Abstract
Skeletal muscle-derived stem cells (MDSCs) can undergo osteogenesis when treated with bone morphogenetic proteins (BMPs), making them a potential cell source for bone tissue engineering. The signaling pathways that regulate BMP4-induced osteogenesis in MDSCs are not well understood, although they may provide a means to better regulate differentiation during bone regeneration. The objective of this study was to characterize the signaling pathways involved in the BMP4-induced osteogenesis of MDSCs. Cells were treated with BMP4 and specific inhibitors to the extracellular signal-regulated kinases 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), and phosphatidyl inositol 3-kinase (PI3K) pathways (PD98059, SB203580, and Ly294002, respectively). Cellular proliferation, expression of osteoblast-related genes, alkaline phosphatase (ALP) activity, and tissue mineralization were measured to determine the role of each pathway in the osteogenic differentiation of MDSCs. Inhibition of the ERK1/2 pathway increased ALP activity and mineralization, whereas inhibition of the p38 MAPK pathway decreased osteogenesis, suggesting opposing roles of these pathways in the BMP4-induced osteogenesis of MDSCs. Inhibition of the PI3K pathway significantly increased mineralization by MDSCs. These findings highlight the involvement of the ERK1/2, p38 MAPK, and PI3K pathways in opposing capacities in MDSC differentiation and warrant further investigation, as it may identify novel therapeutic targets for the development of stem cell-based therapies for bone tissue engineering.
Introduction
Stem cells play a key role in embryonic development, organogenesis, and tissue regeneration in adults.1 Because of their self-renewal potential and ability to differentiate toward various lineages, stem cells have become a key component of tissue engineering approaches. Among the numerous stem cell sources currently studied for their application in regenerative medicine, one can include muscle-derived stem cells (MDSCs). It is an early myogenic progenitor cell that has been isolated from the mouse skeletal muscle using a modified preplate technique.2,3 MDSCs have the ability to differentiate toward skeletal muscle, neural, endothelial, and hematopoietic tissues,3,4 and when treated with bone morphogenetic protein 2 (BMP2) or BMP4, MDSCs are capable of osteogenic and chondrogenic differentiation in vitro, have been used for bone and cartilage formation in vivo, and show promise in the developing field of bone and cartilage tissue engineering.5–9
To undergo cellular differentiation toward a specific lineage, all cells require the involvement of intrinsic signaling pathways, which can be activated by extrinsic signals, such as growth factors. BMPs are biological factors that play a key role in the osteogenic and chondrogenic differentiation of numerous cells in vitro and in vivo. The main BMP signaling pathway utilizes signaling molecules known as Smads,10–12 although BMPs have been also shown to utilize other cell signaling pathways such as the mitogen-activated protein kinase (MAPK) cascades and the phosphatidyl inositol 3-kinase (PI3K).13–21
Inhibitory compounds have been widely used to elucidate the role of the pathways they inhibit. Some of these compounds are pyridinyl imidazole (SB203580) and 2′-amino-3′-methoxyflavone (PD98059), which are inhibitors of the MAPK cascades,22,23 and 2-(4-morpholinyl)-8-phenylchromone (Ly294002), which inhibits the PI3K pathway.24 SB203580 specifically inhibits the MAPK family member known as stress-activated protein kinase 2a, also known as p38.23 PD98059 binds to MAPK kinase 1 (MEK1), preventing its activation by upstream proteins such as Raf, which makes it unable to phosphorylate extracellular signal-regulated kinases (ERK1/2).22 SB203580 thus inhibits the p38 MAPK pathway, and PD98059 inhibits the ERK1/2 pathway. Ly294002 binds to the ATP-binding site of PI3K, preventing it from phosphorylating Akt and thus inhibiting the PI3K-Akt pathway.24
The p38 MAPK pathway has been linked to osteogenesis in cell types such as human osteoblastic cells,25,26 mouse MC3T3-E1 preosteoblastic cells,27,28 and mouse C2C12 cells.14,29 Gallea et al. showed that both the p38 MAPK and ERK1/2 cascades are activated by stimulation of C2C12 cells with BMP2.14 In this specific cell line, blocking the p38 MAPK pathway with SB203580, a p38-specific inhibitor, led to a dose-dependent decrease in alkaline phosphatase (ALP) activity, whereas inhibition of the ERK1/2 cascade by its selective inhibitor PD98059 led to a slight increase in ALP activity. The PI3K-Akt pathway has been also implicated in the differentiation of osteoblasts, myoblasts, chondrocytes, and adipocytes.15,30–34 BMP2 can stimulate PI3K activity in osteogenic cells and its inhibition with the specific inhibitor Ly294002 prevented BMP2-induced ALP activity.15
BMP2 and BMP4 are highly homologous molecules, differing solely in their amino terminal region. Both can bind to BMP receptors type I and type II, which come together to enable BMP receptor type II to phosphorylate BMP receptor type I, leading to Smad activation.10,35 Although many cell signaling studies have been performed with BMP2 stimulation, inhibitors such as PD98059, SB203580, or Ly294002 have been also studied using BMP4.20,21,28,36–38 It has been shown that BMP4-stimulated osteocalcin synthesis is negatively regulated by ERK1/2, whereas p38 MAPK is a positive regulator of its synthesis in MC3T3-E1 cells.36 BMP4-induced ALP activity can be reduced in the same cells with SB203580, also suggesting an important role of p38 MAPK in BMP4-induced osteogeneis.28 Using Ly294002 on human multipotent mesenchymal stromal cells (MSCs), it was determined that the PI3K pathway may play an important role in endogenous BMP osteogenesis.21
To date, the signaling pathways involved in the BMP4-induced osteogenic differentiation of MDSCs are not well known. Elucidating the role of specific signaling pathways in the BMP4-induced osteogenic differentiation of MDSCs may allow for increased regulation of differentiation, which may in turn lead to novel approaches to improve the role of MDSCs for bone tissue engineering. Therefore, this study tested the hypothesis that ERK1/2, p38 MAPK, and PI3K pathways affect BMP4-induced osteogenic differentiation of MDSCs by playing a role in their cell viability, expression of osteoblast-related genes, ALP activity, and tissue mineralization.
