Abstract
The mammary gland and other treelike organs develop their characteristic fractal geometries through branching morphogenesis, a process in which the epithelium bifurcates and invades into the surrounding stroma. Controlling the pattern of branching is critical for engineering these organs. In vivo, the branching process is instructed by stromal–epithelial interactions and adipocytes form the largest component of the fatty stroma that surrounds the mammary epithelium. Here, we used microlithographic approaches to engineer a three-dimensional culture model that enables analysis of the effect of adipocytes on the pattern of branching morphogenesis of mammary epithelial cells. We found that adipocyte-rich stroma induces branching through paracrine signals, including hepatocyte growth factor, but does not affect the branching pattern per se. This tissue engineering approach can be expanded to other organs, and should enable piecemeal analysis of the cellular populations that control patterning during normal development.
Introduction
The treelike structure of the mammary gland forms through branching morphogenesis, a process mediated by epithelial extension and bifurcation into the surrounding adipose-rich stroma. During embryonic development, the mammary epithelial rudiment invades into fat pad precursor tissue. During postnatal development, the gland remains quiescent until puberty, at which time steroid hormones induce the epithelium to invade and branch into the fat pad. Unlike stereotyped organs such as the lung, the pattern of the mammary epithelial tree varies between individuals. It is unclear whether the adipocytes that make up the fat pad influence the final pattern of the mature mammary tree.
Epithelial–stromal interactions are essential for the morphogenesis and functional differentiation of most organs.1 During the branching process cap cells at the leading edge of the pubertal mammary epithelium closely abut adipocytes as they invade the stroma.2 Combining embryonic mammary epithelium with salivary stroma leads to a salivary pattern of branching, suggesting that branching patterns can be dictated by the stromal compartment.3,4 Mouse mammary stroma is dominated by adipocytes, but also includes fibroblasts, vasculature, and immune cells; many of these cell types have been shown to play a role in branching morphogenesis.5–8 Transplantation experiments with embryonic fat pad precursors demonstrated that both embryonic and adult mammary epithelium must interact with a fatty stroma to grow into a characteristic mammary tree; fibroblast-rich mammary mesenchyme induced ductal hyperplasia.9 Indeed, mammary branching morphogenesis is inhibited in transgenic mice lacking white adipose tissue, indicating that the absence of adipocytes stunts ductal growth.10 The interactions between mammary epithelium and the fat pad are likely nonspecific, however, as recombining adult mammary epithelium with adipocytes from other organs (including the pararenal, mesometrial, and interscapular fats) leads to branching morphogenesis.11–13 Unfortunately, these in vivo studies could not distinguish between the signals that induce branching and those that pattern the gland.
Comprehensive analysis of the role of adipocytes in patterning the mammary tree requires techniques that can separate these cells from the rest of the stroma and that can distinguish alterations in the resulting branching pattern. A number of culture models have been developed to recapitulate the morphogenesis and functional differentiation of mammary epithelial cells (reviewed in Nelson and Bissell14). A natural evolution of these models is to introduce the stromal compartment and thereby build a more faithful histological representation of the organ—essentially to engineer mammary tissue interactions in culture. Even though the mammary stroma is populated largely by adipocytes, the analysis of mammary epithelial interactions with stromal cells in culture has focused primarily on fibroblasts, largely because of their effects on tumor development (reviewed in Bhowmick et al.15). The handful of studies examining mammary epithelial interactions with adipocytes have revealed that 3T3-L1 adipocytes16,17 or mammary fat pad explants18,19 affect the proliferation, acinar and ductal morphogenesis, and milk protein synthesis of primary rodent mammary epithelial cells and mammary epithelial cell lines.
This study had two objectives aimed at understanding how adipocytes influence the pattern of branching morphogenesis of mammary epithelial cells. The first objective was to create a method for coculturing mammary epithelial ducts within adipose-rich stroma. To that end, we used microlithographic techniques to build arrays of epithelial tubules of defined geometry within three-dimensional (3D) collagen gels containing differentiated adipocytes. The second objective was to investigate the juxtacrine and paracrine effects of adipocytes on mammary epithelial branching morphogenesis. Because each microfabricated epithelial tubule was initially identical in size and geometry, we could directly compare sites of branching in the presence and absence of adipocytes. We found that adipocytes stimulated branching morphogenesis via paracrine signals, including hepatocyte growth factor (HGF), but did not affect the pattern of branching.
