Abstract
PERK (EIF2AK3) was originally discovered as a major component of the Unfolded Protein Response (UPR). PERK deficiency results in permanent neonatal diabetes, which was initially thought to be caused by a failure to regulate ER stress in insulin-secreting beta cells, culminating in beta cell death. However, subsequent studies found that low beta cell mass was due to reduced cell proliferation, rather than increased apoptosis. Genetic and cellular studies of Perk-deficient beta cells showed that PERK was critically required for ER functions including proinsulin trafficking and quality control, unrelated to the ER stress pathway. Under normal physiological conditions, changes in ER calcium levels, mediated by glucose and other insulin secretagogues, regulate PERK activity for the purpose of controlling insulin biogenesis.
Stress response pathways in cells are manned predominately by proteins that have important physiological and/or developmental functions often unrelated to stress, but are pressed into action in response to an acute stress. Such proteins are typically first discovered in screens for genes that respond to chemical or physical treatment in cultured cells, and then only later found to have primary functions in normal development and physiology of the whole organism. A relevant case in point is the endoplasmic reticulum chaperone protein GRP78/BiP, which was first discovered because its expression is highly induced upon glucose deprivation (1; 2). BiP is also highly induced by pharmacological insults that elicit the ER stress response (3–5). Somewhat later the primary and most important functions of BiP were discovered; BiP is the key ER chaperone required for the normal functions of protein folding, trafficking, and quality control in the ER (6; 7). Studies on the regulation of ER stress response and BiP led to the discovery of Inositol Requiring-1 (IRE1). IRE1 catalyzes unique splicing of specific mRNAs and was found to be a key sensor and mediator of the ER stress response in yeast and mammals. The discovery of IRE1 launched an avalanche of studies on the regulation of the ER stress pathway (see reviews (8–14). Soon thereafter PERK (EIF2AK3) was discovered independently by two groups (15; 16), and was immediately recognized as a sensor of ER stress by virtue of the shared homology of the ER luminal sensor domain with IRE1. However, the normal functions of these genes have taken a considerably longer time to decipher in the context of the whole organism.
Within two years following the discovery of PERK, its deficiency was found to be the cause of the human Wolcott Rallison syndrome (WRS), characterized by permanent neonatal diabetes, exocrine pancreas atrophy, skeletal dysplasias, recurrent hepatitis, and growth retardation (17). Shortly thereafter, PERK KO mice were shown to have the same defects as those seen in WRS (18; 19). The cause of WRS was initially interpreted as a defect in regulating the ER stress pathway. This interpretation has largely persisted in the literature, and PERK deficiency has become the poster-child for ER stress diseases. More explicitly, it was proposed that PERK responds to fluctuating physiological stress to dampen global protein synthesis and to upregulate an ER stress adaptive pathway via the transcription factor ATF4 (CREB2) (Figure 1). If stress became too great, PERK would signal cellular suicide by upregulating GADD153/CHOP, an inducer of apoptotic cell death (20). The molecular explanation for the phenotype of Perk deficient humans and mice was then argued to be due to a failure to repress global protein synthesis and to activate the adaptive pathway climaxing in cell death (18). Seemingly consistent with this hypothesis, beta cell mass was found to be greatly diminished in humans and mice deficient for Perk (18; 19; 21). However subsequent studies failed to confirm the key assumptions of the ER stress hypothesis, and instead pointed to an alternative explanation related to cell proliferation and proinsulin trafficking (22–25).
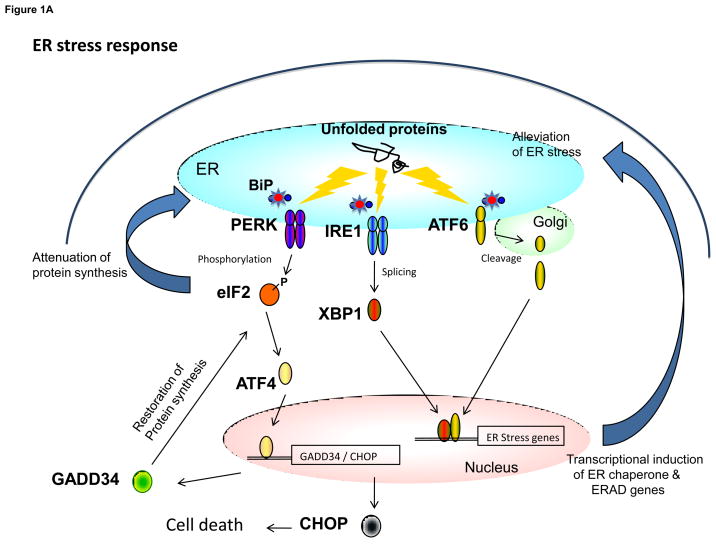
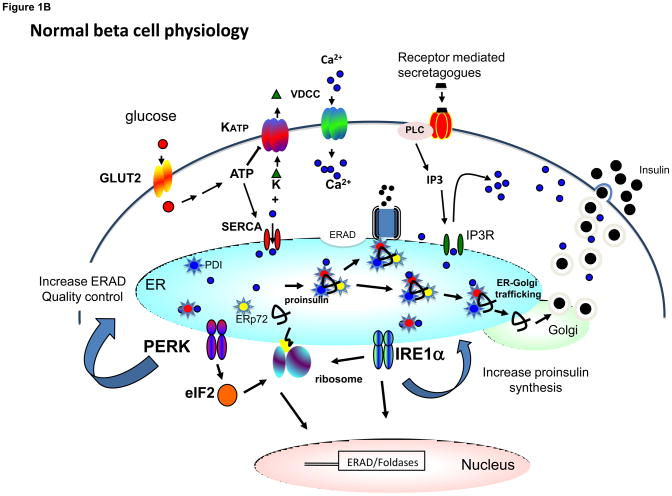
Figure 1.