Experimental Procedures
Isolation and culture of MDSCs
MDSCs were isolated from 3-week-old C57BL/10J mice using a modified preplate technique.2,3 Cells were cultured in phenol red-free proliferation medium (PM) consisting of Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) supplemented with 110 mg/L sodium pyruvate (Sigma-Aldrich), 584 mg/L l-glutamine, 10% fetal bovine serum, 10% horse serum, 1% penicillin/streptomycin (all from Invitrogen), and 0.5% chick embryo extract (Accurate Chemical Co.) at 37°C in a humidified atmosphere of 5% CO2 in air. To determine the minimal dose of BMP4 necessary to have an effect on ALP activity and Alp gene expression, MDSCs were plated at a density of 1500 cells/cm2 and, on the following day, were treated with BMP4 (0, 50, 100, or 200 ng/mL). All subsequent monolayer assays in this study began with cells plated at a density of 1500 cells/cm2 and, on the following day, were treated with or without the optimized concentration of BMP4 (50 ng/mL) and the inhibitors PD98059 (Biomol International), SB203580 (Biomol International), or Ly294002 (Cell Signaling), which are specific inhibitors for the ERK1/2, p38 MAPK, and PI3K pathways, respectively. Inhibitors were dissolved in dimethyl sulfoxide before use, and control cultures received a concentration of 25 μM of dimethyl sulfoxide, which is equivalent to the highest concentration found in the treated cultures. In all assays, cells were incubated with the inhibitors for 1 h before addition of BMP4.
Cell viability
Cell viability was measured in 96-well microtiter flat-bottomed plates. Inhibitors were tested at concentrations of 25 μM (PD98059 and SB203580) or 10 μM (Ly294002) and medium was refreshed every 48 h (100 μL/well). The inhibitor concentrations were chosen based on previously published studies14,17 and preliminary data. Cell viability was assayed with the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) after 4 days of treatment. At the end of the assay, 20 μL of CellTiter 96® AQueous One Solution reagent was added to each well, the plate was incubated for 2 h at 37°C in a humidified, 5% CO2 incubator, and the absorbance was measured at 490 nm.
Quantitative polymerase chain reaction analysis of osteogenic genes
MDSCs were treated as described above for 24 h, after which total RNA was collected using the RNeasy kit (Qiagen). Quantitative real-time PCR (qPCR) analysis was performed with Taqman® One-step RT-PCR Master Mix (Applied Biosystems) as described previously.39 RNA samples (1 μL) were added to sequence-specific primers and Taqman® probes (200 nM per 10 μL reaction) for Alp, Runx2, and Osterix (Osx) genes. All target genes were normalized to 18S (primers and probe from Applied Biosystems). The sequences of the target gene primers and probes were previously published.39 All target gene probes were labeled with FAM as the 5′ reporter dye and TAMRA as the 3′ quencher dye. qPCR assays were carried out in triplicate on an ABI Prism 7900HT sequence detection system in the core facility of the Genomics and Proteomics Core Laboratories of the University of Pittsburgh. Data were analyzed using SDS 2.1 Software from Applied Biosystems.
ALP activity
For ALP enzymatic activity, MDSCs were treated with BMP4 in the presence or absence of the inhibitors PD98059 (25 μM), SB203580 (25 μM), or Ly294002 (10 μM), as described above. Three days after initiation of treatment, the cells were washed once with phosphate-buffered saline, lysed in 0.1% Triton-X in water, and assayed using SIGMA FAST™ p-nitrophenyl phosphate tablets (N-2770; Sigma-Aldrich). Diluted samples of cell lysate were incubated with p-nitrophenyl for 30 min in the dark at room temperature. Following this incubation, absorbance at 405 nm was determined. Total protein was also calculated by assaying diluted samples of cell lysate using the Micro BCA™ protein assay (Pierce) according to manufacturer's instructions. ALP activity was normalized per mg protein and expressed as nanomoles of p-nitrophenyl liberated per microgram of total cellular protein.
Osteogenic pellet culture
To test mineralization, 250,000 MDSCs were cultured as pellets, as previously described,40 in osteogenic medium (OSM) containing phenol red-free DMEM supplemented with 110 mg/L sodium pyruvate, 584 mg/L l-glutamine, 10% fetal bovine serum, 1% penicillin/streptomycin, 10−7 M dexamethasone, 5 × 10−5 M ascorbic-acid-2-phosphate, and 10−2 M β-glycerophosphate and with BMP4 (100 ng/mL) in the presence or absence of pathway inhibitors (25 μM PD98059, 25 μM SB203580, and 10 μM Ly294002) for 21 days. Medium was replaced every 3 days. Preliminary data testing different doses of BMP4 on pellet cultures indicated that BMP4 at a concentration of 100 ng/mL was necessary to induce a measurable amount of mineralization in the MDSC population used in this study. To assess mineralization, all pellets were analyzed at 14 and 21 days using a μCT imaging system (vivaCT 40; Scanco Medical) with the following settings: 55 kVp of energy, 200 ms integration time, and an isotropic voxel size of 10.5 μm.40 Two-dimensional image slices were obtained and contour lines were drawn to define the volume of interest. An appropriate threshold was chosen for the bone voxels by visually matching thresholded areas to grayscale images. The threshold was kept constant throughout the analyses of each pellet. This led to a three-dimensional (3D) reconstruction of the mineralized tissue within the pellets and provided quantitative data on mineralized tissue volume (mm3) and density (mg hydroxyapatite [HA]/cm3). In a subsequent experiment, pellets were made and treated with BMP4 for 21 days, but were only treated with pathway inhibitors for the first 7 days of culture.