Materials and Methods
Cell culture and reagents
3T3-L1 preadipocytes were obtained from the American Type Culture Collection and maintained in growth medium containing Dulbecco's modified Eagle's medium (DMEM; Invitrogen), 10% calf serum (Atlanta Biologicals), and 50 μg/mL gentamicin (Invitrogen). Functionally normal EpH4 mouse mammary epithelial cells20 were cultured in 1:1 DMEM:F12 (HyClone) containing 2% fetal bovine serum (FBS; Atlanta Biologicals), 5 μg/mL insulin (Sigma), and 50 μg/mL gentamicin. Mammary epithelial cells were assayed for functional differentiation in the presence of lactogenic differentiation medium as previously described.21
Adipocyte differentiation
3T3-L1 preadipocytes were induced to differentiate into mature adipocytes at 100% confluence using differentiation medium containing DMEM, 10% FBS, 50 μg/mL gentamicin, 1 μg/mL insulin, 1 μM dexamethasone, and 115 μg/mL 3-isobutyl-1-methylxanthine for 48 h. The differentiation medium was then replaced with medium containing DMEM, 10% FBS, 50 μg/mL gentamicin, and 1 μg/mL insulin every other day for 8 days. Data reported are from passages up through p6; later passages increasingly failed to differentiate, as reported for other preadipocytes.22 For differentiation in collagen, 3T3-L1 cells were re-suspended and mixed in liquid neutralized rat tail collagen (BD Biosciences; 6 mg/mL final concentration) or bovine dermal collagen (Koken; 4 mg/mL final concentration). The 3T3-L1/collagen mixture was allowed to gel at 37°C for 30 min, and then treated with differentiation medium for 48 h. For differentiation in laminin-rich extracellular matrix (ECM), 3T3-L1 cells were re-suspended and mixed in liquid-neutralized collagen containing 5% Matrigel (BD Biosciences). The elastic modulus of these collagen preparations was ∼500 Pa, as determined by rheometry (500 ± 51 Pa for bovine dermal; 473 ± 69 Pa for rat tail).
Microlithography
Microfabricated cultures of mammary epithelial tubules embedded within collagen gels containing adipocytes were formed by replica micromolding using previously described protocols23,24 with the following modifications: Patterned elastomeric stamps of poly(dimethylsiloxane) (Sylgard 184) were rendered nonadhesive by coating with a 1% solution of bovine serum albumin in phosphate-buffered saline (PBS). Stamps were then placed upon a drop of liquid-neutralized collagen containing 3T3-L1 cells at 37°C until gelation. Preadipocytes within gelled samples were induced to differentiate as described above. Stamps were removed after 6 days and a concentrated suspension of mammary epithelial cells was allowed to settle within the micromolded collagen cavities. Excess mammary epithelial cells were rinsed away with the culture medium, and a second layer of collagen gel containing differentiated adipocytes was gently placed on top of the sample.
Adipocyte-conditioned medium
Conditioned medium was prepared from 3T3-L1 cells induced to differentiate as described above. Medium containing DMEM, 10% FBS, 50 μg/mL gentamicin, and 1 μg/mL insulin was exposed to confluent differentiated 3T3-L1 cultures (6–8 days postinduction) for 48 h, centrifuged to remove cellular debris, and filtered through 0.22-μm-diameter syringe filters before adding to mammary epithelial tubules. Unconditioned medium was used as a control. To assay for effects mediated by HGF, the cMet inhibitor PHA665752 (200 nM; Tocris25) was added to tubules at the time of treatment with conditioned medium.
Immunofluorescence staining
Cells were stained for lipid inclusions using Oil red O (Sigma) or Sudan III (Sigma). For Oil red O staining, cells were washed with PBS, fixed in 4% paraformaldehyde in PBS for 15 min at room temperature, washed again with PBS, and incubated with 0.5% Oil red O in isopropanol for 1 h at room temperature. Samples were washed extensively with PBS and allowed to dry before imaging. For Sudan III staining, samples were washed and fixed as above, and then incubated in 0.2% Sudan III in 70% ethanol for 20 min at room temperature. Samples were washed extensively in PBS and counterstained with Hoechst 33342 (Invitrogen) before mounting and imaging. To observe epithelial cytokeratins, samples processed for Sudan III staining were blocked with 10% goat serum (Sigma), incubated with anti-pan-keratin (Dako), washed, and incubated with appropriate Alexa-conjugated secondary antibodies (Invitrogen). Proliferating cells were observed with the Click-iT EdU Imaging Kit (Invitrogen) as previously described.26 Relative percentage of proliferating cells was determined by quantifying frequency maps constructed from fluorescence images as described below.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cells using the RNeasy kit (Qiagen). cDNA was synthesized using the Verso cDNA kit (Thermo Fisher) from equal amounts of RNA. Quantitative real-time polymerase chain reaction (PCR) analysis was performed with the MiniOpticon system (BioRad) using RT Real-Time PCR SYBR green/fluorescein master mix (SA Biosciences). The following primers were used to amplify cDNA sequences: lipoprotein lipase (LPL) forward primer 5′-CGC TCC ATT CAT CTC TTC ATT G-3′ and reverse 5′-GTC GCT TCT CTT GGC TCT G-3′; peroxisome proliferator-activated receptor (PPAR)γ2 forward primer 5′-ATG ACC TGA AGC TCC AAG AAT ACC-3′ and reverse 5′-CCA CAG ACT CGG CAC TCA ATG-3′; β-casein forward primer 5′-GCT CAG GCT CAA ACC ATC TC-3′ and reverse 5′-TGT GGA AGG AAG GGT GCT AC-3′; 18s forward primer 5′-TCA GAT ACC GTC GTA GTT C-3′ and reverse 5′-CCT TTA AGT TTC AGC TTT GC-3′. Amplification was followed by melting curve analysis to verify the presence of a single PCR product.
