Abstract
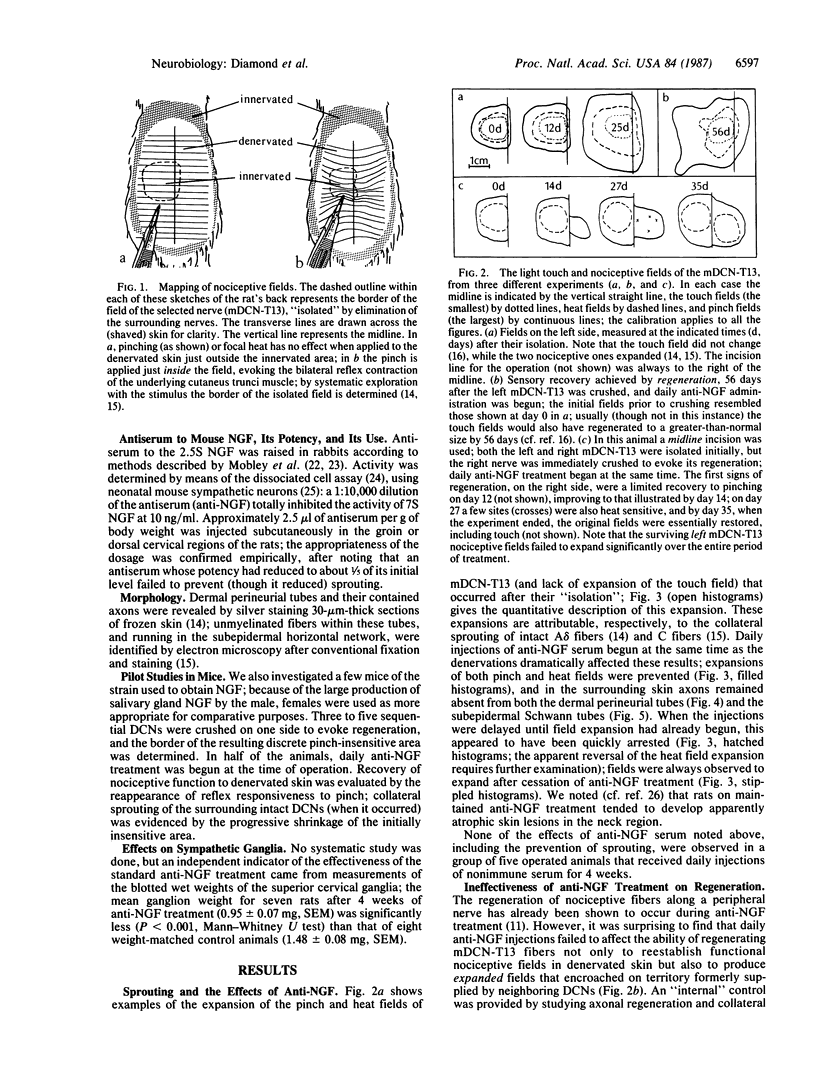
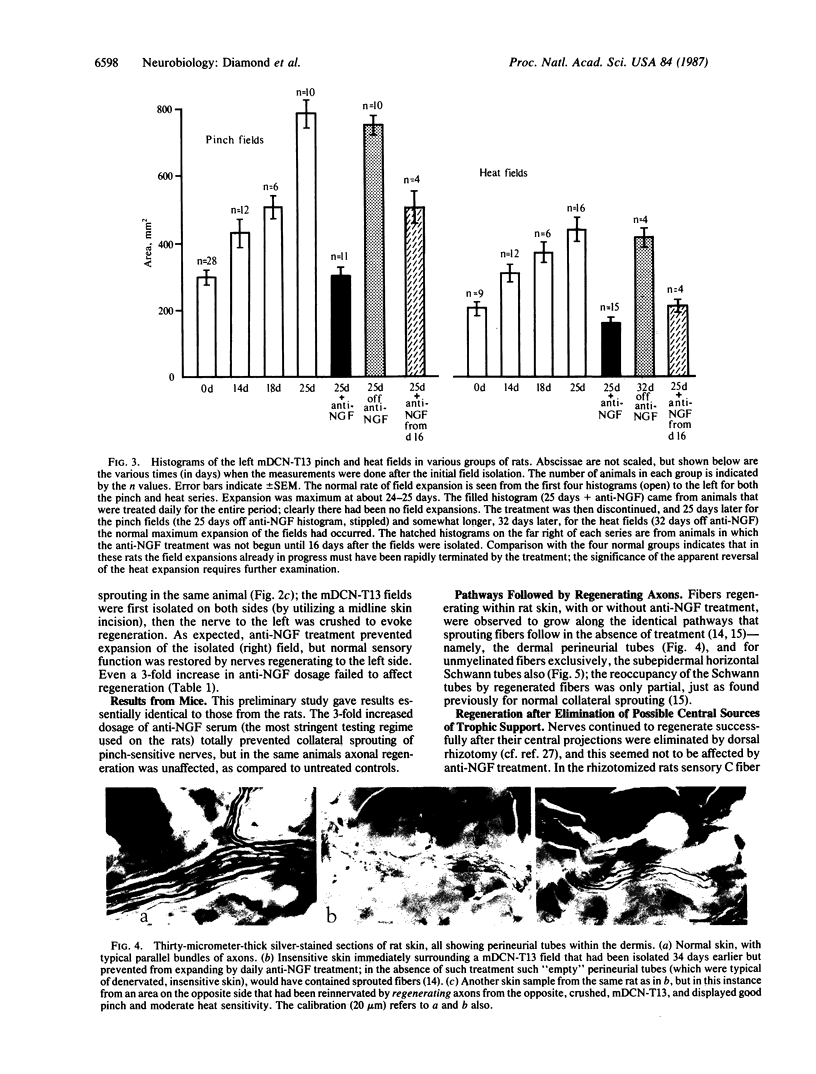
A key role has not yet been identified for beta nerve growth factor (NGF) in the growth responses that continue to be expressed in the sensory neurons of adult animals. We have now examined the effects of daily administration to adult rats (and in a few experiments, mice) of antiserum to NGF on (i) the collateral sprouting of undamaged nociceptive nerves that occurs into denervated adjacent skin and (ii) the regeneration of cutaneous sensory axons that occurs after they are damaged. The results were unexpected. All collateral sprouting was prevented and that already in progress was halted; sprouting resumed when treatment was discontinued. In contrast, the reestablishment, and even enlargement, of cutaneous nerve fields by regenerating axons was unaffected by anti-NGF treatment, even after dorsal rhizotomy was done to eliminate any central trophic support. In denervated skin, regenerating and collaterally sprouting axons utilized the same cellular pathways to establish functionally identical fields, thus displaying apparently identical growth behaviors, yet anti-NGF treatment clearly distinguished between them. We suggest that endogenous NGF is responsible for the collateral sprouting of nociceptive axons, probably reflecting an ongoing function of NGF in the regulation of their fields. This demonstration in the adult sensory system of a defined role for NGF in nerve growth could apply to nerve growth factors generally in the adult nervous system. The regeneration, however, of nociceptive axons (and nonnociceptive one) is not dependent on NGF.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Cozzari C., Calissano P., Levi-Montalcini R. Somatic and behavioral postnatal effects of fetal injections of nerve growth factor antibodies in the rat. Nature. 1981 Jun 4;291(5814):413–415. doi: 10.1038/291413a0. [DOI] [PubMed] [Google Scholar]
- Assouline J. G., Bosch P., Lim R., Kim I. S., Jensen R., Pantazis N. J. Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Brain Res. 1987 Jan;428(1):103–118. doi: 10.1016/0165-3806(87)90087-3. [DOI] [PubMed] [Google Scholar]
- Bjerre B., Björklund A., Mobley W., Rosengren E. Short- and long-term effects of nerve growth factor on the sympathetic nervous system in the adult mouse. Brain Res. 1975 Aug 29;94(2):263–277. doi: 10.1016/0006-8993(75)90061-x. [DOI] [PubMed] [Google Scholar]
- Bjerre B., Björklung A., Edwards D. C. Axonal regeneration of peripheral adrenergic neurons: effects of antiserum to nerve growth factor in mouse. Cell Tissue Res. 1974 May 8;148(4):441–476. doi: 10.1007/BF00221931. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Coughlin M. D., Collins M. B. Nerve growth factor-independent development of embryonic mouse sympathetic neurons in dissociated cell culture. Dev Biol. 1985 Aug;110(2):392–401. doi: 10.1016/0012-1606(85)90098-3. [DOI] [PubMed] [Google Scholar]