The contrasting functions of PERK in the canonical UPR and in the regulation of the insulin secreting beta cell. (a) The UPR-ER stress response is initiated by the accumulation of unfolded proteins in the endoplasmic reticulum that activates the three major arms of the UPR including PERK, IRE1, and ATF6. PERK phosphorylates eIF2α at amino acid residue Serine-51. Phosphorylation at this site results in attenuation of protein synthesis but also in activation of translation of a small number of genes including ATF4. ATF4 increases the transcription of GADD34, which acts as a feedback control to restore normal protein synthesis. ATF4 also activates the transcription of CHOP, which if produced at high levels in conjunction with other factors, may lead to cell death. IRE1 catalyzes the splicing of the transcription factor XBP1, which in turn activates the transcription of genes encoding ER chaperones and other proteins facilitating ERAD function. The activation of ATF6 results in its translocation to the Golgi and generation of the active nuclear form. After trafficking to the nucleus, ATF6 acts in concert with other transcription factors to activate the expression of ER chaperone and ERAD genes. In some cases ATF6 and XBP1 co-activate specific genes. (b) Under normal physiological conditions, PERK activity is dynamically controlled by changes in ER calcium levels. ER calcium levels are heavily influenced by glucose and other insulin secretagogues including those that activate the IP3 receptor localized in the ER membrane. Depending on other factors, glucose can either stimulate calcium uptake through SERCA pump which requires ATP or it can stimulate calcium release from the IP3 receptor via calcium-induced calcium release (CICR). PERK in turn regulates proinsulin trafficking and ERAD. The activity of IRE1α is also regulated by glucose, but in an opposite manner to PERK. In turn, IRE1a regulates proinsulin biogenesis. PERK and IRE1α are likely to activate changes in gene expression as part of their regulation of proinsulin trafficking and biogenesis, but the relevant downstream target genes have not been identified. GLUT2 = glucose transporter-2, KATP = ATP-sensitive potassium channel, VDCC = voltage-dependent calcium channel, PLC = phospholipase C, IP3R = inositol triphosphate receptor, ERAD = ER associated protein degradation, SERCA = Sarcoplasmic-endoplasmic reticulum calcium ATPase - calcium pump, BiP = immunoglobulin binding protein/glucose regulated protein-78.
Beta cell death and ER stress – not the cause of diabetes
Early studies on the cause of diabetes in Perk deficient humans and mice were hampered by confounding effects of other organ dysfunctions. These confounding effects were ultimately resolved by generating tissue-specific Perk KO mice and a beta-cell specific rescuing Perk transgene (24). As expected, the diabetic phenotype was directly traced to absence of PERK expression in the insulin-secreting beta cells. However, upon characterizing these conditional Perk KO mice, it was discovered that beta cell death was not increased, but rather beta cell proliferation was repressed during the embryonic and neonatal periods sufficiently to explain the ten-fold deficiency in beta cell mass observed in juvenile Perk KO mice (Figure 2a) (24). Moreover, a microarray experiment in islets of Perk KO mice showed that the majority of genes differentially regulated were related to the cell cycle and proliferation, whereas ER stress and apoptosis related genes were largely unaffected. A further prediction of the ER stress hypothesis was that in the absence of one of the major regulatory arms, ER stress would become out of control and result in hyperactivation of the other arms of the pathway. However IRE1 and ATF6 and their downstream targets were not activated in Perk KO beta cells (24). Finally the ER stress model predicted that in the absence of PERK, global protein synthesis would be derepressed leading to over synthesis of proteins further exacerbating ER stress. Once again, the Perk KO phenotype of beta cells initially seemed to support this hypothesis as a significant fraction of cells accumulated very high levels of proinsulin in the ER, denoted as an Impacted-ER phenotype (Figure 2b). However, a thorough analysis of synthesis rates and levels of total protein, proinsulin, and insulin did not show that global protein or proinsulin synthesis were derepressed in Perk deficient beta cells (22). Therefore the Impacted ER phenotype of proinsulin over accumulation was not caused by over synthesis.