Imaging and analysis
Tubules were observed 24–48 h after addition of the adipocyte-conditioned medium. Samples were imaged using either a 10 × (numerical aperture [NA] = 0.3) or a 20 × (NA = 0.45) air objective on a Nikon Eclipse Ti-U inverted fluorescence microscope equipped with a Hamamatsu ORCA CCD camera. Frequency maps were created using ImageJ and Photoshop software. First, gray-scale images were converted to black-and-white images using a binarize function. The black-and-white images were then summed to create a composite gray-scale image, which was converted into a color-coded frequency map using the indexed color mode in Photoshop.
Results
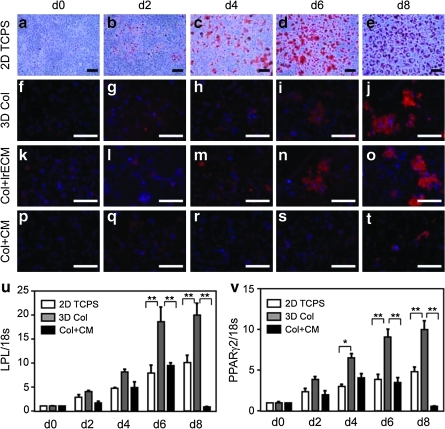
Here we set out to engineer a 3D coculture model of mammary epithelial tubules of precisely defined geometry within adipocyte-rich stroma. This model requires embedding fat cells within a 3D hydrogel; however, the isolation, handling, and culture of mature adipocytes are problematic due to their fragility. Therefore, we decided to differentiate the adipocytes in situ and used the 3T3-L1 murine preadipose cell line,27 a well-characterized model of adipogenesis that forms a fat pad when injected subcutaneously into mice.28 3T3-L1 preadipocytes spontaneously differentiate into mature adipocytes after reaching confluence when cultured on two-dimensional tissue culture-grade polystyrene.29 Differentiation is enhanced in the presence of medium containing dexamethasone (a glucocorticoid agonist), isobutyl-methylxanthine (a cyclic adenosine monophosphate [cAMP] phosphodiesterase inhibitor), and high concentrations of insulin.30 During adipogenesis, the cells round up and accumulate lipid droplets within the cytoplasm, which can be observed by staining with the lipophilic dyes Oil red O or Sudan III (Fig. 1a–e).
FIG. 1.
Time course of adipogenesis. (a–e) Oil red O staining of 3T3-L1 preadipocytes induced to differentiate on two-dimensional (2D) tissue culture polystyrene (TCPS). Sudan III (red) and nuclear (blue) staining of 3T3-L1 cells induced to differentiate in (f–j) three-dimensional collagen (3D Col), (k–o) collagen plus 5% laminin-rich extracellular matrix (Col + lrECM), or (p–t) collagen in the presence of the mammary epithelial-conditioned medium (CM; Col + CM). Extent of differentiation as determined by quantitative reverse transcription (RT)/polymerase chain reaction analysis of (u) lipoprotein lipase (LPL) and (v) peroxisome proliferator-activated receptor (PPAR)-γ2 expression levels; shown are average and standard error of the mean (SEM). *p < 0.01; **p < 0.001. Scale bars, 100 μm.
The ECM of normal adipose tissue in vivo is dominated by fibrillar type I collagen,31 and interactions between the preadipocytes and the surrounding collagen are critical for adipogenesis.32 When embedded in 3D gels of type I collagen and treated with differentiation medium, the preadipocytes formed fatty clusters ∼100-μm in diameter interspersed throughout the gel (Fig. 1f–j). Approximately 90% of the cells contained lipid droplets within the cytoplasm by day 6 after induction of differentiation. Laminin-rich ECM has been reported to enhance adipogenic differentiation in culture33 and in vivo.34 However, adding laminin-rich ECM to the collagen gels did not obviously affect the kinetics of differentiation or the size of the fatty clusters that formed (Fig. 1k–o). To determine whether mammary epithelial cells affected the differentiation of the adipocytes, we embedded 3T3-L1 cells in collagen, induced differentiation, and then treated with the mammary epithelial-conditioned medium. Staining lipid inclusions with Sudan III showed that samples treated with the mammary epithelial-conditioned medium formed fewer and smaller fatty clusters than controls (Fig. 1p–t). Phenotypic differentiation was confirmed by RT-PCR analysis of adipogenic markers, including LPL and the adipogenic transcription factor PPARγ2 (Fig. 1u, v). Expression of LPL and PPARγ2 were both decreased in the samples treated with the mammary epithelial-conditioned medium as compared to 3D collagen controls. These data suggest that mammary epithelial cells either inhibit or delay differentiation of the preadipocytes, and indicate that adipocytes should be engineered into a fatty stroma before introducing the mammary epithelial tubules.
