Abstract
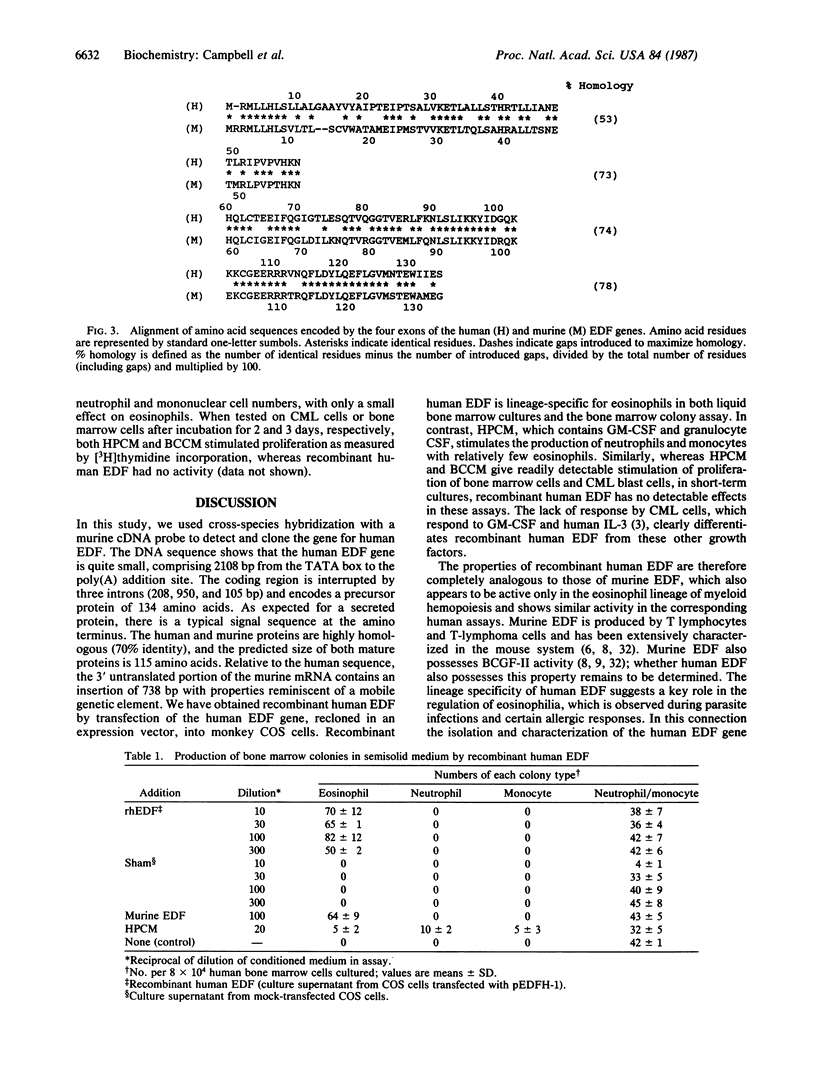
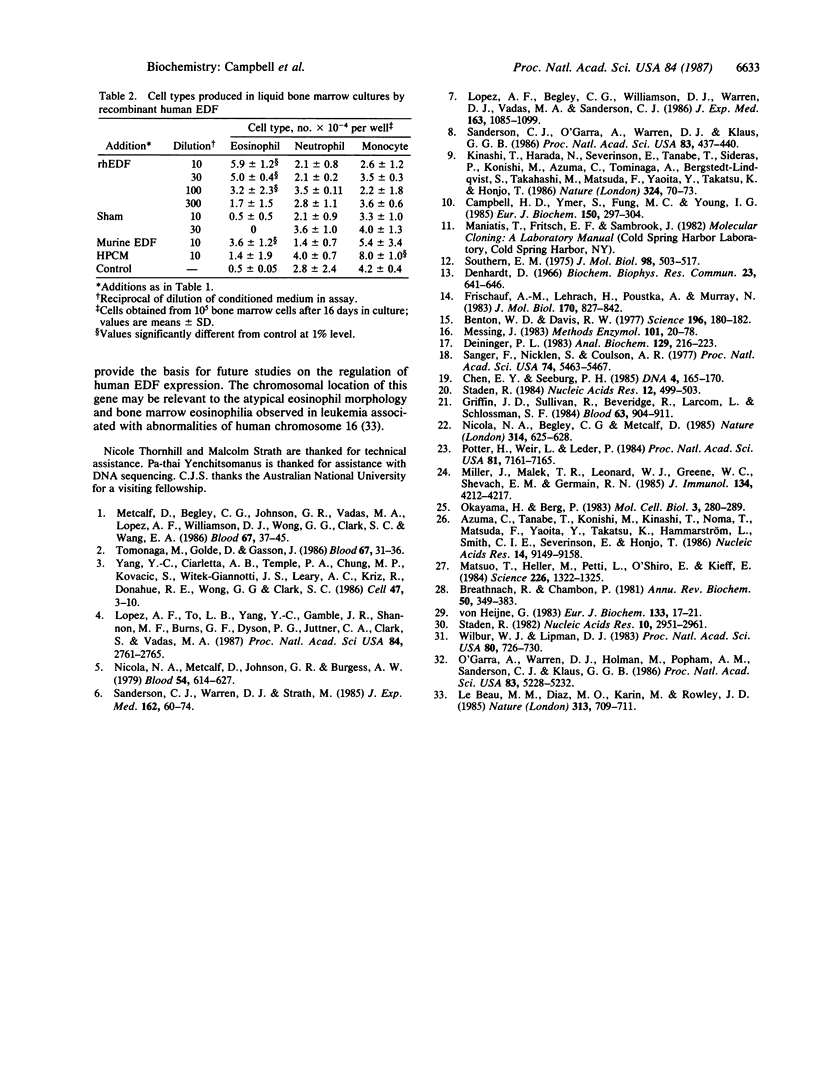
The human eosinophil differentiation factor (EDF) gene was cloned from a genomic library in lambda phage EMBL3A by using a murine EDF cDNA clone as a probe. The DNA sequence of a 3.2-kilobase BamHI fragment spanning the gene was determined. The gene contains three introns. The predicted amino acid sequence of 134 amino acids is identical with that recently reported for human interleukin 5 but shows no significant homology with other known hemopoietic growth regulators. The amino acid sequence shows strong homology (approximately 70% identity) with that of murine EDF. Recombinant human EDF, expressed from the human EDF gene after transfection into monkey COS cells, stimulated the production of eosinophils and eosinophil colonies from normal human bone marrow but had no effect on the production of neutrophils or mononuclear cells (monocytes and lymphoid cells). The apparent specificity of human EDF for the eosinophil lineage in myeloid hemopoiesis contrasts with the properties of human interleukin 3 and granulocyte/macrophage and granulocyte colony-stimulating factors but is directly analogous to the biological properties of murine EDF. Human EDF therefore represents a distinct hemopoietic growth factor that could play a central role in the regulation of eosinophilia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma C., Tanabe T., Konishi M., Kinashi T., Noma T., Matsuda F., Yaoita Y., Takatsu K., Hammarström L., Smith C. I. Cloning of cDNA for human T-cell replacing factor (interleukin-5) and comparison with the murine homologue. Nucleic Acids Res. 1986 Nov 25;14(22):9149–9158. doi: 10.1093/nar/14.22.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Campbell H. D., Ymer S., Fung M. C., Young I. G. Cloning and nucleotide sequence of the murine interleukin-3 gene. Eur J Biochem. 1985 Jul 15;150(2):297–304. doi: 10.1111/j.1432-1033.1985.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Sullivan R., Beveridge R. P., Larcom P., Schlossman S. F. Induction of proliferation of purified human myeloid progenitor cells: a rapid assay for granulocyte colony-stimulating factors. Blood. 1984 Apr;63(4):904–911. [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Diaz M. O., Karin M., Rowley J. D. Metallothionein gene cluster is split by chromosome 16 rearrangements in myelomonocytic leukaemia. Nature. 