Abstract
Because the visual system integrates information across time, an image that moves on the retina would be expected to be perceived as smeared. In this article, we summarize the previous evidence that human observers perceive a smaller extent of smear when retinal image motion results from an eye or head movement, compared to when a physically moving target generates comparable image motion while the eyes and head are still. This evidence indicates that the reduction of perceived motion smear is asymmetrical, occurring only for targets that move against the direction of an eye or head movement. In addition, we present new data to show that no reduction of perceived motion smear occurs for targets that move in either direction during a visually-induced perception of self motion. We propose that low-level extra-retinal eye- and head-movement signals are responsible for the reduction of perceived motion smear, by decreasing the duration of the temporal impulse response. Although retinal as well as extra-retinal mechanisms can reduce the extent of perceived motion smear, available evidence suggests that improved visual functioning may occur only when an extra-retinal mechanism reduces the perception of smear.
Keywords: Motion blur, motion smear, eye movement, extra-retinal signals, temporal impulse response
1. Introduction
The eye frequently is likened to a camera, but the details of this analogy change as photographic technology advances. One way in which the eye behaves like a digital camera is that image information is accumulated by both neural and photoelectric detectors for a significant period of time. When the image is stationary, temporal summation allows the signal-to-noise ratio of the detectors to increase. However, when an image moves either across the retina or a photoelectric detector surface, the information from each point in the image is distributed across multiple detectors, leading to motion blur or smear. This unwanted consequence of temporal summation is presumably one reason why multiple oculomotor mechanisms exist to stabilize gaze and promote stability of the retinal image (Robinson, 1977; Leigh & Zee, 1999). Conceptually similar mechanical stabilization systems have been developed to reduce the influence of jitter and the resulting motion blur in cameras (e.g., Sachs, Nasiri & Goehl, 2007; Wikipedia, 2010). Temporal summation in the visual system results in visual persistence, such that brief stimulation of one region on the retina results in neural activity that continues for a period of time. Several previous authors used the extent of perceived motion smear to estimate the time course and duration of visual persistence (e.g., Bidwell, 1899; McDougall, 1904; Allport, 1970; Geremek et al., 2002).
In a frequently cited paper, Burr (1980) reported that the extent of perceived motion smear produced by a moving random-dot display increases with the duration of motion up to approximately 40 ms, and decreases for longer durations. The explanation offered for this observation by Burr, Ross and Morrone (1986) was that the activation of motion detectors, which summate information along a spatiotemporal trajectory, results in a reduction of perceived motion smear, a process that they called motion-deblurring. Evidence to support this proposed de-blurring mechanism was provided by Watamaniuk (1992), who reported a shorter duration of visible persistence for targets with a consistent direction of apparent motion than for targets in apparent motion with random trajectories. More recently, Nishida and coworkers showed that the perceived color of chromatic targets reflects an integration of color information along the path of motion (Nishida, Watanabe, Kuriki & Tomimoto, 2007; Watanabe & Nishida, 2007). Nevertheless, Burr’s results differ from the more extensive perception of motion smear that was reported by earlier authors, who viewed an isolated visual target in continuous motion (e.g., Bidwell, 1899; McDougall, 1904; Allport, 1970). Subsequent experiments indicated that an important reason for these apparently discrepant observations is that the extent of perceived motion smear exhibits a strong dependence on the spatial density of the moving visual targets (Di Lollo & Hogben, 1985; Chen, Bedell & Ögmen, 1995; Tong, Aydin & Bedell, 2007a). Specifically, as shown in the top two panels of Figure 1, the extent of perceived motion smear decreases as the density of the moving visual stimuli increases. To obtain the data in Figure 1, we followed the paradigm described by Burr (1980), in which observers adjusted the length of a stationary bright line presented after each trial to match the extent of smear that they perceived in the moving stimulus. Several authors attributed the reduction of perceived motion smear with element density to spatio-temporal interactions between visual targets, akin to metacontrast masking (Hogben & Di Lollo, 1985; Farrell, Pavel & Sperling, 1990; Castet, Lorenceau & Bonnet, 1993; Castet, 1994; Chen et al., 1995; Purushothaman, Ögmen, Chen & Bedell, 1998), wherein the persisting neural activity generated at each retinal location by a previous moving target is reduced by subsequent activity from one or more nearby targets. For targets in apparent motion, the duration of visible persistence increases with the spatial separation between successive stimuli (e.g., Dixon & Hammond, 1972; Di Lollo & Hogben, 1985; Farrell et al., 1990; Castet et al., 1993; Castet, 1994). It should be noted, however, that the existence of retinal image smear during target motion is not always deleterious, as motion smear has been shown to foster the detection of target motion (Geisler, 1999; Edwards & Crane, 2007) and the discrimination of the direction of motion (Burr & Ross, 2002; Tong et al., 2007a; Narasimhan, Tripathy & Barrett, 2009)