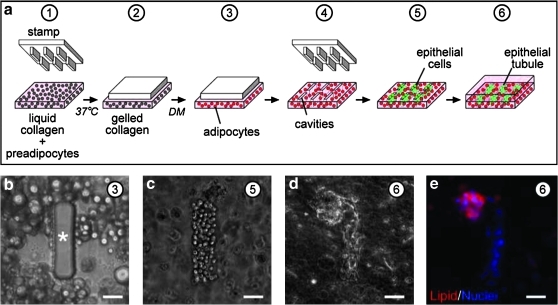
On the basis of the differentiation results, we adapted a microlithographic technique23,24 to engineer mammary epithelial tubules of defined shape and size within collagen gels containing differentiated adipocytes (schematized in Fig. 2a). Liquid neutralized collagen containing a suspension of 3T3-L1 preadipocytes was gelled against a silicone stamp having rectangular posts. The collagen concentrations were chosen to yield a gel with an elastic modulus of ∼500 Pa, within the range of values reported for the normal mouse mammary gland.21,35–37 After gelation, the samples were placed in differentiation medium to induce adipogenesis (Fig. 2b). Stamps were removed upon differentiation of the preadipocytes, and EpH4 murine mammary epithelial cells were seeded into the resulting cavities (Fig. 2c). The mammary epithelial cells spontaneously rearranged to form a tubule after overnight culture (Fig. 2d), similar to our previous studies using collagen without adipocytes.23,24,26 In the presence of mammary epithelial tubules, the 3T3-L1 adipocytes maintained their lipid droplets (Fig. 2e), suggesting that these cells remained phenotypically differentiated.
FIG. 2.
Construction of adipose-rich stroma. (a) Schematic of microlithography procedure used to build adipose-rich stroma. (b) Phase-contrast image of differentiated 3T3-L1 cells within collagen gel molded around poly(dimethylsiloxane) post. Phase-contrast images of mammary epithelial cells (c) immediately after and (d) 24 h after deposition within adipose-rich stroma. (e) Immunofluorescence image of mammary epithelial tubule 24 h after deposition into adipose-rich stroma, stained for nuclei (blue) and lipid inclusions (red). Scale bars, 50 μm. Circled numbers correspond to steps in (a). Asterisk denotes post.
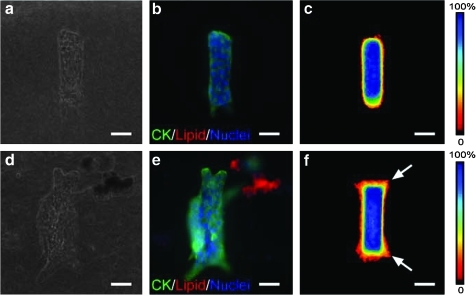
In this model, each epithelial tubule is engineered to be initially identical in size and geometry,23 which permits direct analysis of the sites of branching in the presence and absence of adipocytes. Mammary epithelial tubules remained quiescent and failed to branch when cultured in adipocyte-free collagen gels in the absence of an exogenous stimulus such as epidermal growth factor or HGF (Fig. 3a–c).23 However, when cultured within adipose-rich collagen, the mammary epithelial tubules branched within 24 h even in the absence of exogenous growth factors (Fig. 3d, e). At this time point, the branches that formed consisted of multicellular extensions within the collagen gel, without appreciable lumen formation. Staining for epithelial cytokeratins and stacking images of 50 tubules generated a frequency map of the locations of the mammary epithelial cells; these frequency maps indicated that the epithelial cells branched preferentially from the ends of the tubules (Fig. 3f). Branches continued to extend preferentially from the ends of the tubules at later time points.
FIG. 3.
Effect of fatty stroma on pattern of branching morphogenesis. (a) Phase-contrast and (b) immunofluorescence images of mammary epithelial tubules engineered into collagenous stroma. Shown are staining for epithelial cytokeratins (CK; green), lipid droplets (red), and nuclei (blue). (c) Branching is quantified using frequency maps as described in the text. (d) Phase-contrast and (e) immunofluorescence images and (f) frequency maps of mammary epithelial tubules engineered into fatty stroma. Scale bars, 50 μm. Arrows denote branching.