- Davies A. M., Bandtlow C., Heumann R., Korsching S., Rohrer H., Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. 1987 Mar 26-Apr 1Nature. 326(6111):353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A. The level of nerve growth factor (NGF) as a function of innervation. A correlation radio-immunoassay and bioassay study of the rat iris. Exp Cell Res. 1983 Oct 15;148(2):311–317. doi: 10.1016/0014-4827(83)90155-6. [DOI] [PubMed] [Google Scholar]
- Finn P. J., Ferguson I. A., Renton F. J., Rush R. A. Nerve growth factor immunohistochemistry and biological activity in the rat iris. J Neurocytol. 1986 Apr;15(2):169–176. doi: 10.1007/BF01611653. [DOI] [PubMed] [Google Scholar]
- Foerster A. P. Spontaneous regeneration of cut axons in adult rat brain. J Comp Neurol. 1982 Oct 1;210(4):335–356. doi: 10.1002/cne.902100403. [DOI] [PubMed] [Google Scholar]
- Goedert M., Otten U., Hunt S. P., Bond A., Chapman D., Schlumpf M., Lichtensteiger W. Biochemical and anatomical effects of antibodies against nerve growth factor on developing rat sensory ganglia. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1580–1584. doi: 10.1073/pnas.81.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin P. D., Johnson E. M. Experimental autoimmune model of nerve growth factor deprivation: effects on developing peripheral sympathetic and sensory neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5382–5386. doi: 10.1073/pnas.76.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin P. D., Johnson E. M., Jr Effects of long-term nerve growth factor deprivation on the nervous system of the adult rat: an experimental autoimmune approach. Brain Res. 1980 Sep 29;198(1):27–42. doi: 10.1016/0006-8993(80)90341-8. [DOI] [PubMed] [Google Scholar]
- Green L. A. A quantitative bioassay for nerve growth factor (NGF) activity employing a clonal pheochromocytoma cell line. Brain Res. 1977 Sep 16;133(2):350–353. doi: 10.1016/0006-8993(77)90770-3. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Harper G. P., Thoenen H. Target cells, biological effects, and mechanism of action of nerve growth factor and its antibodies. Annu Rev Pharmacol Toxicol. 1981;21:205–229. doi: 10.1146/annurev.pa.21.040181.001225. [DOI] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Scott J., Thoenen H. Relationship between levels of nerve growth factor (NGF) and its messenger RNA in sympathetic ganglia and peripheral target tissues. EMBO J. 1984 Dec 20;3(13):3183–3189. doi: 10.1002/j.1460-2075.1984.tb02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch K. Guidance of regrowing sensory axons after cutaneous nerve lesions in the cat. J Neurophysiol. 1979 Sep;42(5):1437–1449. doi: 10.1152/jn.1979.42.5.1437. [DOI] [PubMed] [Google Scholar]