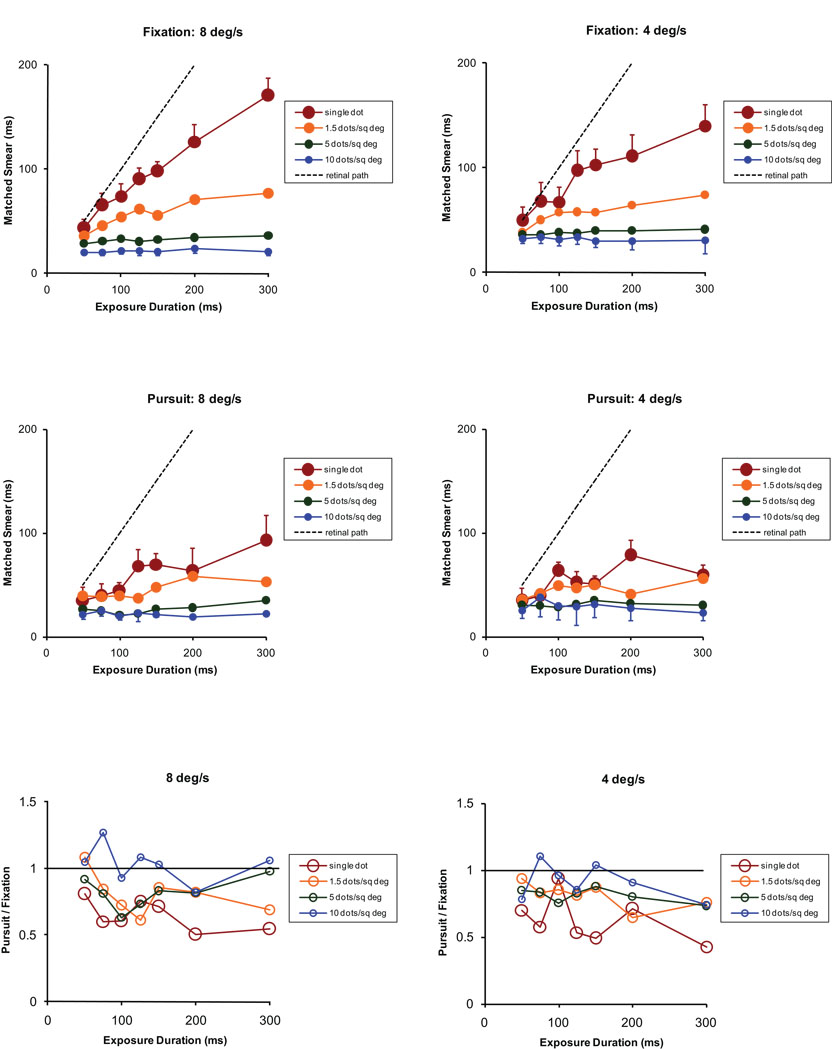
Figure 1.
The extent of perceived motion smear, averaged for 3 normal observers, determined for retinal image motion produced by physical target motion during fixation on a stationary light emitting diode (LED, top row) or by a physically stationary target during horizontal smooth pursuit of the same LED (middle row). The targets were bright random dots with densities that ranged from a single dot to 10 dots/deg2 and had an intensity that was 2 log units above their detection threshold on a dark background. In each panel, symbol size decreases with an increase in dot density. Target duration ranged from 50 to 300 ms, as indicated on the x axis of each plot. During fixation, random dot stimuli were generated on the face of a display oscilloscope (Tong et al., 2007a) and viewed after reflection from a horizontally moving mirror at 4 (right column) or 8 deg/s (left column). Physically stationary random dot targets were presented during horizontal smooth pursuit at 4 or 8 deg/s. From trial to trial, the random-dot target or the pursuit stimulus moved randomly to the right or the left. Horizontal eye position was measured on pursuit trials by infrared limbal tracking, to allow calculation of the retinal image velocity on each pursuit trial. To account for trial-to-trial differences in the velocity of the retinal image during pursuit, the extent of perceived smear is expressed as a duration in ms (duration = matched smear [deg] / calculated image velocity [deg/ms]). Standard errors are shown in each of the four top panels for the dot densities that produced the greatest and least perception of motion smear. The plots in the bottom row show the extent of perceived smear during pursuit divided by the perceived smear during fixation. Values less than one for low dot densities indicate that the extent of perceived motion smear is reduced during pursuit for these stimulus conditions.
2. Perceived motion smear during eye and head movements
If the duration of visual persistence depends only on the spatio-temporal characteristics of retinal events, then it should be immaterial whether retinal image motion results from the movement of a target in space or from the movement of the eyes with respect to a physically stationary target. In fact, the extent of perceived motion smear is considerably less when the eye moves with respect to an isolated visual target that remains stationary in space (Figure 1, middle panels). This reduction of perceived motion smear has been demonstrated to occur during smooth pursuit (Bedell & Lott, 1996; Tong, Patel & Bedell, 2005; Tong, Aydin & Bedell, 2007b), smooth vergence (Bedell, Chung & Patel, 2004), saccades (Bedell & Yang, 2001), and the slow phase of the vestibulo-ocular reflex (Bedell & Patel, 2005; Tong, Patel & Bedell, 2006). The results shown in the bottom panels of Figure 1 indicate that a significant reduction of perceived motion smear during pursuit eye movements occurs only for relatively sparse random-dot stimuli. When the random-dot stimuli are dense, spatio-temporal interactions between neighboring image components decrease the perception of motion smear so much that little or no further reduction occurs in conjunction with the pursuit eye movement.
A natural question is whether the reduction of perceived motion smear during eye movements can be attributed to interactions between components of the retinal image, for example, between the stimuli that the observers judge and the visible edges of the display. A priori, this possibility is unlikely because, as noted above, only interactions between nearby retinal images lead to a reduction of perceived motion smear. Further, in a number of the experiments that documented a reduction of perceived motion smear during eye movement the targets were presented in an otherwise dark visual field (Bedell & Yang, 2001; Bedell & Patel, 2005; Tong et al., 2006), thereby eliminating any possibility for spatio-temporal visual interactions. Clearly, two separate mechanisms can reduce the perception of motion smear when a dense visual image moves on the retina during an eye movement. For visual scenes that are more complicated than random dot patterns, the relative contributions of these two mechanisms may be more complex than suggested by the data in Figure 1. Further, as discussed below in section 6, the two mechanisms that contribute to reducing perceived motion smear may have different functional consequences for vision.