Statistical analysis
All in vitro monolayer experiments (cell viability, qPCR, and ALP activity) were performed in triplicate and repeated three times. One representative experiment is reported as the mean of three treatment replicates ± standard error of mean. For osteogenic pellet culture experiments, data represent four pellets per treatment group. Data for Figures 1, 2, 4, and 5 were analyzed using a one-way, between-subjects analysis of variance and Tukey post hoc analysis. Data for Figure 3 were analyzed using Student's t-tests for each comparison (with and without inhibitor). Data for Figure 6 were analyzed using separate (for volume and density, respectively) two-way, within-subject analysis of variance and Tukey post hoc analysis. All analyses were performed with SPSS statistical software. A p-value of <0.05 was considered significant.
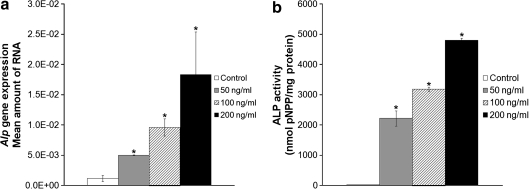
FIG. 1.
Response of muscle-derived stem cells (MDSCs) to bone morphogenetic protein 4 (BMP4) stimulation (0–200 ng/mL). (a) Alp gene expression at 24 h after initiation of BMP4 stimulation. (b) Alkaline phosphatase (ALP) activity at 3 days after initiation of BMP4 stimulation. Bars represent mean ± standard error of mean (SEM); n = 3. *p < 0.05 compared with control.
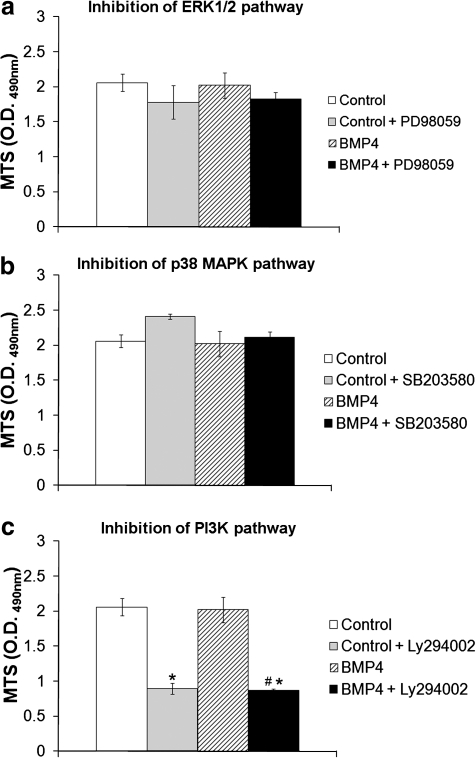
FIG. 2.
Cellular viability of MDSCs with or without BMP4 stimulation (50 ng/mL) and inhibition of the (a) extracellular signal-regulated kinases 1/2 (ERK1/2), (b) p38 mitogen-activated protein kinase (MAPK), and (c) phosphatidyl inositol 3-kinase (PI3K) pathways. Bars represent mean ± SEM; n = 3. *p < 0.05 compared with control. #p < 0.05 compared with BMP4.
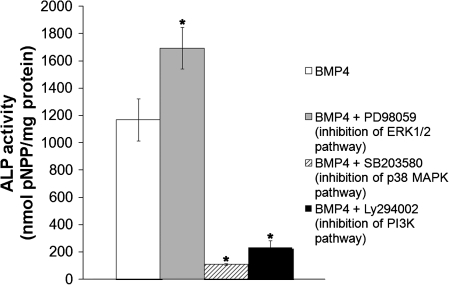
FIG. 4.
ALP activity of MDSCs treated with BMP4 (50 ng/mL) and inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways for 3 days. Bars represent mean ± SEM; n = 3. *p < 0.05 compared with BMP4.
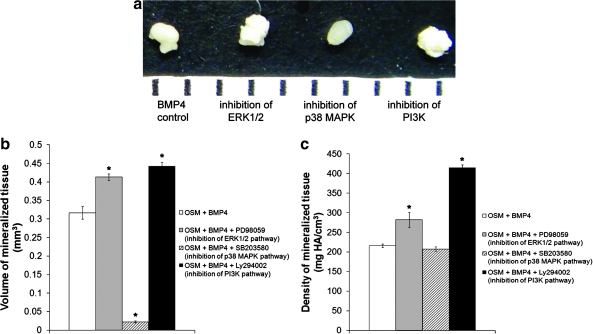
FIG. 5.
Osteogenic pellet culture of MDSCs treated with BMP4 (100 ng/mL) and inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways for 21 days. (a) Macroscopic images of MDSC pellets (1 mm scale). (b) Volume of mineralized tissue and (c) density of mineralized tissue determined by μCT analysis. Bars represent mean ± SEM; n = 4 pellets/group. *p < 0.05 compared with osteogenic medium (OSM) + BMP4. Color images available online at www.liebertonline.com/ten.
