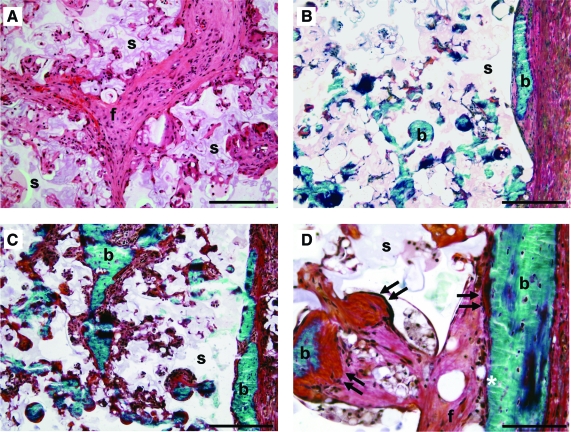
Microscopic evaluation of transverse sections showed that the gelatin hydrogel around the microsphere/scaffold composite was completely resorbed and the PLGA microspheres were degrading to create a porous PPF network. In the ectopic site, the gelatin hydrogel was replaced by a well-vascularized fibrous capsule. There was a mild foreign body reaction to the microsphere/PPF rod as indicated by some inflammatory cells inside the pores of the scaffold. The interconnected pores of the ectopically implanted empty scaffolds were filled with connective tissue (Fig. 4A). Ectopic bone formation and osteoid depositions were seen on the surface and inside the pores of BMP-2-containing implants (Fig. 4B–D). Bone formation was more extensive in the ectopic BMP-2 implants of rats treated with PTH compared to the nontreated animals. Fluorochrome analysis showed the presence of calcein green along the surface and alizarin red at both the surface and in the pores of the implant, indicating that mineralization started at the periphery and progressed toward the center.
FIG. 4.
Histological sections of subcutaneously implanted empty (A) or BMP-2 (B–D) scaffolds in rats that were treated with (A, C, D) or without (B) PTH. Hematoxylin/eosin-stained section (A) showing the interconnected porous polymer network and fibrous tissue (f) in the scaffold. Goldner-stained sections (B–D) showing bone formation (b) and osteoid deposition (arrows) at the surface and in the pores of BMP-2 scaffolds (s). Scale bars represent 500 μm (A, D), 200 μm (B), and 50 μm (C). Color images available online at www.liebertonline.com/ten.
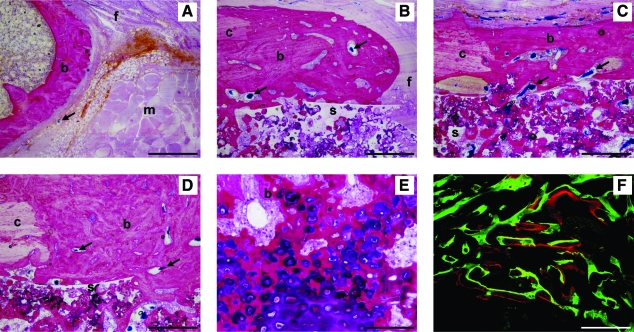
In the orthotopic implant, new bone formation extending from the femur edges was seen in all implants (Fig. 5). The empty defects were filled with inter-positioning muscles, fibrous tissue, and a limited amount of bone (Fig. 5A). Implantation of empty scaffolds resulted in bone formation at the surface and in the scaffold pores at the defect edges, whereas the center was filled with connective tissue (Fig. 5B). In contrast to the empty defects and defects filled with empty scaffold, almost full cortical bridging was seen in the BMP-2 composite containing defects (Fig. 5C, D). A narrow area with dense connective tissue containing chondrocyte-like cells showing signs of endochondral ossification could be observed in the center of most of these defects (Fig. 5E). Overall, the rats treated with PTH showed broadening of the preexisting parts of the femur and newly formed cortices with a more compact structure of the bone (Fig. 5C, D). The calcein green and alizarin red were found throughout the newly formed cortical bone, indicating that mineralization was present in the entire cortical area before 4 weeks and continued over the rest of the follow-up period (Fig. 5F).
FIG. 5.
Histological sections of an empty femoral defect (A) and defects filled with empty (B) or BMP-2 scaffolds (C–F) in rats treated without (A, C) or with (B, D–F) PTH. Methylene blue/basic fuchsin-stained sections (A–E) showing interpositioning of muscles (m), fibrous tissue formation (f), and bone formation (b) extending from the original femur cortices (c) into the defect and the pores of the scaffold (s). The vascular network in the bone and fibrous tissue is observed by the blue Microfil® agent (arrows). Endochondral ossification was seen in the center of the defects of BMP-2-containing implants (E). Fluorochrome analysis of unstained sections (F) showed calceine green and alizarin red deposition in newly formed bone. Scale bars represent 500 μm (A–D), 50 μm (D, E), and 500 μm (F). Color images available online at www.liebertonline.com/ten.