Because the pattern of retinal stimulation is virtually identical in the fixation and eye-movement conditions of the experiments cited in the paragraph above, the reduction of perceived motion smear in the eye-movement condition must be attributed to non-retinal events. Evidence indicates that both voluntary eye movements, such as pursuit, horizontal vergence and saccades, and involuntary eye movements, such as the vestibulo-ocular reflex (VOR), are accompanied by extra-retinal signals (von Helmholtz, 1866; von Holst & Mittelstädt, 1950; Bedell, Klopfenstein & Yuan, 1989; Bridgeman, 1995; Bedell, 2000). These extra-retinal signals are based on a combination of internal efference copy information and inflowing signals of muscle proprioception (Gauthier, Nommay & Vercher, 1990; Bridgeman & Stark, 1991). The extent of perceived motion smear is reduced when a visual target is presented in darkness during a passive smooth eye movement (Tong, Stevenson & Bedell, 2008), which was induced when the observer pushed laterally on a circular wire loop that was held against the upper and lower lids of one eye. This outcome indicates that proprioceptive signals alone are sufficient for a reduction of perceived motion smear to occur. We consider the possible locus for the interaction between retinal information and extra-retinal signals in section 5, below.
Additional evidence that is consistent with a contribution of extra-retinal signals to the reduction of perceived motion smear comes from findings that the perceived extent of smear during eye movements is directionally asymmetric. Specifically, Tong et al. (2005) assessed the extent of perceived smear for a target that moved at various speeds with respect to the eye for 200 ms, either ‘with’ or ‘against’ the direction of smooth pursuit.1 Note that the only difference in the retinal stimulation between these two conditions is the direction of the retinal image motion during pursuit. In agreement with previous studies, when the relative motion of the target was ‘against’ the direction of pursuit, the extent of perceived motion smear was substantially less than when comparable motion of the retinal image occurred during steady fixation. However, no such reduction of perceived motion smear occurred for relative target motion ‘with’ the direction of pursuit. Tong et al. (2007b) evaluated the extent of perceived motion smear for an isolated target that moved, also for 200 ms, in one of several possible directions relative to the direction of smooth pursuit. A reduction of perceived smear occurs whenever the relative motion of the target includes a component ‘against’ the direction of the pursuit eye movement. The directional tuning of the reduction of perceived motion smear is therefore relatively broad, with a full width at half height on the order of 150 deg.
Observers also report a reduced extent of perceived motion smear during head and body movement, even in the absence of any change in the eye position with respect to the head (Tong et al., 2005; 2006). In these studies, the observers fixated on a visual stimulus moving with them in space while they underwent passive, full-body rotation around a vertical axis. The absence of eye movement in this condition results from suppression of the VOR, which has been attributed, at least partly, to the ‘cancellation’ between a supranuclear eye-movement signal for the VOR and an oppositely directed internal signal for smooth pursuit (Barnes, Benson & Prior, 1978; Misslisch et al., 1996). Analogous to the reduction of perceived motion smear that occurs during smooth pursuit eye movements, perceived smear is attenuated only for targets that undergo relative motion ‘against’ the direction of the head and body movement during VOR suppression. In contrast, when the VOR is not suppressed the extent of perceived motion smear is reduced for physical target motion that is either ‘with’ or ‘against’ the direction of the head and body rotation (Tong et al., 2006). These results imply that the asymmetrical reduction of perceived motion smear during VOR suppression should be attributed to extra-retinal information about a change in head position, and not to a covert internal signal for pursuit. Comparison of the results obtained during VOR suppression and during head and body rotation without (VOR) and with a physically stationary fixation stimulus (the visually enhanced VOR, or VVOR) indicates that the reduction of perceived motion smear neither depends on nor requires a change in gaze position (Tong et al., 2006). Rather, the data from this study indicate that a reduction of perceived smear occurs when relative motion of the target is ‘against’ the direction of either an eye or a head movement.
A comparison of the extent of perceived motion smear during combined head and eye movements indicates that perceived smear is reduced significantly more when target motion is ‘against’ the direction of eye compared to head movement (Tong et al., 2006). Further, the reduction of perceived smear increases with the velocity of an oppositely directed vestibular-driven eye movement, but not with the velocity of an oppositely directed head movement (Tong et al., 2006). A greater reduction of perceived motion smear also occurs with an increase in the velocity of non-vestibular smooth eye movements ’against’ the direction of the target’s motion (Tong et al., 2006; Tong et al., 2008; Bedell & Tong, 2009). Because Tong et al. (2006) varied the velocity of the test target in space randomly from trial to trial in the VOR and VVOR conditions, it is possible to evaluate the relationship between perceived motion smear and the velocity of retinal image motion more or less independently of the eye (or head) velocity.2 Regardless of the relative direction between the target motion and the eye (or head) movement, the extent of perceived motion smear, when expressed as a duration, increases significantly as the velocity of the retinal image motion increases (average r = 0.30; for all conditions, p < 0.02). A similar positive relationship between the extent of perceived smear and the velocity of retinal image motion exists during steady fixation (see Figure 1; also Bedell & Lott, 1996; Purushothaman et al., 1998). However, as the eye and retinal image velocity did not vary independently in the experiments that examined smooth pursuit, we are not able to make similar comparisons between the extent of perceived motion smear and the velocity of the retinal image motion during pursuit.