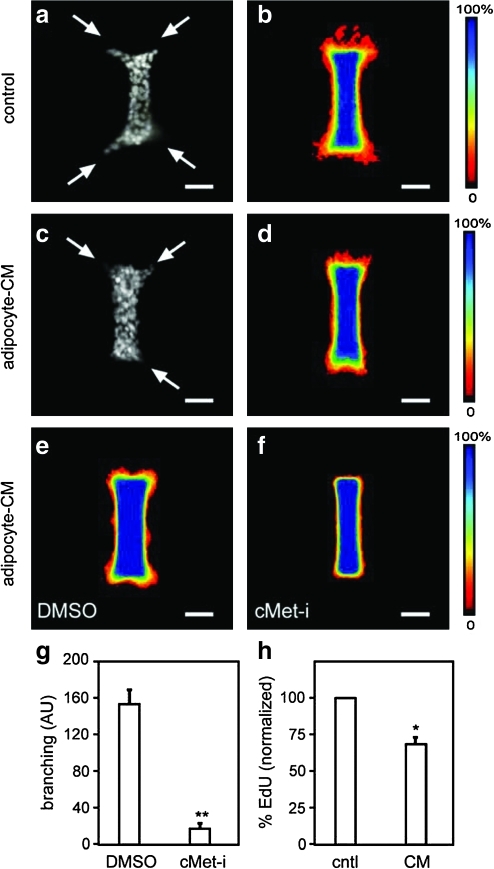
To determine whether adipocytes induced branching through juxtacrine (short-range) or paracrine (long-range) signals, we engineered mammary epithelial tubules in adipocyte-free collagen gels and treated with adipocyte-conditioned medium. Conditioned medium permits examination of adipocyte-derived factors in the absence of feedback from the epithelial cells. We found that 24-h treatment with adipocyte-conditioned medium induced branching morphogenesis of mammary tubules (Fig. 4a, b), with branch sites localized to the ends of the tubules. This pattern of branching from the ends is identical to that of tubules treated with epidermal growth factor or HGF in the absence of adipocytes (Fig. 4c, d).23 Differentiated 3T3-L1 adipocytes synthesize and secrete HGF,38 and a recent study found that depleting HGF activity from adipocyte-conditioned medium would prevent branching morphogenesis of mammary epithelial cells.39 To determine the role of HGF in the engineered system, we treated mammary epithelial tubules with conditioned medium and PHA665752, an inhibitor of the kinase activity of the HGF receptor, cMet.25 Control tubules exhibited robust branching from the ends, but cMet-inhibitor-treated tubules showed a striking and significant reduction in branching (Fig. 4e–g). The HGF-mediated induction of branching is not due to increased proliferation, as the conditioned medium-treated samples showed a modest but significant decrease in incorporation of the thymidine analog 5-ethynyl-2′-deoxyuridine (Fig. 4h), consistent with previous reports.39 Altogether, these data suggest that adipocytes induce branching of mammary epithelial tubules through soluble factors, including HGF, but do not directly determine the sites at which new branches form.
FIG. 4.
Effect of adipose-derived soluble factors on branching morphogenesis. Quantification of branching of mammary epithelial tubules engineered into collagenous stroma and treated with or without adipocyte-CM. Shown are (a) immunofluorescence image of nuclei and (b) frequency maps of control tubules, (c) immunofluorescence image of nuclei and (d) frequency maps of adipocyte-CM-treated tubules. Frequency maps of branching of tubules treated simultaneously with adipocyte-CM and with (e) dimethyl sulfoxide (DMSO) control or (f) cMet inhibitor (cMet-i) PHA665752 (200 nM). (g) Branching was quantified from frequency maps by measuring pixel intensity (arbitrary units, AU) at a fixed position from the ends of the tubules; shown are average and SEM of three independent experiments. (h) Proliferation of tubules treated with control (cntl) or adipocyte-CM, as determined by 5-ethynyl-2′-deoxyuridine (EdU) incorporation; shown are SEM of three independent experiments. *p < 0.05; **p < 0.005. Scale bars, 50 μm. Arrows denote branching.
Discussion
Tissue recombination studies11–13 and analysis of transgenic mice lacking a fat pad10 have revealed that adipose-rich stroma is required for normal branching morphogenesis of the embryonic and postnatal mammary epithelium in vivo. However, animal studies cannot be used to evaluate the role of adipocytes in determining the sites of branching and the final pattern of the mature epithelium. It has thus remained unclear whether adipose tissue acts as a global stimulator or as a chemoattractant in regulating mammary epithelial morphogenesis. Microscale tissue engineering approaches enable the recreation and relative placement of different parenchymal and stromal compartments with micrometer length-scale precision. These models are thus ideally suited to fill the gap between in vivo studies and conventional culture techniques.
Here, we used a microlithographic approach to surround mammary epithelial tubules of defined geometry with clusters of adipocytes within 3D collagen gels. Type I collagen actively regulates adipogenic differentiation32 and is the primary ECM component surrounding adipocytes in vivo.31 Indeed, we found robust differentiation of the preadipocytes when cultured within collagen, as determined by staining with lipophilic dyes and analyzing transcripts of specific markers. However, adipogenesis does not require exogenous collagen per se. Others have investigated a variety of 3D scaffolds, including fiber meshes of polyglycolic acid28 and silk.40 In depth analysis of the differentiation of human preadipocytes within one such scaffold, polyethylene glycol, has revealed that the stiffness of the polymer affects adipogenesis.41 Scaffold stiffness also regulates adipogenic differentiation of human and murine mesenchymal stem cells.42 Therefore, we believe that by increasing the seeding density of the preadipocytes and by further tuning the physical properties of the collagen gel, one could increase the fat content of the engineered stroma from fatty clusters to a more continuous fat pad. However, substratum stiffness also regulates the phenotypic differentiation of mammary epithelial cells,21,37 so the physical properties of the scaffold would need to be permissive for both adipogenesis and mammary tubule formation and branching morphogenesis.