Discussion
This study clearly shows the benefits of combined PTH and BMP-2 treatment on ectopic and orthotopic bone formation in a microsphere/scaffold composite for bone tissue engineering. Although an anabolic effect on the rest of the skeleton was seen, the 10 μg PTH/kg/day treatment had no significant effect on local bone regeneration in unfilled and empty scaffold-filled defects. Implantation of BMP-2-loaded scaffolds resulted in a significant increase of BMD and bone volume in both sites as opposed to the empty scaffolds. The combination of BMP-2-containing scaffolds with daily PTH injections resulted in a further increase of both ectopic and orthotopic bone volumes.
The exact cellular mechanism underlying the anabolic effect of PTH treatment is not fully understood. Histological observations have shown that intermittently administered PTH stimulates new bone formation on existing surfaces by increasing the osteoblast number.5,19–21 Previous studies have suggested that this increase in osteoblast number is not dependent on the proliferation of osteoprogenitor cells and osteoblastogenesis, but the result of PTH effects on existing cells.19,20,22 Likely target cells for short-term PTH effects are bone-lining cells, which are thought to be inactive osteoblasts. These lining cells may undergo hypertrophy and resume matrix synthesis in response to PTH treatment.5,19,21 Another proposed method for the continued effect of long-term PTH treatments is the inhibition of osteoblast apoptosis.5,20
The absence of bone formation in the ectopically implanted empty scaffolds was anticipated, as the synthetic polymers are not osteoinductive. Since PTH is expected to act upon cells committed to the osteoblastic lineage, it was not surprising that PTH was not able to affect ectopic bone formation in these empty scaffolds.23 Although PTH cannot induce bone formation from uncommitted cells, it can enhance bone formation once it has been initiated. Previous bone fracture, distraction, and conduction chamber models have shown a strong dose-related effect of intermittent PTH administration on local bone regeneration.24–27 Despite bone induction at the edges of the unfilled or empty scaffold filled defects and the positive PTH effects on preexistent bone, no significant differences were found between these untreated and PTH-treated defects. The discrepancy between previous studies and these results may be due to the more challenging critical-sized defect model and low PTH dose used in this study.
In contrast to PTH, BMP-2 plays an essential role in the commitment and differentiation of mesenchymal stem cells toward the osteoblastic lineage. Its high osteoinductive potential is clearly demonstrated by its ability to induce bone formation in an ectopic implantation site.28,29 The BMP-induced bone formation occurs through endochondral (through a cartilage intermediate) and/or intramembranous (direct) ossification and results in woven bone, which is later remodeled into normal bone.1,28 In our study, after implantation of the microsphere/PPF/gelatin delivery vehicle, clear signs of endochondral and intramembranous ossification were also seen at the ectopic or orthotopic location.
Despite the nonsignificant effect of PTH alone, PTH significantly enhanced the BMP-2-induced bone formation at both ectopic and orthotopic locations. This clearly shows that the different mechanisms of action of BMP-2 and PTH enhance each other when used as a combination therapy. Whereas BMP-2 induces bone formation by committing mesenchymal stem cells toward the osteoblastic lineage, PTH could act upon these committed cells to prolong the matrix-synthesizing function. Further, the antiapoptotic effect of PTH could also have enhanced bone formation by expanding the osteoblast life span by counteracting the possible apoptotic effects of BMP-2 on osteoblasts.20,30 Unfortunately, further histological analysis (e.g., counting of the osteoblast number) of the mechanism underlying the anabolic effect in the combination therapy was impossible due to the woven aspect of the newly formed bone.
Compared to previous studies, the ectopic results obtained in our study corresponded with previous findings of PTH-mediated enhancement of ectopic BMP-induced bone formation in a collagen sponge.8,9 In contrast to the clearly enhanced ectopic effect, no significant differences could be observed between a PTH/BMP-7 combination and PTH alone in a previous study.31 This nonsignificant effect might be caused by their use of a less challenging partial thickness metaphysic defect model. Since the spontaneous regeneration response was capable of healing the nontreated defects as well, no significant effect of BMP-7 could be shown compared to the control group. Consequently, PTH enhanced both spontaneous and BMP-7-assisted healing response and no significant differences in bone formation were observed between the PTH/BMP-7 combination and PTH alone. In the critical-sized defect model in this study, the normal bone regeneration response resulted in limited amounts of bone formation at the defect edges, which was not significantly enhanced by PTH. However, bone regeneration was significantly stimulated by the BMP-2-releasing implants and PTH could act upon this to synergistically enhance the healing response.