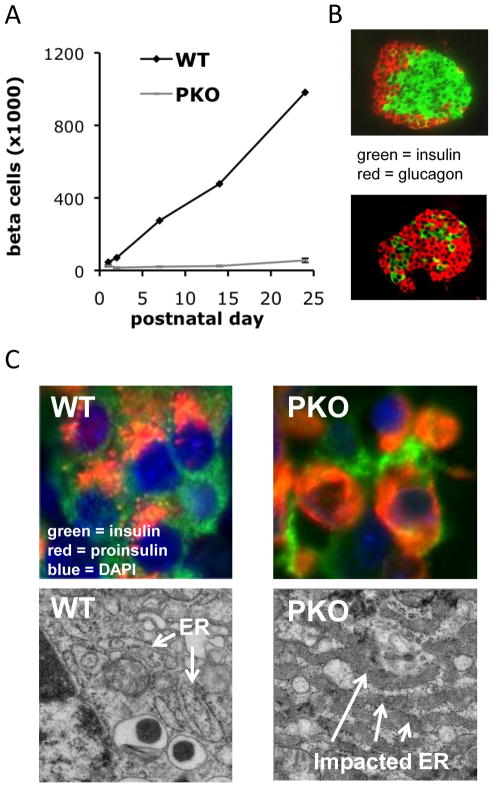
Figure 2.
PERK deficiency results in diminished proliferation of beta cells and a severe defect in proinsulin trafficking. (a) Beta cell mass normally increases 20–40 fold during the first four postnatal weeks in mice (24). In contrast, beta cell mass increases only about 2-fold in Perk KO mice. (b) Images of wildtype and Perk KO islets at the 3rd postnatal week are shown. (c) Proinsulin (red) normally accumulates in the ER-Golgi Intermediate Compartment and Golgi adjacent to the nucleus (blue) whereas insulin (green) is dispersed through the cytoplasm. In a large fraction of beta cells in Perk KO (PKO) mice, proinsulin accumulates in the ER and is not trafficked to the Golgi (24). (c) The ER in wildtype beta cells exhibits an elongated, tubular structure dotted with ribosomes, whereas the ER in Perk KO mice is highly distended with high electron density due to the accumulation of a large amount of proinsulin and other client proteins.
A knock-in mutation of the regulatory phosphorylation site of eIF2α (Ser51Ala) also displays a severe Impacted ER phenotype with over accumulation of proinsulin in beta cells (26). Like the PERK KO mice, eIF2αS51A mice do not exhibit activation of the other arms of the ER stress pathway, but in contrast to Perk deficient beta cells, a derepression of proinsulin synthesis is observed (27). The molecular phenotype of Perk deficient beta cells and the eIF2α Ser51Ala knock-in mutant are likely to be somewhat different, since three other known eIF2α kinases can phosphorylate eIF2α, and the alanine substitution in eIF2α (Ser51Ala) may have additional effects on eIF2 function beyond blocking phosphorylation at this site. Whatever the reason for the differences in phenotype between mutations in Perk and in its eIF2α substrate, the hypothesis that PERK is first and foremost a translation attenuator of protein synthesis is not supported by studies in the insulin secreting beta cells (22).
Over accumulation of proinsulin caused by defects in proinsulin quality control and trafficking
If over accumulation of proinsulin in the ER of PERK deficient beta cells is not caused by over synthesis, then what alternative explanations remain? One possibility is that the export of proinsulin out of the ER to distal secretory compartments may be blocked. In addition, impaired quality control and ER associated protein degradation (ERAD) could also cause proinsulin and other secretory and plasma membrane proteins to accumulate in the ER. To examine these possibilities, the anterograde trafficking of proinsulin and vesicular stomatitis virus protein G (VSVG) was examined in Perk deficient cells (22). Normally, proinsulin and VSVG are rapidly folded in the ER and transported to the Golgi. Trafficking of these proteins from the ER to the Golgi was found to be blocked in Perk deficient cells. Retrotranslocation of misfolded proteins out of the ER, a key step in the ERAD pathway, was also found to be ablated in Perk impaired cells. Potentially, defects in anterograde trafficking could cause dysfunctions in ER quality control and ERAD. To circumvent the potential negative influence of defective anterograde trafficking, an ER retention signal (KDEL) was inserted at the carboxy terminus of proinsulin to limit its fate to either being degraded via ERAD or retained in the ER. In Perk deficient cells, a gross accumulation of proinsulin was observed in the ER, suggesting that the ERAD pathway was impaired (22).