1985 Feb 21;313(6004):709–711. doi: 10.1038/313709a0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Begley C. G., Williamson D. J., Warren D. J., Vadas M. A., Sanderson C. J. Murine eosinophil differentiation factor. An eosinophil-specific colony-stimulating factor with activity for human cells. J Exp Med. 1986 May 1;163(5):1085–1099. doi: 10.1084/jem.163.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., To L. B., Yang Y. C., Gamble J. R., Shannon M. F., Burns G. F., Dyson P. G., Juttner C. A., Clark S., Vadas M. A. Stimulation of proliferation, differentiation, and function of human cells by primate interleukin 3. Proc Natl Acad Sci U S A. 1987 May;84(9):2761–2765. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Heller M., Petti L., O'Shiro E., Kieff E. Persistence of the entire Epstein-Barr virus genome integrated into human lymphocyte DNA. Science. 1984 Dec 14;226(4680):1322–1325. doi: 10.1126/science.6095452. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Vadas M. A., Lopez A. F., Williamson D. J., Wong G. G., Clark S. C., Wang E. A. Biologic properties in vitro of a recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1986 Jan;67(1):37–45. [PubMed] [Google Scholar]
- Miller J., Malek T. R., Leonard W. J., Greene W. C., Shevach E. M., Germain R. N. Nucleotide sequence and expression of a mouse interleukin 2 receptor cDNA. J Immunol. 1985 Jun;134(6):4212–4217. [PubMed] [Google Scholar]
- Nicola N. A., Begley C. G., Metcalf D. Identification of the human analogue of a regulator that induces differentiation in murine leukaemic cells. Nature. 1985 Apr 18;314(6012):625–628. doi: 10.1038/314625a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Johnson G. R., Burgess A. W. Separation of functionally distinct human granulocyte-macrophage colony-stimulating factors. Blood. 1979 Sep;54(3):614–627. [PubMed] [Google Scholar]
- O'Garra A., Warren D. J., Holman M., Popham A. M., Sanderson C. J., Klaus G. G. Interleukin 4 (B-cell growth factor II/eosinophil differentiation factor) is a mitogen and differentiation factor for preactivated murine B lymphocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5228–5232. doi: 10.1073/pnas.83.14.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. A computer program to enter DNA gel reading data into a computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):499–503. doi: 10.1093/nar/12.1part2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga M., Golde D. W., Gasson J. C. Biosynthetic (recombinant) human granulocyte-macrophage colony-stimulating factor: effect on normal bone marrow and leukemia cell lines. Blood. 1986 Jan;67(1):31–36. [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]