Our engineering strategy was constrained by the finding that mammary epithelial cells inhibit adipogenic differentiation, which has also been observed in other epithelial cell lines and attributed to secreted factors.43–45 The factors responsible for inhibiting adipogenesis have not been defined, but a teleological explanation would suggest that the inhibition may be related to the time course of development in vivo, in that the fat pad is present before the epithelium invades, both in the embryo and during puberty.46 Previous investigations of cocultures of mammary epithelial cells and adipocytes or preadipocytes have also differentiated the preadipocytes separately before combining the two cell types, although the reasons for this approach were not discussed.19,39 These studies have found that adipocytes profoundly influence the morphology and function of mammary epithelial cells, by affecting proliferation and enhancing alveolar morphogenesis and functional differentiation.19,39 In our model system, adipocytes affected proliferation but not lactogenic differentiation of the mammary epithelial cells (Supplemental Fig. S1, available online at www.liebertonline.com/ten).
We used the engineered coculture model to investigate the effect of adipocytes in patterning new branch sites during branching morphogenesis of the mammary epithelium. We found that mammary epithelial tubules are induced to branch when embedded in adipose-rich stroma or treated with adipocyte-conditioned medium, suggesting that the induction of branching is mediated by paracrine signaling. Under both conditions, the new branches initiated from the ends of the tubules. This pattern is identical to that observed in the absence of adipocytes and is due in part to concentration gradients of autocrine inhibitors such as transforming growth factor-β.23 These data suggest that branch sites are independent of the cellular stromal microenvironment. Nonetheless, one could imagine stromal conditions that alter the diffusion of transforming growth factor-β or the mechanical properties of the matrix and thereby affect induction of branching. Adipocytes synthesize and secrete several growth factors that can stimulate branching of the mammary epithelium, including HGF,38,39 and inhibiting signaling through the cMet receptor by blocking its kinase activity disrupts adipocyte-mediated induction of branching of the engineered tubules. Strain-specific side-branching patterns have been attributed to the stroma rather than the epithelium,47,48 although the factors and/or properties involved remain elusive. Further, past studies have determined that the morphology of branches depends on the type of stroma that they encounter, with epithelium forming spiky protrusions when branching through collagen fibrils and rounded bulbous buds when adjacent to fatty stroma.49 Therefore, it is also possible that adipocytes may influence the final geometry of the gland by informing the directions in which new branches invade, rather than determining the sites from which they sprout along the trunk.
Pubertal branching morphogenesis of the mammary gland in vivo is stimulated by steroid hormones, including estrogen, which acts on estrogen receptors located in the stroma to induce production of mitogens including HGF.50,51 The stromal compartment of the mammary gland contains adipocytes, fibroblasts, blood vessels, lymphatics, nerves, and immune cells. Previous culture models have clearly demonstrated that the ability to induce branching is not limited to one stromal cell type. A mixed population of preadipocytes and adipocytes differentiated from human adipose-derived stem cells was recently found to induce branching of MCF10A breast epithelial cells, also apparently through HGF.39 Fibroblast-conditioned medium has been shown to induce tubulogenesis and branching morphogenesis of TAC-2 murine mammary epithelial cells (a subline of NMuMG cells) through secretion of HGF,52,53 and coculture with fibroblasts induces branching of primary mammary organoids.7 These culture models could not address branch site selection, but our data suggest that the patterning of branch sites is an intrinsic function of the epithelium and may be independent of the cellular source of the stromal stimulus. It is possible that the levels of growth factor secreted by these different stromal cell types differ, which could differentially affect the extent of branching of the mammary epithelium.
In conclusion, we have used microlithography to build a 3D coculture model consisting of adipose-rich collagen surrounding mammary epithelial tubules of precise geometry. This model is useful for examining the principles underlying normal mammary development, as well as future efforts to engineer breast tissue for reconstructive purposes.54 This study contributes to a growing family of microscale tissue engineering approaches55,56 demonstrating the power of recapitulating tissue structure in culture.
Supplementary Material
Acknowledgments
We thank Nikolce Gjorevski for rheometry and Joe Tien for helpful discussions. This work was supported in part by the NIH (GM083997 and CA128660), Susan G. Komen for the Cure, and the David & Lucile Packard Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Disclosure Statement
No competing financial interests exist.