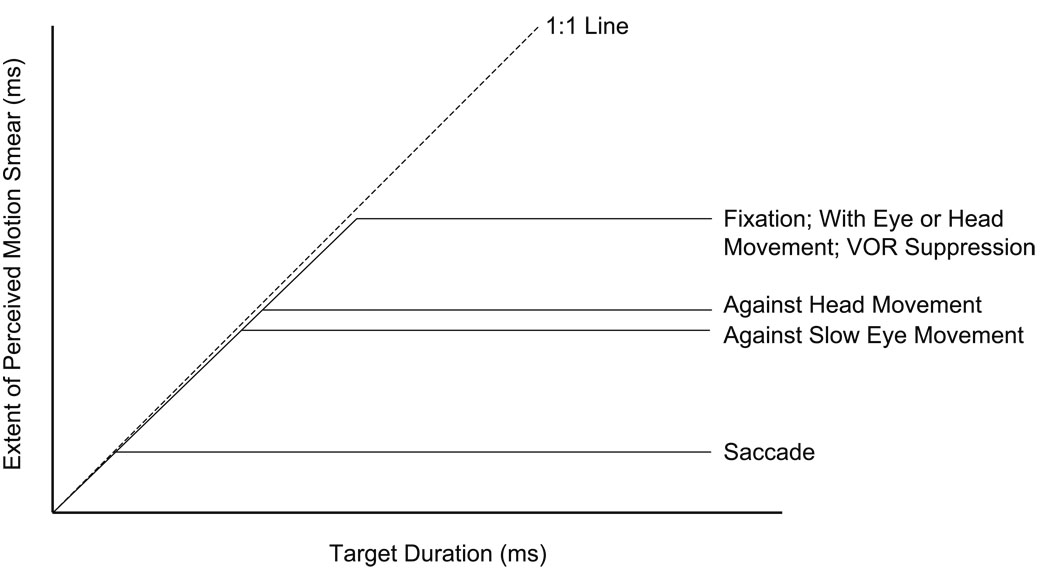
Figure 2 presents an idealized summary of the change in perceived motion smear with target duration for different eye- and head-movement conditions, along with the presumed relationship between perceived smear and the duration of visual persistence. The figure shows that the extent of perceived smear increases with the duration of the target, until the duration of visual persistence is reached. For target durations longer than the duration of visual persistence, the perceived extent of motion smear remains constant. A reduction of perceived smear by extra-retinal eye or head movement signals therefore appears in the Figure as a lower asymptotic value of perceived smear and therefore a reduced duration of visual persistence. For simplicity, the effects of changing the eye, head and retinal-image velocity that were discussed in the paragraph above are not represented in the Figure.
Figure 2.
A stylized summary representation of how the extent of perceived motion smear varies with the duration of the retinal image motion for different viewing conditions. For each viewing condition, perceived smear is shown to increase linearly with the duration of the target motion until the duration of visual persistence is reached (horizontal lines). Although this increase is shown to have slope of 1, trial-to-trial variability in the duration of visible persistence will reduce the actual slope, particularly as the asymptotic value of persistence is approached. The duration of visual persistence provides an upper limit for perceived motion smear in each of the viewing conditions. Perceived motion smear for target motion ‘with’ the direction of an eye or head movement is assumed to follow the line labeled “Fixation.” The duration of visual persistence for a target that moves ‘against’ the direction of different types of slow eye movements (smooth pursuit, the vestibulo-ocular reflex, and vergence) is assumed to be the same. The slightly longer duration of persistence that is depicted for target motion ‘against’ the direction of head movement than ‘against’ slow eye movements is based on the results of Tong et al. (2006). Based on the discussion of possible mechanisms to reduce perceived motion smear in section 4 of the text, differences in the duration of visual persistence for the various viewing conditions are assumed to result from differences in the duration of the temporal impulse response.
We suggested previously that the observed directional asymmetry in the reduction of perceived motion smear may be attributed to a strategy adopted by the visual system to reduce perceived smear only when the retinal image motion during eye and/or head movements is consistent with that expected from a physically stationary object in the environment (Tong et al., 2005; Tong et al., 2006). To avoid the necessity for time-consuming quantitative neural calculations, we proposed that the visual system largely ignores the speed of the retinal image motion during eye and/or head movements and instead categorizes the direction of motion as consistent or inconsistent with that expected for a physically stationary object. Specifically, extra-retinal eye and/or head movement signals act to reduce perceived smear to the extent that the direction of motion is consistent with that expected from an object that is stationary in the environment. On the other hand, relative image motion ‘with’ the direction of a head or eye movement is consistent with a physically moving target (Tong et al., 2005; Tong et al., 2006). As motion smear provides a useful cue for detecting and discriminating the trajectory of a target in physical motion (Geisler, 1999; Burr & Ross, 2002; Tong et al., 2007; Edwards & Crane, 2007), the visual system would be expected to benefit if the perception of motion smear is unattenuated for a target that moves in the same direction as an eye or head movement.