PERK gene dosage modulates glucose homeostasis and diabetic progression
The Akita insulin mutation, first described in mice and more recently found in humans (28–30), causes diabetes due to insulin insufficiency. The molecular basis for the insulin insufficiency was initially attributed to beta cell death (31; 32), but more recently the cause has been pinpointed to an induction of ER associated protein degradation, which increases the degradation of not only of the Akita insulin protein but also the normal wild-type insulin (22; 33). Cell death does eventually reduce beta cell mass but not until after an early phase of repressed insulin secretion and compensatory beta cell hyperplasia when hyperglycemia is first detected (22). Introduction of the global PERK KO into the Akita insulin mutant mouse results in the more severe and early onset form of diabetes seen in the global Perk KO mice. However, unexpectedly, mice that are doubly heterozygous for Akita and Perk (Ins2Akita/+; Perk +/−) progress more slowly to frank diabetes, whereas the addition of an extra gene copy of Perk specifically to beta cells hastens diabetic progression (Figure 3) (22). Paradoxically then, Perk gene dosage is negatively correlated with the severity and progression of diabetes in the Akita mouse, whereas the complete absence of Perk results in permanent neonatal diabetes. Together these studies suggest that PERK positively regulates ERAD and proinsulin turnover; therefore reducing, but not eliminating, this function allows more wild type proinsulin to escape the negative influence of the Akita proinsulin and become secreted. Two other ER resident proteins, P58/DNAJc3 and ERO1β, which participate in ERAD and proinsulin trafficking, have also been shown to modulate diabetic progression in the Akita mouse (34–36) demonstrating the importance of this processing in insulin biogenesis.
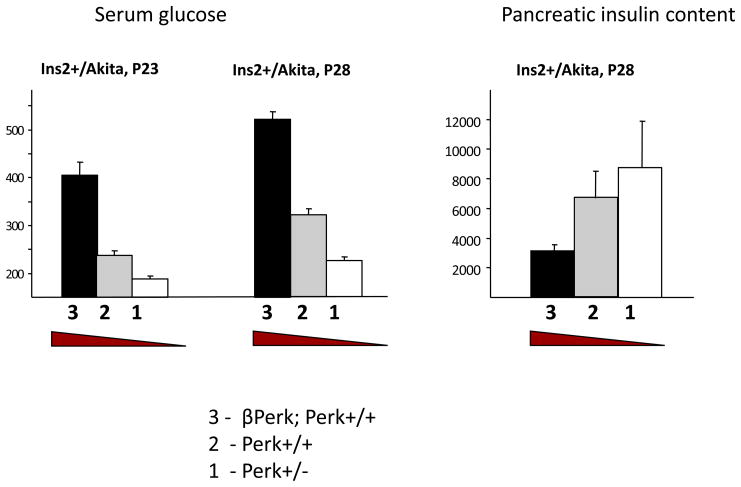
Figure 3.
Diabetic progression in Akita insulin mutant mouse is ameliorated by reducing Perk gene dosage. The effect of three Perk genotypes – Perk +/−, Perk +/+, and βPerk; Perk +/+ on the diabetic progression of the insulin Akita mouse (Ins2+/Akita) was investigated (22). At postnatal day 23 the serum glucose level of Akita mice begins to increase and five days later (P28) all mice exceed 200 mg/dl. However, the diabetic progression during this period is inversely related to Perk gene dosage. The Perk dependent progression of diabetes in the Akita mouse is correlated with differences in pancreatic insulin content.
The amelioration of diabetes in the Akita mouse affected by reducing Perk dosage prompted us to examine beta cell functions more closely in Perk heterozygous mice. Although humans and mice heterozygous for Perk are ostensibly normal, Perk+/− mice exhibit significantly lower serum glucose levels and higher pancreatic insulin content (22). Moreover, Perk+/− mice eventually become moderately glucose intolerant (18). The amount of PERK in beta cells, and by extension the level of PERK activity, therefore impacts beta cell function, suggesting that physiological modulation of PERK activity may be important in regulating beta cell functions.
ER calcium dynamics regulates PERK activity
The activities of PERK, IRE1, and ATF6 are negatively regulated by BiP/GRP78, which binds their ER luminal domains and inhibits oligomerization and activation (22). Upon treatment with an ER stress elicitor (e.g. DTT, thapsigargin, or tunicamycin), BiP disassociates from them, allowing their activation. According to the ER stress hypothesis, these elicitors cause misfolding of ER client proteins and the misfolded proteins compete for the attention of BiP, resulting in disassociation of BiP from PERK, IRE1, and ATF6. Whether they are activated as a direct consequence of inducing protein misfolding by these drugs, however, is controversial. Detailed molecular studies by Ron Prywes and colleagues have discredited this competition model and instead propose that ER stressors stimulate the disassociation of BiP by some other means (37), perhaps through interactions with co-chaperones.