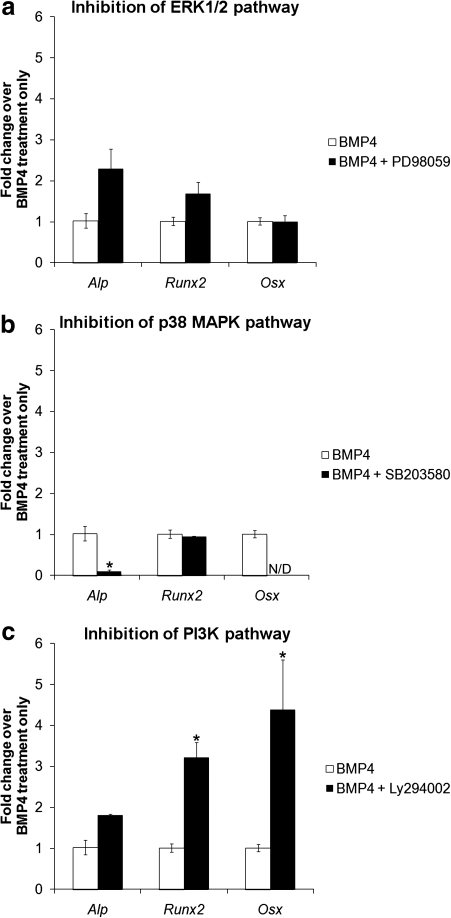
FIG. 3.
Alp, Runx2, and Osx gene expression in MDSCs treated with BMP4 (50 ng/mL) in the presence or absence of the inhibitors to the (a) ERK1/2, (b) p38 MAPK, and (c) PI3K pathways. Bars represent mean ± SEM; n = 3. *p < 0.05 compared with BMP4.
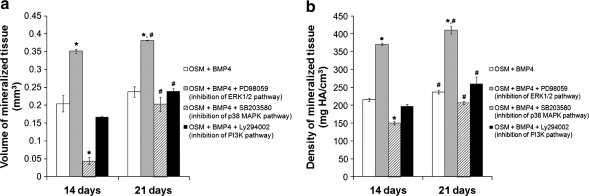
FIG. 6.
Osteogenic pellet culture of MDSCs treated with BMP4 (100 ng/mL) for 21 days and inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways for the first 7 days of culture. (a) Volume of mineralized tissue and (b) density of mineralized tissue determined by μCT analysis. Bars represent mean ± SEM; n = 4 pellets/group. *p < 0.05 compared with OSM + BMP4. #p < 0.05 compared with 14 days.
Results
Response of MDSCs to BMP4 stimulation
Twenty-four hours after the initiation of BMP4 treatment (50, 100, or 200 ng/mL), a dose response was observed for Alp gene expression and ALP activity. Both were significantly higher than untreated control at all BMP4 concentrations tested (Fig. 1a, b, *p < 0.05). The lowest BMP4 concentration necessary to have a significant effect on MDSCs, 50 ng/mL, was chosen for all other assays involving monolayer culture.
Effect of pathway inhibitors on cell viability
Specific inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways were added to untreated (control) or BMP4-treated MDSCs and the cell viability was measured after 4 days (Fig. 2). Cell viability was not significantly increased in BMP4-treated cells compared with untreated control cells. Inhibition of the ERK1/2 pathway had no effect on the viability of MDSCs (Fig. 2a), and inhibition of the p38 MAPK pathway similarly did not affect MDSC viability (Fig. 2b). Inhibition of the PI3K pathway in MDSCs displayed a significant decrease in cell viability and this was evident when both control cells and BMP4-treated cells were incubated with the pathway inhibitor (Fig. 2c, *p < 0.05 vs. control, #p < 0.05 vs. BMP4).
Effect of pathway inhibitors on osteogenic differentiation
Osteogenic gene expression
Inhibition of the ERK1/2 pathway by addition of PD98059 to BMP4-treated MDSCs did not show a significant effect on Alp, Runx2, or Osx gene expression (Fig. 3a). However, a trend of increased fold change over BMP4 treatment only was observed for both Alp and Runx2 gene expression (Fig. 3a). A significant decrease in Alp gene expression was seen as a result of inhibiting the p38 MAPK pathway during BMP4 stimulation (Fig. 3b, *p < 0.05). A decrease in Osx gene expression upon addition of SB203580 to BMP4 was also observed, as levels were nondetectable by qPCR (Fig. 3b, N/D). Following inhibition of the PI3K pathway with Ly294002, an increase in Alp, Runx2, and Osx gene expression was observed in comparison to BMP4 treatment only (Fig. 3c). This increase was statistically significant for Runx2 and Osx gene expression (Fig. 3c, *p < 0.05).
ALP activity
Inhibition of the ERK1/2 pathway led to a significant increase in ALP activity in MDSCs that were treated with BMP4 (Fig. 4, *p < 0.05 vs. BMP4). The opposite was seen with inhibition of the p38 MAPK pathway, where a significant decrease in ALP activity was found (Fig. 4, *p < 0.05 vs. BMP4). Inhibition of the PI3K pathway in BMP4-treated MDSCs significantly decreased ALP activity (Fig. 4, *p < 0.05 vs. BMP4).