- Jackson P. C., Diamond J. Temporal and spatial constraints on the collateral sprouting of low-threshold mechanosensory nerves in the skin of rats. J Comp Neurol. 1984 Jul 1;226(3):336–345. doi: 10.1002/cne.902260304. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Yip H. K. Central nervous system and peripheral nerve growth factor provide trophic support critical to mature sensory neuronal survival. 1985 Apr 25-May 1Nature. 314(6013):751–752. doi: 10.1038/314751a0. [DOI] [PubMed] [Google Scholar]
- Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981 Sep 1;25(2):137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Bell W. O., Black I. B. Interactions between the sympathetic and sensory innervation of the iris. J Neurosci. 1983 Jun;3(6):1301–1307. doi: 10.1523/JNEUROSCI.03-06-01301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A. Parasympathetic, sympathetic, and sensory interactions in the iris: nerve growth factor regulates cholinergic ciliary ganglion innervation in vivo. J Neurosci. 1985 Oct;5(10):2719–2725. doi: 10.1523/JNEUROSCI.05-10-02719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S., Thoenen H. Nerve growth factor supply for sensory neurons: site of origin and competition with the sympathetic nervous system. Neurosci Lett. 1985 Mar 15;54(2-3):201–205. doi: 10.1016/s0304-3940(85)80079-3. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Longo F. M., Hayman E. G., Davis G. E., Ruoslahti E., Engvall E., Manthorpe M., Varon S. Neurite-promoting factors and extracellular matrix components accumulating in vivo within nerve regeneration chambers. Brain Res. 1984 Aug 20;309(1):105–117. doi: 10.1016/0006-8993(84)91014-x. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Rutkowski J. L., Tennekoon G. I., Buchanan K., Johnston M. V. Choline acetyltransferase activity in striatum of neonatal rats increased by nerve growth factor. Science. 1985 Jul 19;229(4710):284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Schenker A., Shooter E. M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976 Dec 14;15(25):5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Nixon B. J., Doucette R., Jackson P. C., Diamond J. Impulse activity evokes precocious sprouting of nociceptive nerves into denervated skin. Somatosens Res. 1984;2(2):97–126. doi: 10.1080/07367244.1984.11800553. [DOI] [PubMed] [Google Scholar]
- Rich K. M., Yip H. K., Osborne P. A., Schmidt R. E., Johnson E. M., Jr Role of nerve growth factor in the adult dorsal root ganglia neuron and its response to injury. J Comp Neurol. 1984 Nov 20;230(1):110–118. doi: 10.1002/cne.902300110. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Ebendal T. Nerve growth activities in rat peripheral nerve. Brain Res. 1982 Aug 19;246(1):57–64. doi: 10.1016/0006-8993(82)90141-x. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Riopelle R. J. Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci. 1986 Aug;6(8):2312–2321. doi: 10.1523/JNEUROSCI.06-08-02312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. M., Riopelle R. J. Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J Neurosci. 