What then regulates PERK activity in the beta cell and finally what role does PERK play in regulating beta cell functions? Physiologically relevant factors have been discovered that activate PERK in beta cells including low glucose levels and high levels of the fatty acid palmitate (19; 38–40). Interestingly, both of these conditions decrease the level of ER calcium stores similar to the more severe effect of treating cells with thapsigargin, which inhibits the SERCA calcium pump (41).
The mechanism of ER calcium regulation of PERK activity is unknown, but may be related to the fact that BiP is a major calcium binding protein in the ER (42), and its binding to PERK may be dependent upon the relatively high level of calcium present in the ER. Calcium dynamically regulates the ATPase activity of BiP (42), which in turn influences its chaperone functions and interactions with ER luminal proteins. We have proposed that PERK acts primarily as an ER calcium sensor as mediated by BiP (22) (Figure 1b). Under this hypothesis, then, PERK dynamically senses changes in ER calcium levels that are stimulated by events outside of the ER lumen and often initiated by signaling events at the plasma membrane. ER calcium levels are particularly impacted in a complex manner by glucose (43–46). Upon glucose stimulation, the ER releases calcium during the first few minutes but then rapidly sequesters calcium to an even higher level than under low glucose conditions. In parallel, eIF2α is dephosphorylated in beta cells upon glucose treatment and is restored as glucose is diminished (47; 48). This suggests that PERK may be sensing glucose through changes in ER calcium levels, raising the intriguing possibility that the primary function of PERK in beta cells is to modulate proinsulin quality control and trafficking in concert with changes in circulating glucose levels. A whole host of other metabolites and factors including fatty acids can also alter ER calcium levels and potentially regulate PERK activity. A key question is whether the normal physiological fluctuations in these factors are sufficiently large to affect a change in PERK activity. At least for glucose, substantial changes in eIF2α phosphorylation have been detected in the pancreata of mice fasted and then injected with a bolus of glucose (19).
Phosphorylation of eIF2α is mostly dependent upon PERK in beta cells, but the rapid dephosphorylation seen upon glucose treatment is mediated by protein phosphatase-1 (47). PP1 activity is negatively regulated by Ppp1r1a (I-1) (47) and positively regulated by GADD34 and CReP (49), which specifically recruit PP1 to eIF2α to affect its dephosphorylation. Increasing evidence supports the hypothesis that glucose-dependent induction of protein synthesis in beta cells is partly regulated by dephosphorylation of eIF2α by PP1 as mediated by one or more of these PP1 coactivators and repressor proteins. Although the gross beta cell dysfunctions seen in Perk deficient beta cells are not caused by derepression of protein and/or proinsulin synthesis, PERK may nonetheless participate in concert with PP1 to regulate glucose-dependent protein synthesis.
Other arms of the UPR embrace pancreatic functions
Mammals have two IRE1 genes, IRE1α and IRE1β. IRE1α is expressed in most tissues including the pancreatic beta cells (50). Suppression of IRE1α in beta cells results in decreased proinsulin biosynthesis and insulin content but does not impact global protein synthesis or insulin secretion (50). Moreover IRE1α is activated by high glucose and other insulin secretagogues including GLP-1. Although BiP disassociates from IRE1α when cells are treated with toxic ER stress elicitors, its activation by the insulin secretagogues occurs without the disassociation of BiP (50). The major function of IRE1α in the ER stress response is to catalyze splicing of XBP1 mRNA, which is required for efficient translation of XBP1 (51; 52). XBP1 in turn activates the transcription of several ER chaperones and ERAD genes in concert with ATF6 (53). Activation of IRE1α by glucose does not result in an increase in the splicing of XBP1 mRNA nor is there a robust induction of the ER stress response genes (50). The mechanism of proinsulin synthesis by IRE1α is therefore unclear but appears not to be mediated through the ER stress pathway and splicing of XBP1.
Severe exocrine pancreatic defects are seen in mice deficient for three UPR genes including XBP1 (54), PERK (18; 19; 55) and ATF4 (55). XBP1 and ATF4 KO mice exhibit early fetal and neonatal growth defects in the exocrine pancreas (54; 55), whereas the exocrine pancreas develops and functions normally in Perk deficient mice until postnatal day 18 where it begins to atrophy due to oncotic cell death (55). Although ATF4 is critically regulated by PERK in the ER stress response, the underlying cause of the exocrine pancreatic defects in mice deficient for ATF4 is unrelated to PERK (55). ATF6, ATF4, XBP1, and CHOP deficient mice exhibit normal islet and beta cell development and are normoglycemic, suggesting that they are not required for normal beta cell functions. In summary, the principal mediators of the ER stress response, with the exception of PERK and IRE1α, are not required for normal beta cell functions although ATF4 and XBP1 are required for exocrine pancreatic development. Not surprisingly then, the normal functions of PERK and IRE1α in beta cells are unrelated to the ER stress pathway and instead both are focused on different aspects of beta cell proliferation and/or proinsulin synthesis and trafficking.