References
- 1.Nelson C.M. Bissell M.J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams J.M. Daniel C.W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 3.Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- 4.Sakakura T. Nishizuka Y. Dawe C.J. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- 5.Lilla J.N. Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol. 2010;337:124. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouon-Evans V. Rothenberg M.E. Pollard J.W. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 7.Simian M. Hirai Y. Navre M. Werb Z. Lochter A. Bissell M.J. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 2001;128:3117. doi: 10.1242/dev.128.16.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternlicht M.D. Kouros-Mehr H. Lu P. Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakakura T. Sakagami Y. Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91:202. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 10.Couldrey C. Moitra J. Vinson C. Anver M. Nagashima K. Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223:459. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino K. Transplantability of mammary gland in brown fat pads of mice. Nature. 1967;213:194. doi: 10.1038/213194a0. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K. Morphogenesis and growth potentiality of mammary glands in mice. I. Transplantability and growth potentiality of mammary tissue of virgin mice. J Natl Cancer Inst. 1962;29:835. [PubMed] [Google Scholar]
- 13.Hoshino K. Mammary transplantation and its histogenesis. In: Yokoyama A., editor; Mizuno H., editor; Nagasawa H., editor. Physiology of Mammary Glands. Baltimore, MD: University Park Press; 1980. pp. 163–228. [Google Scholar]
- 14.Nelson C.M. Bissell M.J. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmick N.A. Neilson E.G. Moses H.L. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine J.F. Stockdale F.E. 3T3-L1 adipocytes promote the growth of mammary epithelium. Exp Cell Res. 1984;151:112. doi: 10.1016/0014-4827(84)90361-6. [DOI] [PubMed] [Google Scholar]
- 17.Levine J.F. Stockdale F.E. Cell-cell interactions promote mammary epithelial cell differentiation. J Cell Biol. 1985;100:1415. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovey R.C. MacKenzie D.D. McFadden T.B. The proliferation of mouse mammary epithelial cells in response to specific mitogens is modulated by the mammary fat pad in vitro. In Vitro Cell Dev Biol Anim. 1998;34:385. doi: 10.1007/s11626-998-0020-2. [DOI] [PubMed] [Google Scholar]
- 19.Zangani D. Darcy K.M. Shoemaker S. Ip M.M. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247:399. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]
- 20.Reichmann E. Ball R. Groner B. Friis R.R. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcaraz J. Xu R. Mori H. Nelson C.M. Mroue R. Spencer V.A. Brownfield D. Radisky D.C. Bustamante C. Bissell M.J. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daya S. Loughlin A.J. Macqueen H.A. Culture and differentiation of preadipocytes in two-dimensional and three-dimensional in vitro systems. Differentiation. 2007;75:360. doi: 10.1111/j.1432-0436.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson C.M. Vanduijn M.M. Inman J.L. Fletcher D.A. Bissell M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson C.M. Inman J.L. Bissell M.J. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat Protoc. 2008;3:674. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puri N. Khramtsov A. Ahmed S. Nallasura V. Hetzel J.T. Jagadeeswaran R. Karczmar G. Salgia R. A selective small molecule inhibitor of c-Met, PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67:3529. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 26.Mori H. Gjorevski N. Inman J.L. Bissell M.J. Nelson C.M. Self-organization of engineered epithelial tubules by differential cellular motility. Proc Natl Acad Sci U S A. 2009;106:14890. doi: 10.1073/pnas.0901269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green H. Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113. [Google Scholar]
- 28.Fischbach C. Spruss T. Weiser B. Neubauer M. Becker C. Hacker M. Gopferich A. Blunk T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Green H. Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 30.Cornelius P. MacDougald O.A. Lane M.D. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 31.Napolitano L. The differentiation of white adipose cells: an electron microscope study. J Cell Biol. 1963;18:663. doi: 10.1083/jcb.18.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun T.H. Hotary K.B. Sabeh F. Saltiel A.R. Allen E.D. Weiss S.J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez Fernandez J.L. Ben-Ze'ev A. Regulation of fibronectin, integrin and cytoskeleton expression in differentiating adipocytes: inhibition by extracellular matrix and polylysine. Differentiation. 1989;42:65. doi: 10.1111/j.1432-0436.1989.