The influence of extra-retinal information on perception for only one direction of target motion is consistent with the reported asymmetrical effects of extraretinal eye-movement signals vs. the retinal image motion of a background stimulus on the perceived speed of a tracked visual target (e.g., Brenner & van den Berg, 1994). Other studies suggest an asymmetric effect of extra-retinal information from pursuit on the perceived speed of an untracked target (Wertheim & van Gelder, 1990; Turano & Heidenreich, 1999; Freeman, 2001; Turano & Masoff, 2001; but see also Souman, Hooge & Wertheim, 2006). Specifically, in most of these studies extra-retinal signals appear to contribute to the perceived speed of an untracked target primarily when the target moves in the opposite direction of the observer’s pursuit eye movement. However, in contrast to the influence of extra-retinal eye and head movement signals on perceived motion smear, to correctly recover the physical speed (or the location) of a target in space, the target’s retinal image velocity (or its position) must be combined with quantitative information about eye and head movements. For this reason, we do not assume that the neural mechanism that reduces perceived motion smear is responsible also for determining the speed and location of visual targets during eye and head movements. In agreement with this distinction, Tong et al (2007b) found that the direction of perceived motion smear exhibits almost no compensation for the direction of the eye movement during pursuit.
3. Perceived motion smear during visually induced self motion
Under normal conditions of illumination, information about head motion is provided by visual as well as vestibular signals. In particular, motion of the visual field with respect to a stationary observer produces a perception of self motion that is indistinguishable from real physical motion (Brandt, Dichgans & Koenig, 1973; Dichgans & Brandt, 1978). Previous studies indicated that vestibular and visual signals for self motion are combined in the responses of some neurons in the vestibular nucleus (Waespe & Henn, 1977; 1979; but see Bryan & Angelaki, 2009). We therefore asked whether the perception of motion smear would be reduced during perceived self motion that was induced purely by visual stimulation.
3.1. Experimental methods
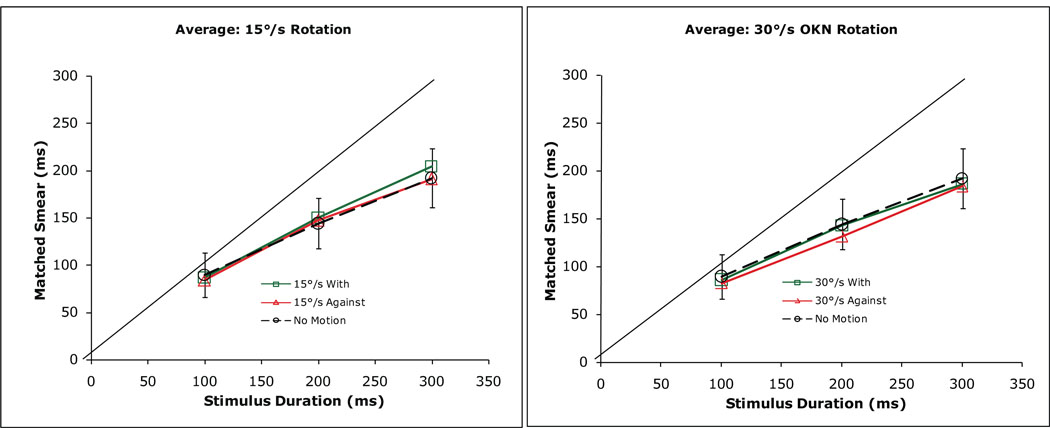
To address this question, observers sat inside an 1.5 m diameter cloth optokinetic drum, the interior of which was covered by alternating 1 cm gray and white vertical stripes, corresponding to a spatial frequency of 0.65 cpd at the 74 cm viewing distance. The mean luminance of the interior of the drum was 0.47 cd/m2. Continuous horizontal clockwise or anti-clockwise rotation of the drum at 15 or 30 deg/s produced a compelling perception of self motion in the opposite direction of the drum’s rotation. The observers’ task was to adjust the length of a bright line to match the extent of perceived motion smear, produced by a red laser spot that moved physically for between 100 and 300 ms ‘with’ or ‘against’ the direction of the drum rotation. The moving laser spot was presented 2.3 deg below a physically stationary fixation stimulus and the subsequently presented bright matching line was 2.3 deg above the fixation stimulus. The fixation stimulus, the moving laser spot, and the adjustable comparison line all were presented near the center of an 11 deg by 35 deg stationary homogeneous rectangular screen, mounted just inside the surface of the moving optokinetic drum. Each of the four normal observers who participated in this study perceived this screen to move along with them in the opposite direction of the drum’s rotation. During the experiment, the right eye was occluded and the horizontal position of the observers’ left eye was recorded on each trial at a sampling frequency of 1 KHz using infrared limbal detection. The change in eye position during the presentation of the moving laser spot was combined with the physical motion of the spot to determine the velocity of image motion on the retina. Trials on which the eye velocity was greater than 2 deg/s were discarded.3 From trial to trial, the moving laser spot had a velocity between 10 and 60 deg/s, either ‘with’ or ‘against’ the direction of the observer’s perceived self motion.
3.2. Experimental results and discussion
The extent of perceived motion smear, expressed in ms and averaged for the four observers, did not differ significantly for motion ‘with’ vs. ‘against’ the direction of the observers’ visually induced self motion (Fdf=1,3 = 4.54, p = 0.12; see Figure 3). Neither did the extent of perceived motion smear differ significantly during rotation of the optokinetic drum and a comparison condition in which the drum did not move and the observers perceived themselves to remain stationary (Fdf=2,6 = 1.67, p = 0.29). Two of the observers in this study participated in previous experiments, in which they reported an approximately 40% reduction in the extent of perceived motion smear for targets that moved for 200 ms ‘against’ the direction of smooth pursuit, compared to targets that underwent the same retinal image motion during fixation (Tong et al., 2006; 2007a).