Glucose extremes elicit a complex response from PERK, IRE1, and ATF6
As diabetics know all too well, opposite extremes in glucose levels present different but debilitating health consequences. At the cellular level, glucose deprivation induces the expression of BiP (a.k.a. glucose regulated protein-78) (1; 2). Subsequently the activation of ATF6 was found to be responsible for induction of BiP transcription in glucose deprived cells. PERK is also strongly activated in beta cells in the presence of very low glucose conditions (38), and the translation of ATF4 and CHOP is induced as a likely consequence (56). However, IRE1α and XBP1 splicing are relatively repressed in beta cells under these conditions. Thus two of three arms of the UPR are induced by glucose deprivation in cell culture. The relevance of glucose deprivation to beta cells in situ, however, is unclear as glucose concentrations below 20 mg/dl (1.1 mM) are associated with hypoglycemic coma and death. At glucose concentrations closer to physiological low levels (3.3–5.5mM), glucose-deprivation response genes (e.g. BiP and GRP94) remain relatively unchanged compared to high glucose concentrations in beta cells (57). Nonetheless, a large number of genes exhibit highly significant differences in expression in beta cells cultured for 24hrs in low (5.5mM) glucose compared to high glucose (25.0mM) (57; 58). Not surprisingly, genes supporting secretion functions are upregulated in high glucose. With the exception of two genes, P58/DNAJc3 and CHOP, ER stress pathway genes are not significantly impacted. Both P58/DNAJc3 and CHOP are induced in the classic ER stress response, but surprisingly they exhibit opposite responses to low versus high glucose, i.e. P58/DNAJc3 is induced by high glucose and CHOP is induced by low glucose. Under this more physiologically relevant range of glucose, CHOP mRNA level is regulated by FOXO1 (58) and probably not by ATF4, as it is in the ER stress pathway. As P58/DNAJc3 is an important ER co-chaperone (35), its induction by high glucose is consistent with the induction of a large number of other genes in support of insulin synthesis and secretion. PERK and IRE1α activity are also regulated in the normal physiological range of glucose, but in opposite directions – PERK is repressed in high glucose whereas IRE1α is activated by high glucose. In conclusion, the response of beta cells to physiological changes in glucose concentration is complex and does not correspond to a canonical ER stress response seen when beta cells or other cell types are completely deprived of glucose.
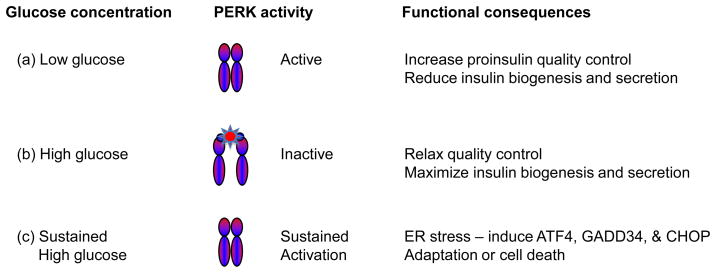
In untreated Type 2 diabetic individuals, circulating glucose may increase to a sustained level (>20mM), which degrades beta cell functions and triggers cell death (9). Recent studies have suggested that unresolved ER stress may be the molecular basis for this glucose toxicity to beta cells. In support of this hypothesis, long term treatment of beta cells with sustained or intermittent high levels of glucose (e.g. 25 mM) leads to an ER stress response (50; 59–61) with activation of PERK, IRE1α and ATF6. The effect of chronic exposure to high glucose on PERK activation (59) is particularly revealing as it is opposite to that seen when beta cells are acutely shifted from low to high glucose. This suggests that the mechanism of PERK activation may change during the transition from acute to longterm high glucose exposure (Figure 4). One possibility is that the activation of PERK switches from being dominated by the level of ER calcium under normal physiological conditions to being activated by unfolded proteins, which may accumulate in the ER during sustained glucose exposure. Glucose toxicity also increases oxidative stress in beta cells, and the NRF-2 transcription factor regulates adaptation to increased reactive oxygen species (ROS) in beta cells (62; 63). Translocation of NRF-2 to the nucleus is regulated by its phosphorylation by PERK(64), and therefore PERK may have a dual regulatory role in mediating adaptation to glucose toxicity via both the eIF2α and NRF-2 pathways.
Figure 4.
