Abstract
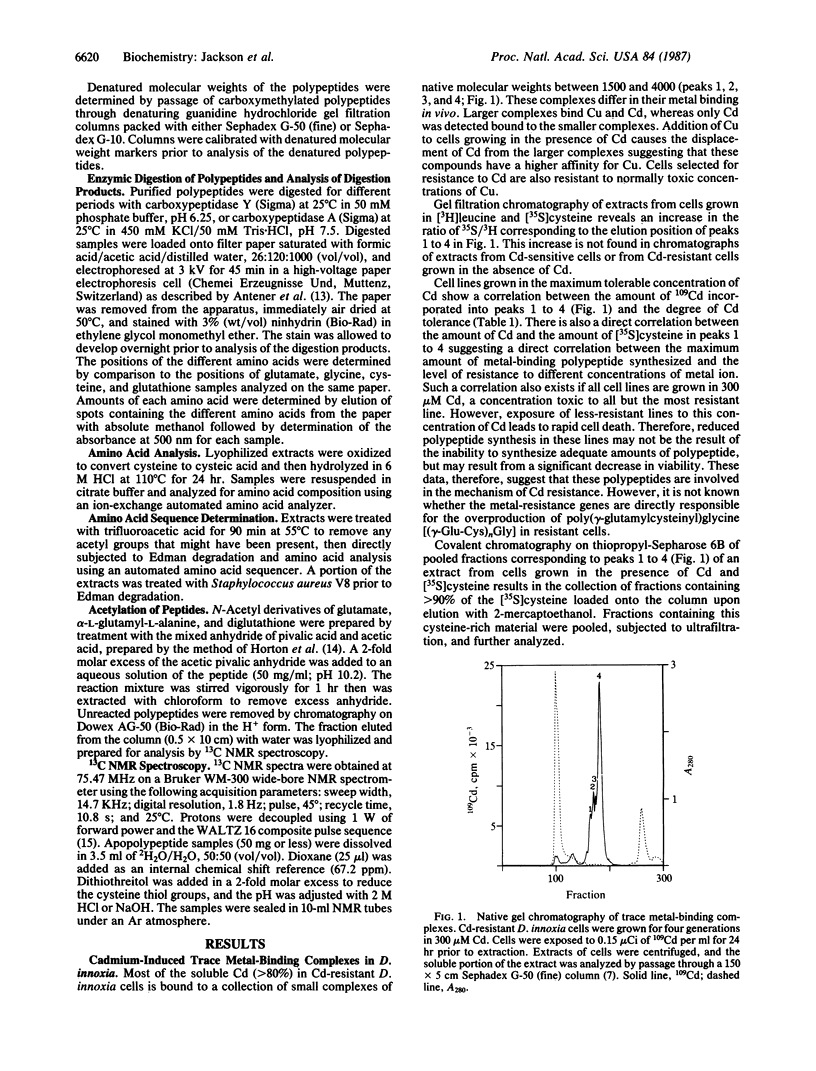
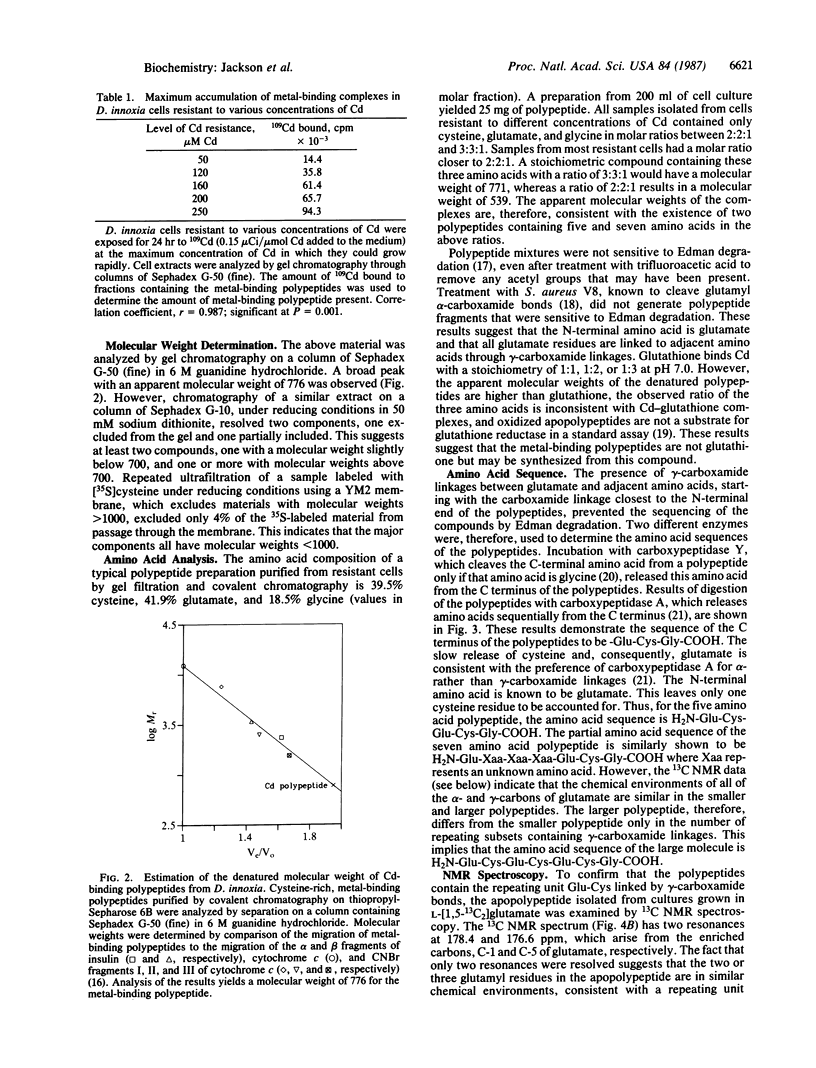
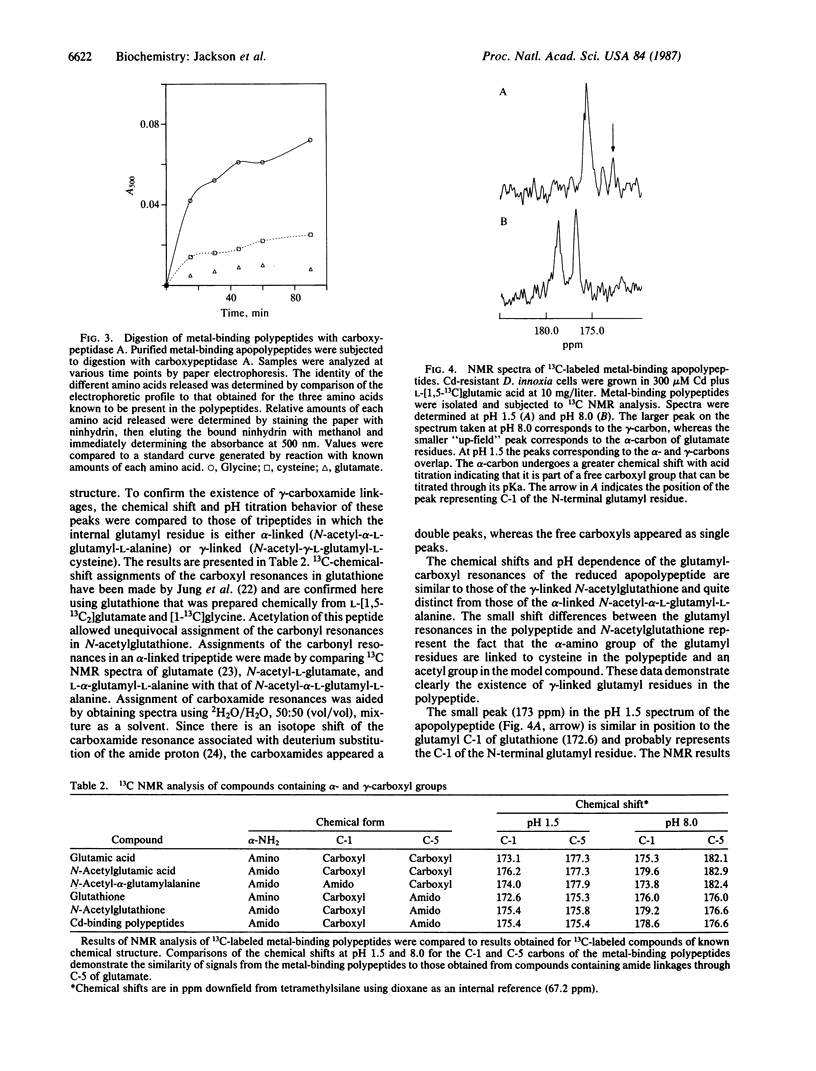
Angiosperms can be selected for the ability to grow in the presence of normally toxic concentrations of certain trace metal ions. Addition of Cd and Cu to Cd-resistant Datura innoxia cell cultures results in the rapid synthesis and accumulation of sulfur-rich, metal-binding polypeptides. The structure of these compounds was determined using amino acid analysis, 13C NMR, and site-specific enzymic digestion. These compounds are poly(gamma-glutamylcysteinyl)glycines. Greater than 80% of the cellular Cd is bound to the bis and tris forms in Cd-resistant cells. There is a direct correlation between the maximum accumulation of the metal-binding polypeptides and the concentration of toxic ions to which the cells are resistant. In the presence of metal ions, the polypeptides form multimeric aggregates that can be resolved by gel chromatography. Cd binds to both the high and low molecular weight aggregates, whereas Cu preferentially binds to the higher molecular