1984 Jul;4(7):1683–1689. doi: 10.1523/JNEUROSCI.04-07-01683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush R. A. Immunohistochemical localization of endogenous nerve growth factor. Nature. 1984 Nov 22;312(5992):364–367. doi: 10.1038/312364a0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Pearson J., Johnson E. M. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982 Jul 29;244(2):378–381. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Pearson J., Johnson E. M. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982 Jul 29;244(2):378–381. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2714–2718. doi: 10.1073/pnas.83.8.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Studies on the regulation of beta-nerve growth factor gene expression in the rat iris: the level of mRNA-encoding nerve growth factor is increased in irises placed in explant cultures in vitro, but not in irises deprived of sensory or sympathetic innervation in vivo. J Cell Biol. 1986 May;102(5):1940–1948. doi: 10.1083/jcb.102.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. A., Young J. Z. Regeneration of fibre diameter after cross-unions of visceral and somatic nerves. J Anat. 1945 Apr;79(Pt 2):48–65. [PMC free article] [PubMed] [Google Scholar]
- So K. F., Aguayo A. J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985 Mar 4;328(2):349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Palay S. L. Altered axons and axon terminals in the lateral vestibular nucleus of the rat. Possible example of axonal remodeling. Lab Invest. 1971 Dec;25(6):653–671. [PubMed] [Google Scholar]
- Sparrow J. R., Kiernan J. A. Endoneurial vascular permeability in degenerating and regenerating peripheral nerves. Acta Neuropathol. 1981;53(3):181–188. doi: 10.1007/BF00688020. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K. J., Reichardt L. F., Bixby J. L. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol. 1986 Dec;103(6 Pt 2):2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara N. Synaptic plasticity in the mammalian central nervous system. Annu Rev Neurosci. 1981;4:351–379. doi: 10.1146/annurev.ne.04.030181.002031. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Whittemore S. R., Ebendal T., Lärkfors L., Olson L., Seiger A., Strömberg I., Persson H. Development and regional expression of beta nerve growth factor messenger RNA and protein in the rat central nervous system. Proc Natl Acad Sci U S A. 1986 Feb;83(3):817–821. doi: 10.1073/pnas.83.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windebank A. J., Poduslo J. F. Neuronal growth factors produced by adult peripheral nerve after injury. Brain Res. 1986 Oct 15;385(1):197–200. doi: 10.1016/0006-8993(86)91567-2. [DOI] [PubMed] [Google Scholar]
- Yodlowski M. L., Fredieu J. R., Landis S. C. Neonatal 6-hydroxydopamine treatment eliminates cholinergic sympathetic innervation and induces sensory sprouting in rat sweat glands. J Neurosci. 1984 Jun;4(6):1535–1548. doi: 10.1523/JNEUROSCI.04-06-01535.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]