Figure 3.
The extent of perceived motion smear, averaged for 4 normal observers, for retinal image motion produced by physical target motion ‘with’ (green squares) and ‘against’ (red triangles) the direction of the perceived self motion that was produced by constant velocity rotation of a full-field optokinetic (OKN) drum, within which the observer sat. The left and right panels plot the extent of perceived smear during motion of the OKN drum at 15 and 30 deg/s, respectively. For comparison, the extent of perceived motion smear is shown also in each panel while the OKN drum was physically stationary (black circles) and the observer did not experience self motion. Error bars represent ±1 SE across the 4 observers. The dashed line in each panel indicates an extent of perceived motion smear that is equal to the duration of the moving stimulus.
In combination with our previous results (Tong et al., 2005; 2006; Bedell & Patel, 2005), the outcome of this experiment indicates clearly that vestibular and visual signals about self motion do not exert a similar influence on the perception of motion smear. It is not obvious why visual information about self motion should be less privileged than vestibular information in this respect. However, this distinction is consistent with observations that visual and vestibular signals of self motion remain segregated at some levels of neural processing (Brandt et al., 1998; Kleinschmidt et al., 2002; Meng & Angelaki, 2010).
4. The temporal impulse response and the reduction of perceived motion smear
As noted above, previous authors measured the extent of perceived motion smear as a way to assess the duration of visual persistence (e.g., Bidwell, 1899; McDougall, 1904; Allport, 1970; Geremek et al., 2002). Visual persistence can be estimated also in terms of the duration of the temporal impulse response function (TIRF: e.g., Blommaert & Roufs, 1987; Zhang, Cantor & Schor, 2008). The TIRF can be estimated from psychophysical two-flash sensitivity data (e.g., Ikeda, 1965; Rashbass, 1970; Burr & Morrone, 1993) or by inverse Fourier transformation of the psychophysical temporal contrast sensitivity function (e.g., Kelly, 1971; Roufs, 1972; Georgeson, 1987; Swanson, Ueno, Smith & Pokorny,1987). Burr & Morrone (1996) reported that, in addition to a substantial loss of sensitivity to luminance modulation, the duration of the TIRF decreases during saccadic eye movements. Although little or no change in visual sensitivity occurs during slow eye movements (Starr, Angel & Yeates, 1969; Bedell & Lott, 1996; Schütz, Braun & Gegenfurtner, 2007; but also see below), on the basis of Burr’s and Morrone’s results we entertained the possibility that a similar decrease in the duration of the TIRF might account for the reduction of perceived motion smear that we observed during slow eye and head movements. Moreover, to account for the directional asymmetry in the reduction of perceived motion smear during eye and head movements, we expected that this decrease in the duration of the TIRF would occur primarily for stimuli that move ‘against’ the direction of the eye or head movement.
Flipse et al. (1988) examined contrast sensitivity for vertical grating stimuli with comparable average velocities of retinal image motion during fixation and pursuit. During the fixation of a stationary target, retinal image motion was produced by triangular, back-and-forth motion of the grating on an oscilloscope screen. During pursuit of the moving grating, retinal image motion occurred when the pursuit gain was less than one, and therefore was ‘against’ the direction of the eye movement. For a spatial frequency of 0.5 cpd, contrast sensitivity was similar in the two conditions except for a slight reduction in peak sensitivity during pursuit, which spanned temporal frequencies of retinal image motion between approximately 5 – 20 Hz. On the other hand, contrast sensitivity for a spatial frequency of 8 cpd was approximately twice as high during pursuit compared to fixation when the retinal image velocity had a temporal frequency of 20 – 25 Hz. Flipse and Maas (1996) reported no similar advantage of contrast sensitivity for a moving 6 cpd grating during passive head movements, compared to equivalent retinal stimulation while the head remained stationary.
Subsequently, Schütz, Braun and Gegenfurtner (2008; 2009a; 2009b) reported a small but significant improvement of sensitivity for flashed and flickering chromatic stimuli and for luminance targets with spatial frequencies greater than approximately 3 cpd during pursuit compared to fixation. Schütz et al. (2009a) observed a similar increase in sensitivity during the slow phases of optokinetic nystagmus, but not during the vestibulo-ocular reflex. Terao et al. (2010) also found increased chromatic temporal sensitivity during pursuit, which was restricted to the condition in which the motion of the chromatically modulated target was ‘against’ the direction of the pursuit eye movement. For luminance-defined stimuli of low spatial frequency, Schütz et al. (2007; 2008) reported a slight reduction in sensitivity when the targets were presented during pursuit.