Glucose dynamically regulates PERK activity in the insulin secreting beta cell. Within the normal physiological range of serum glucose, PERK activity is relatively high after an overnight fast but is rapidly reduced by the administration of high glucose (19). We hypothesize that increased PERK activity during fasting (low glucose) increases quality control that serves to limit the anterograde trafficking proinsulin and insulin secretion. However, sustained high levels of circulating glucose negatively impact the function of beta cells, and a high level of PERK activity is seen. We speculate that one of the consequences of glucotoxicity is the accumulation of unfolded proteins in the ER that activate PERK and the other arms of the ER stress pathway.
In summary, we can define three distinct glucose conditions for which metabolic adaptation occurs: glucose deprivation (0–1 mM), normal range of physiologic glucose, and sustained high glucose (>20mM) for several days. Except for the absence of activation of IRE1 during glucose deprivation, an ER stress response is seen at both extremes: acute glucose deprivation or chronically high glucose. However, there appears to be absence of an ER stress response within the dynamic range of glucose seen in normal individuals or in diabetic patients whose glucose is reasonably well controlled. Among these three glucose conditions, which have been investigated extensively, the relevance of complete glucose deprivation to the function of beta cells in situ is questionable. The response of cells to no glucose is decidedly different from physiologically low glucose, and therefore the myriad of glucose deprivation studies may not provide clues as to how beta cells adapt to fasting or reactive hypoglycemia.
PERK regulates different genes under different conditions
The complex response of PERK to different glucose conditions suggests that the downstream consequences of PERK activation may differ substantially and may depend upon the level and duration of activation as well as on the expression of other regulatory factors that act synergistically to affect metabolic adaptation. The consequences of activating PERK at different levels in the liver have been investigated using a genetically engineered PERK whose activity can be finely controlled by the administration of an antibiotic (65). Interestingly, although a few genes exhibited a monophasic change in expression over large range of PERK activation in the liver, several genes showed a biphasic response with high expression at intermediate levels of PERK activity but low at either extreme of PERK activation (65). This complexity is not surprising given the fact that phosphorylation of eIF2α can have two seemingly opposite effects: repression of global protein synthesis and activation of translation of specific mRNAs. Importantly, all of the genes that have been described to date whose translation is differentially regulated by eIF2α kinases encode transcription factors (13; 66), and therefore the large number of genes whose mRNA expression is impacted by the eIF2α kinases are likely to stem from differential translation of one or more transcription factors that regulate them. Although a few such transcription factors (e.g. ATF4 and ATF5) have been identified as translational targets of PERK during ER stress, none appear to mediate the function of PERK in regulating proinsulin trafficking in the beta cell, and the search continues for the relevant mediators of PERK beta cell functions.
Future studies of PERK’s role in regulating beta cell functions will need to consider the complexity of PERK regulation and functions under both normal and stress conditions. How PERK’s activity is modulated and what PERK regulates is likely to be substantially different during stress associated with severe hypoglycemia or sustained hyperglycemia as compared to normoglycemic conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 2.Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch WJ, Garrels JI, Thomas GP, Lin JJ, Feramisco JR. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983;258:7102–7111. [PubMed] [Google Scholar]
- 4.Olden K, Pratt RM, Jaworski C, Yamada KM. Evidence for role of glycoprotein carbohydrates in membrane transport: specific inhibition by tunicamycin. Proc Natl Acad Sci U S A. 1979;76:791–795. doi: 10.1073/pnas.76.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resendez E, Jr, Attenello JW, Grafsky A, Chang CS, Lee AS. Calcium ionophore A23187 induces expression of glucose-regulated genes and their heterologous fusion genes. Mol Cell Biol. 1985;5:1212–1219. doi: 10.1128/mcb.5.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med. 2004;71:289–297. [PubMed] [Google Scholar]
- 7.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009;9:763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 10.Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 17:107–112. doi: 10.1097/MED.0b013e3283372843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. 2009;146:743–750. doi: 10.1093/jb/mvp166. [DOI] [PubMed] [Google Scholar]