In conclusion, this study clearly shows that BMP-2-induced osteogenesis can be enhanced by intermittent administration of PTH. Although PTH alone did not significantly improve bone formation, it can be beneficial for local bone regeneration in combination with BMP-2. On the basis of this study, PTH/BMP-2 combination therapy could be considered for the restoration of large bone defects in orthopedic surgery. However, due to the physiological and anatomical differences between humans and different animal species for bone regeneration, future studies in more relevant large animal models and clinical trials will be needed to maximize the clinical efficacy of PTH/BMP-2 combination therapies.32,33
Acknowledgments
The authors wish to thank Dr. Wang and Mr. Greutzmacher from the Tissue Engineering and Biomaterials Laboratory for their assistance with polymer chemistry; Dr. Ritman, Mrs. Beighley, and Mr. Vercnocke from the Physiological Imaging Research Laboratory for their assistance with μCT imaging; and Mrs. Burges and Mr. Herrick from the Bone Histomorphometry Laboratory for their assistance with histology. This study was supported by the National Institutes of Health (R01 AR45871 and R01 EB03060) and The Netherlands Organization for Health Research and Development ZonMW (Agiko 920–03-325).
Disclosure Statement
No competing financial interests exist.
References
- 1.Wozney J.M. Overview of bone morphogenetic proteins. Spine. 2002;27:S2. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 2.Burkus J.K. Transfeldt E.E. Kitchel S.H. Watkins R.G. Balderston R.A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 3.Friedlaender G.E. Perry C.R. Cole J.D. Cook S.D. Cierny G. Muschler G.F. Zych G.A. Calhoun J.H. LaForte A.J. Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S151. [PMC free article] [PubMed] [Google Scholar]
- 4.Govender S. Csimma C. Genant H.K. Valentin-Opran A. Amit Y. Arbel R. Aro H. Atar D. Bishay M. Borner M.G. Chiron P. Choong P. Cinats J. Courtenay B. Feibel R. Geulette B. Gravel C. Haas N. Raschke M. Hammacher E. van der Velde D. Hardy P. Holt M. Josten C. Ketterl R.L. Lindeque B. Lob G. Mathevon H. McCoy G. Marsh D. Miller R. Munting E. Oevre S. Nordsletten L. Patel A. Pohl A. Rennie W. Reynders P. Rommens P.M. Rondia J. Rossouw W.C. Daneel P.J. Ruff S. Ruter A. Santavirta S. Schildhauer T.A. Gekle C. Schnettler R. Segal D. Seiler H. Snowdowne R.B. Stapert J. Taglang G. Verdonk R. Vogels L. Weckbach A. Wentzensen A. Wisniewski T. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Lotinun S. Sibonga J.D. Turner R.T. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002;17:29. doi: 10.1385/ENDO:17:1:29. [DOI] [PubMed] [Google Scholar]
- 6.Qin L. Raggatt L.J. Partridge N.C. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Neer R.M. Arnaud C.D. Zanchetta J.R. Prince R. Gaich G.A. Reginster J.Y. Hodsman A.B. Eriksen E.F. Ish-Shalom S. Genant H.K. Wang O. Mitlak B.H. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi H. Saito N. Kinoshita T. Wakabayashi S. Tsutsumimoto T. Otsuru S. Takaoka K. Enhancement of recombinant human bone morphogenetic protein-2 (rhBMP-2)-induced new bone formation by concurrent treatment with parathyroid hormone and a phosphodiesterase inhibitor, pentoxifylline. J Bone Miner Metab. 2004;22:329. doi: 10.1007/s00774-003-0490-y. [DOI] [PubMed] [Google Scholar]
- 9.Kabasawa Y. Asahina I. Gunji A. Omura K. Administration of parathyroid hormone, prostaglandin E2, or 1-alpha,25-dihydroxyvitamin D3 restores the bone inductive activity of rhBMP-2 in aged rats. DNA Cell Biol. 2003;22:541. doi: 10.1089/104454903322405428. [DOI] [PubMed] [Google Scholar]
- 10.Kempen D.H. Lu L. Hefferan T.E. Creemers L.B. Maran A. Classic K.L. Dhert W.J. Yaszemski M.J. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempen D.H. Lu L. Heijink A. Hefferan T.E. Creemers L.B. Maran A. Yaszemski M.J. Dhert W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Kempen D.H. Yaszemski M.J. Heijink A. Hefferan T.E. Creemers L.B. Britson J. Maran A. Classic K.L. Dhert W.J. Lu L. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J Control Release. 