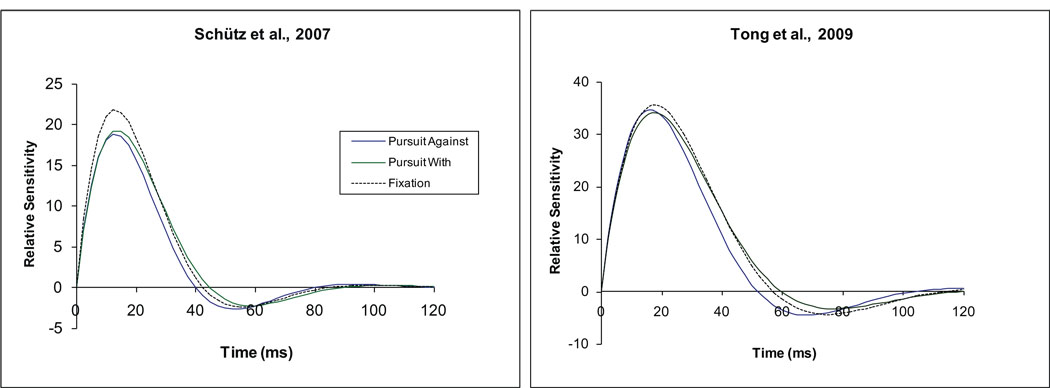
Although Tong et al. (2009) found no significant difference in the sensitivity for horizontal lines flashed during horizontal pursuit and fixation, the TIRFs fit to their 2-pulse data indicated that the duration of the impulse response was reduced significantly during pursuit. Both Schütz, Delipetkos et al. (2007) and Tong et al. (2009) examined temporal contrast sensitivity for 1 cpd vertical gratings during horizontal pursuit. For gratings that produced the same temporal frequency of retinal image motion, Schütz et al. reported poorer contrast sensitivity when the target moved ‘against’ compared to ‘with’ the direction of the pursuit eye movement. Tong et al. (2009) found a similar, non-significant effect of the direction of target motion on contrast sensitivity. A potentially important difference between these studies was the location of the visual stimuli, which were centered 4 deg above or below the fovea in the study by Schütz, Delipetkos et al. (2007) and overlapped the fovea in the study by Tong et al. In both of these studies, the duration of the estimated TIRF was briefer for motion of the target ‘against’ compared to ‘with’ the direction of pursuit, although the effect was statistically significant only in the study by Tong et al. (2009). The TIRFs determined from the averaged temporal contrast sensitivity data in these two studies are compared in Figure 4.
Figure 4.
Temporal impulse response functions for 1 cpd stimuli that move ‘with’ or ‘against’ the direction of a pursuit eye movement and during fixation, estimated from the temporal contrast sensitivity data obtained by Schütz, Delipetkos et al. (2007: left panel) and Tong et al. (2009: right panel). The inverse Fourier transform of the displayed functions produced the optimal least-squares fits to the reported average temporal contrast sensitivity data for each direction of grating motion during pursuit or fixation. Details of the curve fitting procedures can be found in Bedell et al. (2008). Differences in the amplitude and the duration of the TIRFs from the two studies are likely to reflect differences in the extent and the retinal location of the 1-cpd stimuli.
Bedell and Tong (2009) reported that the extent of perceived motion smear is reduced in observers with infantile nystagmus (IN), more so than during normal pursuit and selectively for targets that move relatively ‘against’ the direction of the IN slow phase. Observers with IN also exhibit a decrease in the duration of the TIRF, compared to the TIRF determined in normal observers (Bedell et al., 2008). Consistent with the greater reduction of perceived motion smear during IN compared to normal pursuit, the duration of the TIRF is reduced during IN more than during normal pursuit (Bedell et al., 2008).
An obvious difficulty with attributing the reduction of perceived motion smear to a decrease in the duration of the TIRF during eye movements is that the measured changes in the TIRF are relatively small. For example, the data of Tong et al. (2009) indicate that during pursuit the duration of the first positive lobe of the TIRF decreases by approximately 10% compared to fixation. In contrast, the extent of perceived motion smear for a target with a duration of 150 – 200 ms decreases by an average of approximately 35% during smooth eye movements compared to fixation (Bedell & Lott, 1996; Bedell et al., 2004; Tong et al., 2005; Tong et al., 2007b). An important difference between the methods in these two groups of studies is that the extent of perceived motion smear was determined using supra-threshold visual stimuli, whereas the comparisons of the TIRF during eye movements and fixation have so far used only threshold visual stimuli. Some authors noted that the shape of the estimated TIRF depends on the contrast of the presented visual stimuli (Georgeson, 1987; Stromeyer & Martini, 2003). As the characteristics of the TIRF depend on the detectability of the stimulus, it is plausible that differences between the TIRF for targets that move ‘with’ and ‘against’ the direction of an eye (or head) movement could be more substantial for suprathreshold than for threshold visual stimuli. The characteristics of the TIRF vary with other parameters of the visual stimulus as well, such as background luminance, spatial frequency, and wavelength (Ikeda, 1965; Watson & Nachmias, 1977; Georgeson, 1987; Swanson, Ueno, Smith & Pokorny, 1987; Burr & Morrone, 1993; Shinomori & Werner, 2008). To address the hypothesized relationship between the extent of perceived motion smear and the TIRF, it would be worthwhile to compare the changes in perceived smear and the duration of the TIRF that occur for stimuli that vary along these dimensions.