- 12.Scheuner D, Kaufman RJ. The unfolded protein response. a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9:2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 14.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 17.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 18.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 21.Stewart FJ, Carson DJ, Thomas PS, Humphreys M, Thornton C, Nevin NC. Wolcott-Rallison syndrome associated with congenital malformations and a mosaic deletion 15q 11–12. Clin Genet. 1996;49:152–155. doi: 10.1111/j.1399-0004.1996.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, McGrath B, Cavener DR. PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PLoS ONE. 2009;4:e8008. doi: 10.1371/journal.pone.0008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Feng D, Wei J, Gupta S, McGrath BC, Cavener DR. Acute ablation of PERK results in ER dysfunctions followed by reduced insulin secretion and cell proliferation. BMC Cell Biol. 2009;10:61. doi: 10.1186/1471-2121-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 27.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, Hansen T, Federici L, Pesavento R, Cadario F, Federici G, Ghirri P, Arvan P, Iafusco D, Barbetti F. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008;118:2148–2156. doi: 10.1172/JCI33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 30.Stoy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217. doi: 10.1177/153537020322801018. [DOI] [PubMed] [Google Scholar]
- 32.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodish I, Liu M, Rajpal G, Larkin D, Holz RW, Adams A, Liu L, Arvan P. Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem. 2010;285:685–694. doi: 10.1074/jbc.M109.038042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, Harding HP, Goodman AG, Harant H, Garrison JL, Taunton J, Katze MG, Ron D. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 35.Petrova K, Oyadomari S, Hendershot LM, Ron D. Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DNAJc3. Embo J. 2008;27:2862–2872. doi: 10.1038/emboj.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-{beta}, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol. 188:821–832. doi: 10.1083/jcb.200911086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen J, Snapp EL, Lippincott-Schwartz J, Prywes R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol. 2005;25:921–932. doi: 10.1128/MCB.25.3.921-932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J. 2008;410:485–493. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- 39.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 40.Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem. 2007;282:3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- 41.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 42.Lievremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- 43.Gylfe E, Hellman B. Glucose-stimulated sequestration of Ca2+ in clonal insulin-releasing cells. Evidence for an opposing effect of muscarinic-receptor activation. Biochem J. 1986;233:865–870. doi: 10.1042/bj2330865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellman B, Gylfe E, Bergsten P. Mobilization of different pools of glucose-incorporated calcium in pancreatic beta-cells after muscarinic receptor activation. Adv Exp Med Biol. 1986;211:325–341. doi: 10.1007/978-1-4684-5314-0_30. [DOI] [PubMed] [Google Scholar]
- 45.Gilon P, Arredouani A, Gailly P, Gromada J, Henquin JC. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J Biol Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 46.Lemmens R, Larsson O, Berggren PO, Islam MS. Ca2+-induced Ca2+ release from the endoplasmic reticulum amplifies the Ca2+ signal mediated by activation of voltage-gated L-type Ca2+ channels in pancreatic beta-cells. J Biol Chem. 2001;276:9971–9977. doi: 10.1074/jbc.M009463200. [DOI] [PubMed] [Google Scholar]
- 47.Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, Tsukamoto K, Beullens M, Kaufman RJ, Bollen M, Schuit FC. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology. 2007;148:609–617. doi: 10.1210/en.2006-1012. [DOI] [PubMed] [Google Scholar]
- 48.Gomez E, Powell ML, Greenman IC, Herbert TP. Glucose-stimulated protein synthesis in pancreatic beta-cells parallels an increase in the availability of the translational ternary complex (eIF2-GTP. Met-tRNAi) and the dephosphorylation of eIF2 alpha. J Biol Chem. 2004;279:53937–53946. doi: 10.1074/jbc.M408682200. [DOI] [PubMed] [Google Scholar]
- 49.Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106:1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 54.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. Embo J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 2007;8:38. doi: 10.1186/1471-2121-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenman IC, Gomez E, Moore CE, Herbert TP. Distinct glucose-dependent stress responses revealed by translational profiling in pancreatic beta-cells. J Endocrinol. 2007;192:179–187. doi: 10.1677/joe.1.06898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb GC, Akbar MS, Zhao C, Steiner DF. Expression profiling of pancreatic beta cells: glucose regulation of secretory and metabolic pathway genes. Proc Natl Acad Sci U S A. 2000;97:5773–5778. doi: 10.1073/pnas.100126597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez SC, Cras-Meneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes. 2006;55:1581–1591. doi: 10.2337/db05-0678. [DOI] [PubMed] [Google Scholar]
- 59.Hou ZQ, Li HL, Gao L, Pan L, Zhao JJ, Li GW. Involvement of chronic stresses in rat islet and INS-1 cell glucotoxicity induced by intermittent high glucose. Mol Cell Endocrinol. 2008;291:71–78. doi: 10.1016/j.mce.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Seo HY, Kim YD, Lee KM, Min AK, Kim MK, Kim HS, Won KC, Park JY, Lee KU, Choi HS, Park KG, Lee IK. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008;149:3832–3841. doi: 10.1210/en.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but Not Protein-disulfide Isomerase, Partially Reverses Hyperglycemia-induced Inhibition of Insulin Synthesis and Secretion in Pancreatic {beta}-Cells. J Biol Chem. 2009;284:5289–5298. doi: 10.1074/jbc.M805477200. [DOI] [PubMed] [Google Scholar]
- 62.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pugazhenthi S, Akhov L, Selvaraj G, Wang M, Alam J. Regulation of heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway in mouse beta-cells. Am J Physiol Endocrinol Metab. 2007;293:E645–655. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- 64.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]