Abstract
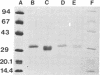
A complementary DNA clone from human placenta, encoding human extracellular superoxide dismutase (EC-SOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1), has recently been isolated and characterized. An expression plasmid, based on the EC-SOD complementary DNA, was transfected into Chinese hamster ovary cells (CHO-K1). The transfected cells secreted human EC-SOD to the culture medium. The secreted recombinant (r) EC-SOD was isolated in high yield with a three-step procedure beginning with immobilized monoclonal anti-EC-SOD antibodies. The properties of the rEC-SOD were compared with native (n) EC-SOD isolated from human umbilical cords. The specific activities and amino-terminal amino acid sequences were identical. The amino acid compositions were virtually identical and very similar to the composition deduced from the complementary DNA sequence. Both rEC-SOD and nEC-SOD contained 4 Cu and 4 Zn atoms per molecule, and the presence of Zn in EC-SOD is thus now established. The rEC-SOD produced is type C, since its affinity for heparin-Sepharose was identical to that of nEC-SOD type C. Both enzymes bound to concanavalin A, lentil lectin, and wheat germ lectin and are thus glycoproteins. rEC-SOD and nEC-SOD seem to have the same subunit structure and composition as analyzed by polyacrylamide gel electrophoresis and gel chromatography.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Engström A., Engström P., Tao Z. J., Carlsson A., Bennich H. Insect immunity. The primary structure of the antibacterial protein attacin F and its relation to two native attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2065–2070. doi: 10.1002/j.1460-2075.1984.tb02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergil B., Ohlsson R. Phosphorylation of proteins in rat liver. Endogenous phosphorylation and dephosphorylation of proteins from smooth and rough endoplasmic reticulum and free ribosomes. Eur J Biochem. 1974 Jul 1;46(1):13–25. doi: 10.1111/j.1432-1033.1974.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987 Feb 15;242(1):55–59. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Properties of extracellular superoxide dismutase from human lung. Biochem J. 1984 May 15;220(1):269–272. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J Biol Chem. 1976 Dec 10;251(23):7504–7507. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]