2009;134:169. doi: 10.1016/j.jconrel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S. Lu L. Yaszemski M.J. Bone-tissue-engineering material poly(propylene fumarate): correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules. 2006;7:1976. doi: 10.1021/bm060096a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldham J.B. Lu L. Zhu X. Porter B.D. Hefferan T.E. Larson D.R. Currier B.L. Mikos A.G. Yaszemski M.J. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng. 2000;122:289. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 15.Kempen D.H. Kruyt M.C. Lu L. Wilson C.E. Florschutz A.V. Creemers L.B. Yaszemski M.J. Dhert W.J. Effect of autologous bone marrow stromal cell seeding and bone morphogenetic protein-2 delivery on ectopic bone formation in a microsphere/poly(propylene fumarate) composite. Tissue Eng Part A. 2009;15:587. doi: 10.1089/ten.tea.2007.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M. Tabata Y. Hong L. Miyamoto S. Hashimoto N. Ikada Y. Bone regeneration by transforming growth factor beta1 released from a biodegradable hydrogel. J Control Release. 2000;64:133. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M. Takahashi Y. Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen S.M. Demirkaya O. Ritman E.L. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. Am J Physiol. 1998;275:H1103. doi: 10.1152/ajpheart.1998.275.3.H1103. [DOI] [PubMed] [Google Scholar]
- 19.Dobnig H. Turner R.T. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 20.Jilka R.L. Weinstein R.S. Bellido T. Roberson P. Parfitt A.M. Manolagas S.C. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leaffer D. Sweeney M. Kellerman L.A. Avnur Z. Krstenansky J.L. Vickery B.H. Caulfield J.P. Modulation of osteogenic cell ultrastructure by RS-23581, an analog of human parathyroid hormone (PTH)-related peptide-(1–34), and bovine PTH-(1–34) Endocrinology. 1995;136:3624. doi: 10.1210/endo.136.8.7628402. [DOI] [PubMed] [Google Scholar]
- 22.Onyia J.E. Bidwell J. Herring J. Hulman J. Hock J.M. In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone. 1995;17:479. doi: 10.1016/8756-3282(95)00332-2. [DOI] [PubMed] [Google Scholar]
- 23.Kempen D.H. Lu L. Kim C. Zhu X. Dhert W.J. Currier B.L. Yaszemski M.J. Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly(propylene fumarate) J Biomed Mater Res A. 2006;77:103. doi: 10.1002/jbm.a.30336. [DOI] [PubMed] [Google Scholar]
- 24.Alkhiary Y.M. Gerstenfeld L.C. Krall E. Westmore M. Sato M. Mitlak B.H. Einhorn T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34) J Bone Joint Surg Am. 2005;87:731. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 25.Seebach C. Skripitz R. Andreassen T.T. Aspenberg P. Intermittent parathyroid hormone (1–34) enhances mechanical strength and density of new bone after distraction osteogenesis in rats. J Orthop Res. 2004;22:472. doi: 10.1016/j.orthres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Skripitz R. Andreassen T.T. Aspenberg P. Strong effect of PTH (1–34) on regenerating bone: a time sequence study in rats. Acta Orthop Scand. 2000;71:619. doi: 10.1080/000164700317362271. [DOI] [PubMed] [Google Scholar]
- 27.Skripitz R. Andreassen T.T. Aspenberg P. Parathyroid hormone (1–34) increases the density of rat cancellous bone in a bone chamber. A dose-response study. J Bone Joint Surg Br. 2000;82:138. doi: 10.1302/0301-620x.82b1.9729. [DOI] [PubMed] [Google Scholar]
- 28.Wang E.A. Rosen V. D'Alessandro J.S. Bauduy M. Cordes P. Harada T. Israel D.I. Hewick R.M. Kerns K.M. LaPan P., et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990;87:2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wozney J.M. Rosen V. Celeste A.J. Mitsock L.M. Whitters M.J. Kriz R.W. Hewick R.M. Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 30.Hay E. Lemonnier J. Fromigue O. Marie P.J. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- 31.Morgan E.F. Mason Z.D. Bishop G. Davis A.D. Wigner N.A. Gerstenfeld L.C. Einhorn T.A. Combined effects of recombinant human BMP-7 (rhBMP-7) and parathyroid hormone (1–34) in metaphyseal bone healing. Bone. 2008;43:1031. doi: 10.1016/j.bone.2008.07.251. [DOI] [PubMed] [Google Scholar]
- 32.Aerssens J. Boonen S. Lowet G. Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 33.Seeherman H. Li R. Wozney J. A review of preclinical program development for evaluating injectable carriers for osteogenic factors. J Bone Joint Surg Am. 2003;85-A(Suppl 3):96. doi: 10.2106/00004623-200300003-00016. [DOI] [PubMed] [Google Scholar]