Mineralization
Cell pellets are shown in Figure 5a. From macroscopic observation, pellets cultured with BMP4 and the inhibitor to either the ERK1/2 or the PI3K pathway appear less translucent than pellets that received BMP4 only or BMP4 and the inhibitor to the p38 MAPK pathway. These differences were quantified by μCT analyses to obtain mineralized tissue volume and density. The pellets treated with BMP4 and inhibitors to the ERK1/2 and PI3K pathways had significantly more mineralized tissue volume (Fig. 5b, *p < 0.05 vs. OSM + BMP4) and density (Fig. 5c, *p < 0.05 vs. OSM + BMP4) than the pellets treated with BMP4 only. Inhibition of the p38 MAPK pathway significantly decreased mineralized tissue volume (Fig. 5b, *p < 0.05 vs. OSM + BMP4). However, mineralized tissue density was not affected (Fig. 5c).
To determine whether a sustained inhibition of the pathways was necessary to affect mineralization, pellets were treated with BMP4 for the 21-day duration of the experiment, but were treated with inhibitors to specific pathways for only the first 7 days of culture. μCT analysis showed that the pellets that received the inhibitor to the ERK1/2 pathway for 7 days had significantly greater mineralized tissue volume and density than the pellets that were treated with BMP4 only, and this increase remained after 21 days of pellet culture (Fig. 6a, b, *p < 0.05 vs. OSM + BMP4). Inhibition of the p38 MAPK pathway for the first 7 days of pellet culture showed a significant decrease in mineralized tissue volume and density compared with BMP4 treatment only at 14 days (Fig. 6a, b, *p < 0.05 vs. OSM + BMP4). Interestingly, there was a significant increase in mineralization in this group between 14 and 21 days of culture, making it no longer different from control (Fig. 6a, b, #p < 0.05 vs. 14 days). Unlike continuous inhibition of the PI3K pathway for 21 days, inhibition of this pathway for only the first 7 days of pellet culture did not lead to differences in mineralized tissue volume or density compared with the pellets treated with BMP4 only (Fig. 6a, b).
A summary of all these results has been included in Table 1 to provide an overview of the effect of each pathway on the osteogenic differentiation of MDSCs.
Table 1.
Overview of the Effect of Different Pathway Inhibitors on the Bone Morphogenetic Protein 4-Induced Osteogenic Differentiation of Muscle-Derived Stem Cells
| |
|
|
Assay results |
|
|
||
|---|---|---|---|---|---|---|---|
| |
|
|
Gene expression |
|
|
||
| Inhibitor used | Pathway inhibited | Proliferation | Alp | Runx2 | Osx | ALP activity | Mineralization |
| PD98059 | ERK1/2 | — | ↑ | ↑ | — | ↑a | ↑a |
| SB203580 | p38 MAPK | — | ↓a | — | ↓a | ↓a | ↓a |
| Ly294002 | PI3K | ↓a | ↑ | ↑a | ↑a | ↓a | ↑a |
Increase or decrease that is statistically significant (p < 0.05).
ALP, alkaline phosphatase; ERK, extracellular signal-regulated kinases; MAPK, mitogen-activated protein kinase; PI3K, phosphatidyl inositol 3-kinase.
Discussion
In this study, the BMP4 signaling of MDSCs was characterized by studying an MDSC population that readily undergoes osteogenic differentiation when treated with BMP4. By taking advantage of specific chemical inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways, such as PD98059, SB203580, and Ly294002, respectively, it was possible to elucidate the involvement of these different pathways in the BMP4-induced osteogenesis of MDSCs.
In studying the osteogenic differentiation of MDSCs induced by BMP4, it was also important to determine how this growth factor affected cell metabolic activity, and the involvement of the different pathways analyzed in this study. However, when BMP4 was added to the PM, the MDSC population used was unaffected. This may be explained by an inverse relationship between proliferation and differentiation, where molecules that promote differentiation may also prevent cell cycle reentry. Although MDSCs readily responded to BMP4 by undergoing osteogenic differentiation, they did not increase their proliferation. By using chemical inhibitors, it was determined that the viability of MDSCs was affected by inhibition of the PI3K pathway but not by inhibition of the p38 MAPK or ERK1/2 pathway. Activation of the PI3K/Akt signaling pathway by growth factors leads to the phosphorylation of the BCL-2 family member BAD, which in turn prevents apoptosis and promotes cell survival.41,42 Activation of the ERK1/2 pathway also follows a similar mechanism.43 Reduced viability was thus expected after blockade of the PI3K signaling pathway with Ly294002 or inhibition of the ERK1/2 signaling pathway with PD98059. On the other hand, activation of p38 MAPK is known to induce exit from the cell cycle and to lead to the differentiation of various cell types.44,45 Its inhibition by SB203580 has increased the proliferation of mammalian cardiomyocytes, although these cells are considered terminally differentiated and incapable of proliferation.46 In the MDSCs used in this study, reduced cellular viability was only seen with Ly294002 and has previously been reported in other adult muscle cells.47 Inhibition of the ERK1/2 and p38 MAPK pathways may not play a significant role in the cellular viability of MDSCs, or a higher concentration of the inhibitors may be needed to have an effect.