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi N. Toriyama K. Nicodemou-Lena E. Inou K. Torii S. Kitagawa Y. Reconstituted basement membrane potentiates in vivo adipogenesis of 3T3-F442A cells. Cytotechnology. 1999;31:215. doi: 10.1023/A:1008198731341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levental I. Levental K.R. Klein E.A. Assoian R. Miller R.T. Wells R.G. Janmey P.A. A simple indentation device for measuring micrometer-scale tissue stiffness. J Phys Condens Matter. 2010;22:194120. doi: 10.1088/0953-8984/22/19/194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levental K.R. Yu H. Kass L. Lakins J.N. Egeblad M. Erler J.T. Fong S.F. Csiszar K. Giaccia A. Weninger W. Yamauchi M. Gasser D.L. Weaver V.M. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paszek M.J. Zahir N. Johnson K.R. Lakins J.N. Rozenberg G.I. Gefen A. Reinhart-King C.A. Margulies S.S. Dembo M. Boettiger D. Hammer D.A. Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Rahimi N. Saulnier R. Nakamura T. Park M. Elliott B. Role of hepatocyte growth factor in breast cancer: a novel mitogenic factor secreted by adipocytes. DNA Cell Biol. 1994;13:1189. doi: 10.1089/dna.1994.13.1189. [DOI] [PubMed] [Google Scholar]
- 39.Wang X. Zhang X. Sun L. Subramanian B. Maffini M.V. Soto A.M. Sonnenschein C. Kaplan D.L. Preadipocytes stimulate ductal morphogenesis and functional differentiation of human mammary epithelial cells in 3D silk scaffolds. Tissue Eng Part A. 2009;15:3087. doi: 10.1089/ten.tea.2008.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauney J.R. Nguyen T. Gillen K. Kirker-Head C. Gimble J.M. Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stacey D.H. Hanson S.E. Lahvis G. Gutowski K.A. Masters K.S. In vitro adipogenic differentiation of preadipocytes varies with differentiation stimulus, culture dimensionality, and scaffold composition. Tissue Eng Part A. 2009;15:3389. doi: 10.1089/ten.TEA.2008.0293. [DOI] [PubMed] [Google Scholar]
- 42.Huebsch N. Arany P.R. Mao A.S. Shvartsman D. Ali O.A. Bencherif S.A. Rivera-Feliciano J. Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Julianelli V.L. Guerra L.N. Calvo J.C. Cell-cell communication between mouse mammary epithelial cells and 3T3-L1 preadipocytes: effect on triglyceride accumulation and cell proliferation. Biocell. 2007;31:237. [PubMed] [Google Scholar]
- 44.Calvo J.C. Chernick S. Rodbard D. Mouse mammary epithelium produces a soluble heat-sensitive macromolecule that inhibits differentiation of 3T3-L1 preadipocytes. Proc Soc Exp Biol Med. 1992;201:174. doi: 10.3181/00379727-201-43496. [DOI] [PubMed] [Google Scholar]
- 45.Yang C.C. Ellis S.E. Xu F. Burg K.J. In vitro regulation of adipogenesis: tunable engineered tissues. J Tissue Eng Regen Med. 2007;1:146. doi: 10.1002/term.17. [DOI] [PubMed] [Google Scholar]
- 46.Sakakura T. Mammary embryogenesis. The Mammary Gland: Development, Regulation, and Function. In: Neville M.C., editor; Daniel C.W., editor. New York: Plenum Press; 1987. pp. 37–65. [Google Scholar]
- 47.Naylor M.J. Ormandy C.J. Mouse strain-specific patterns of mammary epithelial ductal side branching are elicited by stromal factors. Dev Dyn. 2002;225:100. doi: 10.1002/dvdy.10133. [DOI] [PubMed] [Google Scholar]
- 48.Yant J. Buluwela L. Niranjan B. Gusterson B. Kamalati T. In vivo effects of hepatocyte growth factor/scatter factor on mouse mammary gland development. Exp Cell Res. 1998;241:476. doi: 10.1006/excr.1998.4028. [DOI] [PubMed] [Google Scholar]
- 49.Daniel C.W. Berger J.J. Strickland P. Garcia R. Similar growth pattern of mouse mammary epithelium cultivated in collagen matrix in vivo and in vitro. Dev Biol. 1984;104:57. doi: 10.1016/0012-1606(84)90036-8. [DOI] [PubMed] [Google Scholar]
- 50.Cunha G.R. Young P. Hom Y.K. Cooke P.S. Taylor J.A. Lubahn D.B. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2:393. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H.Z. Bennett J.M. Smith K.T. Sunil N. Haslam S.Z. Estrogen mediates mammary epithelial cell proliferation in serum-free culture indirectly via mammary stroma-derived hepatocyte growth factor. Endocrinology. 2002;143:3427. doi: 10.1210/en.2002-220007. [DOI] [PubMed] [Google Scholar]
- 52.Soriano J.V. Pepper M.S. Nakamura T. Orci L. Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J Cell Sci. 1995;108(Pt 2):413. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- 53.Soriano J.V. Pepper M.S. Orci L. Montesano R. Roles of hepatocyte growth factor/scatter factor and transforming growth factor-beta1 in mammary gland ductal morphogenesis. J Mammary Gland Biol Neoplasia. 1998;3:133. doi: 10.1023/a:1018790705727. [DOI] [PubMed] [Google Scholar]
- 54.Patrick C.W. Breast tissue engineering. Annu Rev Biomed Eng. 2004;6:109. doi: 10.1146/annurev.bioeng.6.040803.140032. [DOI] [PubMed] [Google Scholar]
- 55.Nelson C.M. Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Khetani S.R. Bhatia S.N. Engineering tissues for in vitro applications. Curr Opin Biotechnol. 2006;17:524. doi: 10.1016/j.copbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.