weight forms. The presence of gamma-carboxamide linkages between glutamyl and adjacent cysteinyl residues indicates that these polypeptides are products of biosynthetic pathways. Poly(gamma-glutamylcysteinyl)glycines bind metals and, in this respect, appear to be functional analogs of the protein metallothionein. However, in the absence of supraoptimal concentrations of trace metal ions, the functions of metallothionein in animals and microorganisms and poly(gamma-glutamylcysteinyl)glycines in plants may differ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLE R. D., STEIN W. H., MOORE S. On the cysteine content of human hemoglobin. J Biol Chem. 1958 Dec;233(6):1359–1363. [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Hayashi R., Hata T. Action of yeast proteinase C on synthetic peptides and poly- ,L-amino acids. Biochim Biophys Acta. 1972 May 18;263(3):673–679. doi: 10.1016/0005-2795(72)90049-9. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. J., Roth E. J., McClure P. R., Naranjo C. M. Selection, Isolation, and Characterization of Cadmium-Resistant Datura innoxia Suspension Cultures. Plant Physiol. 1984 Aug;75(4):914–918. doi: 10.1104/pp.75.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Breitmaier E., Voelter W. Dissoziationsgleichgewichte von Glutathion. Eine Fourier-Transform- 13 C-NMR spektroskopische Untersuchung der PH-Abhängigkeit der Ladungsverteilung. Eur J Biochem. 1972 Jan 21;24(3):438–445. doi: 10.1111/j.1432-1033.1972.tb19704.x. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Richards V. Human fetal liver contains both zinc- and copper-rich forms of metallothionein. J Biol Chem. 1980 Jun 10;255(11):5380–5383. [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Steffens J. C., Hunt D. F., Williams B. G. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem. 1986 Oct 25;261(30):13879–13882. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Walker T. E., Han C. H., Kollman V. H., London R. E., Matwiyoff N. A. 13C nuclear magnetic resonance studies of the biosynthesis by Microbacterium ammoniaphilum of L-glutamate selectively enriched with carbon-13. J Biol Chem. 1982 Feb 10;257(3):1189–1195. [PubMed] [Google Scholar]