The role of the ERK1/2, p38 MAPK, and PI3K pathways was also investigated with respect to the role they play in the osteogenic differentiation of MDSCs. Genes such as Alp, Runx2, and Osx were investigated, as they are key early indicators of osteogenic differentiation. The inhibition of the ERK1/2 pathway during BMP4 stimulation did not significantly affect osteogenic gene expression, although it showed a trend toward increasing Alp compared with BMP4 treatment only. It did, however, increase ALP activity after 3 days of stimulation with BMP4. This effect has been also shown in C2C12 cells and human MSCs, suggesting that high ERK activity negatively regulates BMP stimulation of ALP.14,17 In the present study, the effect of pathway inhibitors on matrix mineralization was also studied. Inhibition of the ERK1/2 pathway in BMP4-treated MDSCs affected mineralization by significantly increasing it, compared with BMP4 treatment only. The BMP4-induced synthesis of osteocalcin, a bone-type extracellular matrix protein, in MC3T3-E1 cells has been increased by inhibition of the ERK1/2 pathway with PD98059,36 suggesting that the ERK1/2 pathway is a negative regulator of osteogenesis. The role of ERK1/2 as a negative regulator of matrix mineralization has been also recently shown in human MSCs in a 3D collagen type I culture48 and in an in vivo calvaria model.49 Interestingly, the present study showed that when the pathway was inhibited in BMP4-treated pellets for only 7 days and the pellets then cultured with BMP4 only, the increased mineralization was still evident at 21 days. This suggests that blocking the ERK1/2 pathway at early time points during osteogenesis may be important to promote mineralization. The continued increase in mineralization over time indicates that pretreatment of cells with PD98059 may be sufficient to promote bone formation, a technique that may be valuable in the development of novel bone tissue engineering approaches. From the results presented in this study, the ERK1/2 pathway was found to be a negative regulator of the BMP4-induced osteogenesis of MDSCs.
Studies investigating the role of the p38 MAPK pathway in osteogenic differentiation are at times conflicting. A report on the inhibition of p38 MAPK activity in C2C12 cells treated with BMP2 showed an increased osteogenic effect, suggesting an inhibitory role of p38 MAPK on osteogenesis.29 In other studies that employed either BMP2 or BMP4 on primary calvarial osteoblasts, primary bone marrow osteoprogenitor cells, normal human osteoblasts, or the murine osteoblast line MC3T3-E1, it was found that p38 MAPK is necessary for BMP-induced osteogenic differentiation.25–28,36,50 Recently, it has been also found that the p38 MAPK pathway plays a positive role in BMP-induced Osx expression.51,52 In the present study on MDSCs, Alp and Osx gene expression was also shown to be dependent on p38 MAPK activation. Mineralization of MDSCs was decreased as a result of p38 MAPK inhibition, again suggesting that the p38 MAPK pathway is necessary for the osteogenic differentiation of MDSCs. This was further confirmed when the p38 MAPK pathway was inhibited in BMP4-treated MDSC pellets for only 7 days. Although a decrease in mineralization was evident at 14 days, mineralization recovered and approached that seen in MDSC pellets that received BMP4 only stimulation for 21 days. Hence, MDSCs that had the p38 MAPK pathway initially inhibited were able to undergo mineralization once the inhibition was removed.
The PI3K pathway has not been as widely studied as the MAPK pathways in BMP signaling, especially its role in matrix mineralization. In MDSCs, at the gene expression level, inhibition of the PI3K pathway increased the expression of Runx2 and Osx, whereas Alp gene expression was not significantly affected. At the protein level, ALP activity was significantly decreased when PI3K was inhibited in BMP4-treated cells. This decrease in BMP-stimulated ALP activity with Ly294002 is in accordance with previous studies utilizing BMP2 on human MSCs and mouse calvaria cells15,17 or endogenous BMPs, including BMP4, on human MSCs.21 However, the nonsignificant effect of Ly294002 on Alp gene expression after BMP stimulation is not in accordance with previous studies.17,21 The reason why Ly294002 may not have had an effect on the expression of Alp, but decreased ALP activity, may be due to the timing of the assays. Gene expression was measured after 24 h of stimulation, whereas ALP activity at the protein level was measured after 3 days. The effect of Ly29402 on gene expression may have occurred earlier or later than 24 h and may have caused it to be different than the protein level. Another interesting finding in this study is that when MDSCs were cultured as pellets in OSM, the inhibition of the PI3K pathway significantly increased matrix mineralization when compared with BMP4 only treatment. This suggests that PI3K may play a negative role in matrix mineralization. This finding is in accordance with that seen in vascular smooth muscle cells, where Akt signaling was found to have an inhibitory activity on the matrix calcification of these cells.53 Interestingly, inhibition of the PI3K pathway for only 7 days in MDSCs cultured as pellets in OSM did not affect mineralization. Thus, a continuous inhibition may be necessary. Taken together, the findings from this study suggest that PI3K may play a role in the BMP4-induced osteogenic differentiation of MDSCs, although more studies are necessary to elucidate its role in Alp gene expression. PI3K was found to play an important role in matrix mineralization and may be a potential target to promote bone formation in tissue engineering approaches.
Many chemical inhibitors are commercially available, although they may have different affinities for certain protein kinases.54 The inhibitors PD98059, SB203580, and Ly294002 are commonly used in the literature and were thus selected for this study, although other inhibitors could be used in their place.54 PD184352 and U0126 could be used instead of PD98059. SB202190 is structurally similar and has a similar specificity to SB203580. In addition to Ly294002, the PI3K pathway can also be inhibited by Wortmannin and Quercetin. Future studies on MDSCs could use these different chemical inhibitors to more clearly define the role of the ERK1/2, p38 MAPK, and PI3K pathways on BMP4-induced osteogenesis. It should also be noted that chemical inhibitors could affect more than one pathway because of cross-talk between pathways. ERK1/2, p38 MAPK, and PI3K have all been linked with BMP-activated Smads.15,17,26,55 As well, different cell types may respond differently to BMPs and pathway inhibitors. Thus, the role of these signaling pathways in BMP-induced osteogenesis is not a clearly defined process, but can be complicated by their individual and cooperative roles in the regulation of osteogenic markers.
This study was the first step toward understanding how BMP4 signals in MDSCs. By using well-established chemical inhibitors to the ERK1/2, p38 MAPK, and PI3K pathways, we demonstrated that the ERK1/2 pathway plays an inhibitory role in the osteogenic differentiation of MDSCs, especially in their mineralization. On the other hand, the p38 MAPK pathway plays an important stimulatory role in osteogenesis. Another interesting finding in this study is that the PI3K pathway may play an inhibitory role in the matrix mineralization of MDSCs. Future studies may involve preconditioning MDSCs with the ERK1/2 pathway inhibitor to increase their osteogenic differentiation in vitro and potentially enhance their ability to form bone in vivo. The inhibition of the PI3K pathway may also be a potential target. These studies will be especially useful to increase the matrix formation of cells that show less than optimal osteogenic potential. Therefore, further investigation into how the ERK1/2, p38 MAPK, and PI3K pathways affect MDSC differentiation in monolayer, pellet culture, or other 3D environments such as tissue engineering scaffolds is warranted, as it may identify novel therapeutic strategies for the development of stem cell-based therapies for bone tissue engineering.
Acknowledgments
The authors thank Dr. Arvydas Usas for help with the microCT. This work was supported in part by a National Institutes of Health grant (1 RO1 DE13420-06) to J. Huard. This work was also supported by the William F. and Jean W. Donaldson Chair at Children's Hospital of Pittsburgh and by the Henry J. Mankin Endowed Chair in Orthopaedic Surgery at the University of Pittsburgh.
Disclosure Statement
Dr. Johnny Huard has received remuneration from Cook Myosite, Inc., for consulting services and for royalties received from technology licensing during the period of time of this research. No competing financial interests exist for the other authors.
References
- 1.Weissman I.L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Gharaibeh B. Lu A. Tebbets J. Zheng B. Feduska J. Crisan M., et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 3.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R., et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B. Zheng B. Jankowski R.J. Kimura S. Ikezawa M. Deasy B., et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 5.Corsi K.A. Schwarz E.M. Mooney D.J. Huard J. Regenerative medicine in orthopaedic surgery. J Orthop Res. 2007;25:1261. doi: 10.1002/jor.20432. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda R. Usas A. Kubo S. Corsi K. Peng H. Rose T., et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.Y. Qu-Petersen Z. Cao B. Kimura S. Jankowski R. Cummins J., et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng H. Wright V. Usas A. Gearhart B. Shen H.C. Cummins J., et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright V. Peng H. Usas A. Young B. Gearhart B. Cummins J., et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 10.Heldin C.H. Miyazono K. ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 11.Kretzschmar M. Massague J. SMADs: mediators and regulators of TGF-beta signaling. Curr Opin Genet Dev. 1998;8:103. doi: 10.1016/s0959-437x(98)80069-5. [DOI] [PubMed] [Google Scholar]
- 12.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 13.Dang Z.C. Lowik C.W. Differential effects of PD98059 and U0126 on osteogenesis and adipogenesis. J Cell Biochem. 2004;92:525. doi: 10.1002/jcb.20087. [DOI] [PubMed] [Google Scholar]
- 14.Gallea S. Lallemand F. Atfi A. Rawadi G. Ramez V. Spinella-Jaegle S., et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh-Choudhury N. Abboud S.L. Nishimura R. Celeste A. Mahimainathan L. Choudhury G.G. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 16.Nohe A. Keating E. Knaus P. Petersen N.O. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Osyczka A.M. Leboy P.S. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology. 2005;146:3428. doi: 10.1210/en.2005-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoba L.N. Lee J.C. Inhibition of phosphatidylinositol 3-kinase and p70S6 kinase blocks osteogenic protein-1 induction of alkaline phosphatase activity in fetal rat calvaria cells. J Cell Biochem. 2003;88:1247. doi: 10.1002/jcb.10474. [DOI] [PubMed] [Google Scholar]
- 19.Simmons C.A. Matlis S. Thornton A.J. Chen S. Wang C.Y. Mooney D.J. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36:1087. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 20.Billings P.C. Fiori J.L. Bentwood J.L. O'Connell M.P. Jiao X. Nussbaum B., et al. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23:305. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seib F.P. Franke M. Jing D. Werner C. Bornhauser M. Endogenous bone morphogenetic proteins in human bone marrow-derived multipotent mesenchymal stromal cells. Eur J Cell Biol. 2009;88:257. doi: 10.1016/j.ejcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Alessi D.R. Cuenda A. Cohen P. Dudley D.T. Saltiel A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 23.Cuenda A. Rouse J. Doza Y.N. Meier R. Cohen P. Gallagher T.F., et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 24.Vlahos C.J. Matter W.F. Hui K.Y. Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241. [PubMed] [Google Scholar]
- 25.Lai C.F. Cheng S.L. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J Biol Chem. 2002;277:15514. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 26.Noth U. Tuli R. Seghatoleslami R. Howard M. Shah A. Hall D.J., et al. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 27.Guicheux J. Lemonnier J. Ghayor C. Suzuki A. Palmer G. Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Y. Wu Z.J. Yao H.Y. Yu X.D. Guo Z.K. Chen X.S., et al. Activation of p38 mitogen-activated protein kinase contribute to BMP4-induced alkaline phosphatase expression in MC3T3-E1 preosteoblast. Chin Med J (Engl) 2006;119:324. [PubMed] [Google Scholar]
- 29.Vinals F. Lopez-Rovira T. Rosa J.L. Ventura F. Inhibition of PI3K/p70 S6K and p38 MAPK cascades increases osteoblastic differentiation induced by BMP-2. FEBS Lett. 2002;510:99. doi: 10.1016/s0014-5793(01)03236-7. [DOI] [PubMed] [Google Scholar]
- 30.Fujita T. Azuma Y. Fukuyama R. Hattori Y. Yoshida C. Koida M., et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita T. Fukuyama R. Enomoto H. Komori T. Dexamethasone inhibits insulin-induced chondrogenesis of ATDC5 cells by preventing PI3K-Akt signaling and DNA binding of Runx2. J Cell Biochem. 2004;93:374. doi: 10.1002/jcb.20192. [DOI] [PubMed] [Google Scholar]
- 32.Hidaka K. Kanematsu T. Takeuchi H. Nakata M. Kikkawa U. Hirata M. Involvement of the phosphoinositide 3-kinase/protein kinase B signaling pathway in insulin/IGF-I-induced chondrogenesis of the mouse embryonal carcinoma-derived cell line ATDC5. Int J Biochem Cell Biol. 2001;33:1094. doi: 10.1016/s1357-2725(01)00067-x. [DOI] [PubMed] [Google Scholar]
- 33.Kaliman P. Vinals F. Testar X. Palacin M. Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 34.Sakaue H. Ogawa W. Matsumoto M. Kuroda S. Takata M. Sugimoto T., et al. Posttranscriptional control of adipocyte differentiation through activation of phosphoinositide 3-kinase. J Biol Chem. 1998;273:28945. doi: 10.1074/jbc.273.44.28945. [DOI] [PubMed] [Google Scholar]
- 35.Canalis E. Economides A.N. Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 36.Kozawa O. Hatakeyama D. Uematsu T. Divergent regulation by p44/p42 MAP kinase and p38 MAP kinase of bone morphogenetic protein-4-stimulated osteocalcin synthesis in osteoblasts. J Cell Biochem. 2002;84:583. [PubMed] [Google Scholar]
- 37.Kozawa O. Matsuno H. Uematsu T. Involvement of p70 S6 kinase in bone morphogenetic protein signaling: vascular endothelial growth factor synthesis by bone morphogenetic protein-4 in osteoblasts. J Cell Biochem. 2001;81:430. doi: 10.1002/1097-4644(20010601)81:3<430::aid-jcb1056>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Tazoe M. Mogi M. Goto S. Togari A. Involvement of p38MAP kinase in bone morphogenetic protein-4-induced osteoprotegerin in mouse bone-marrow-derived stromal cells. Arch Oral Biol. 2003;48:615. doi: 10.1016/s0003-9969(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 39.Jadlowiec J. Koch H. Zhang X. Campbell P.G. Seyedain M. Sfeir C. Phosphophoryn regulates the gene expression and differentiation of NIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/MAPK signaling pathway. J Biol Chem. 2004;279:53323. doi: 10.1074/jbc.M404934200. [DOI] [PubMed] [Google Scholar]
- 40.Corsi K.A. Pollett J.B. Phillippi J.A. Usas A. Li G. Huard J. Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex. J Bone Miner Res. 2007;22:1592. doi: 10.1359/jbmr.070702. [DOI] [PubMed] [Google Scholar]
- 41.Datta S.R. Brunet A. Greenberg M.E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 42.Song G. Ouyang G. Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartel F.V. Holl M. Arshad M. Aslam M. Gunduz D. Weyand M., et al. Transient hypoxia induces ERK-dependent anti-apoptotic cell survival in endothelial cells. Am J Physiol Cell Physiol. 2010;298:C1501. doi: 10.1152/ajpcell.00333.2009. [DOI] [PubMed] [Google Scholar]
- 44.Ambrosino C. Nebreda A.R. Cell cycle regulation by p38 MAP kinases. Biol Cell. 2001;93:47. doi: 10.1016/s0248-4900(01)01124-8. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z. Woodring P.J. Bhakta K.S. Tamura K. Wen F. Feramisco J.R., et al. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engel F.B. Schebesta M. Duong M.T. Lu G. Ren S. Madwed J.B., et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elia D. Madhala D. Ardon E. Reshef R. Halevy O. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007;1773:1438. doi: 10.1016/j.bbamcr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Lund A.W. Stegemann J.P. Plopper G.E. Inhibition of ERK promotes collagen gel compaction and fibrillogenesis to amplify the osteogenesis of human mesenchymal stem cells in three dimensional, collagen I culture. Stem Cells Dev. 2008;18:331. doi: 10.1089/scd.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kono S.J. Oshima Y. Hoshi K. Bonewald L.F. Oda H. Nakamura K., et al. Erk pathways negatively regulate matrix mineralization. Bone. 2007;40:68. doi: 10.1016/j.bone.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Hu Y. Chan E. Wang S.X. Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068. doi: 10.1210/en.2002-220863. [DOI] [PubMed] [Google Scholar]
- 51.Celil A.B. Hollinger J.O. Campbell P.G. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem. 2005;95:518. doi: 10.1002/jcb.20429. [DOI] [PubMed] [Google Scholar]
- 52.Wang X. Goh C.H. Li B. p38 mitogen-activated protein kinase regulates osteoblast differentiation through osterix. Endocrinology. 2007;148:1629. doi: 10.1210/en.2006-1000. [DOI] [PubMed] [Google Scholar]
- 53.Collett G.D. Sage A.P. Kirton J.P. Alexander M.Y. Gilmore A.P. Canfield A.E. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res. 2007;100:502. doi: 10.1161/01.RES.0000258854.03388.02. [DOI] [PubMed] [Google Scholar]
- 54.Davies S.P. Reddy H. Caivano M. Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kretzschmar M. Doody J. Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389:618. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]