5. Possible neural mechanisms to reduce perceived motion smear
Burr and Morrone (1996) suggested that the change in the temporal impulse response during saccades could be explained by an influence of extra-retinal signals that accompany the saccade on neural contrast gain control. To account for the reduced sensitivity to low spatial frequency luminance patterns during saccades but not to chromatic or high spatial frequency patterns (Volkman, Riggs, White & Moore, 1978; Burr, Morrone & Ross, 1994; Uchikawa & Sato, 1995), Burr et al. (1994) proposed specifically that the gain of the magnocellular pathway is reduced by extra-retinal signals during saccades. Physiological studies indicate that the response gain of nonlinear magnocellular neurons is reduced as the stimulus contrast increases, particularly for stimuli of low temporal frequency (e.g., Benardete, Kaplan & Knight, 1992; Carandini, Heeger & Movshon, 1997). If extra-retinal signals during saccades produce a comparable reduction of low-temporal-frequency response gain, then the TIRF would be expected to shift toward a higher temporal frequency, resulting in a decrease in its duration. Schütz et al. (2008; 2009b) attributed improved sensitivity for chromatic and high-spatial-frequency targets during pursuit to an increase in the gain of the parvocellular pathway, with no change of the magnocellular pathway gain. However, it is not entirely apparent why an overall increase in parvocellular pathway gain (with or without a change in the gain of the magnocellular pathway) should result in a decrease in the duration of the TIRF.
Burr, Morgan & Morrone (1999) favored a subcortical or early cortical site for the proposed change in contrast gain that they proposed is responsible for saccadic suppression and, presumably, for the decreased duration of the TIRF during saccades. In agreement with the idea that extra-retinal eye movement signals mediate a change in the TIRF, Reppas et al. (2002) reported that the impulse response function, assessed for magnocellular neurons in the primate LGN using a full-field flickering stimulus, is of shorter duration during saccadic eye movements than during fixation. On the other hand, Schütz et al. (2008) proposed that the changes in visual sensitivity during pursuit eye movements may be mediated at a cortical locus. One site that they suggested is the medial superior temporal area (MST), where visual information and extra-retinal eye movement signals are combined. In addition to information about versional eye movements (Newsome, Wurtz & Komatsu, 1988; Churchland & Lisberger, 2005), some MST neurons also receive information about vergence eye movements (Akao et al., 2005) and vestibular signals (e.g., Thier & Erickson, 1992; Duffy, 1998; Bremmer et al, 1999). The directional tuning for the reduction of perceived motion smear is on the order of 150 deg (Tong et al., 2007b), which is comparable to the directional tuning reported for individual motion-sensitive neurons in cortical area MST (Squatrito & Maioli, 1997).
A separate question is at what neural level the extra-retinal signals originate that are responsible for reducing perceived motion smear and, presumably, for the changes in visual sensitivity during eye movements. Schütz et al. (2008) demonstrated that chromatic sensitivity improves before the onset of pursuit, which might be interpreted to indicate that extra-retinal eye movement information is available to affect visual sensitivity in the form of an efference copy signal before the eye movement. However, as pointed out by Pola (2004; 2007; also Zhang et al., 2008) in the context of perceived visual direction near the time of a saccade, these results could reflect the interaction of extra-retinal signals that become available at or even after the start of the eye movement with a persisting visual signal from before the onset of eye movement.
To summarize the results presented above, similar reductions of perceived motion smear occur in association with versional and vergence eye movements, voluntary and involuntary eye movements, active and passive eye movements, and passive head movements (see Fig. 2). No reduction of perceived smear occurs during head movement when the VOR is suppressed and the eyes do not move with respect to the head. We show here that signals of self-motion produced by large-field visual stimulation do not produce a reduction of perceived motion smear, despite the convergence of vestibular and optic flow information onto neurons in MST, as well as other cortical sites (e.g., Schlack, Hoffmann & Bremmer, 2002; Wall & Smith, 2008). A parsimonious interpretation of these results is that the extra-retinal eye and head movement signals that are responsible for reducing perceived motion smear are generated at a relatively low level, by some combination of muscle proprioception and vestibular and efference copy signals in the brainstem.
6. Functional consequences of the reduction of perceived motion smear
Burr and Morgan (1997) compared blur discrimination thresholds for moving stimuli that were presented with varying amounts of physical blur during steady fixation for either 40 or 150 ms. Based on the finding that blur discrimination becomes poorer as the blur of the stimulus increases (e.g., Watt & Morgan, 1983; Pääkkönen & Morgan, 1994) and Burr’s (1980) observation that perceived motion blur is reduced for stimulus durations longer than 40 ms, it might be anticipated that observers would be able to detect smaller changes in stimulus blur for long- than short-duration stimuli. Although Burr and Morgan found slightly poorer blur discrimination for their 40-ms than 150-ms stimuli, a similar effect occurred for both stationary and moving visual targets. These results led them to conclude that discrimination is limited by the physical rather than the perceived extent of motion smear. Several other studies showed that visual functions such as spatial interval acuity (Morgan & Benton, 1989), stereoacuity (Ramamurthy, Bedell & Patel, 2005) and direction-of-motion discrimination (Burr & Ross, 2002) change during physical target motion as would be expected in the presence of the motion smear that is produced by the temporal integration of retinal information. A different result was reported by Tong et al. (2007a), who found that direction-of-motion discrimination for moving random-dot stimuli varied with the dot density according to the extent of the perceived motion smear.
Each of the preceding studies evaluated the effects of motion blur on visual functioning during steady fixation. In contrast, the studies cited in section 4 above (Flipse et al., 1988; Schütz et al., 2008; 2009a; 2009b) indicate that visual contrast sensitivity differs for visual targets with the same parameters of retinal image motion during fixation and eye movement, and according to the direction of the target’s motion with respect to the direction of the eye movement. A functional improvement of spatial contrast sensitivity in conjunction with a reduction of perceived motion smear by extra-retinal eye (and perhaps head: Bedell, Tong, Patel & White, 2008) movement signals clearly would be beneficial for observers with eye movement disorders such as IN. However, as one can not readily produce comparable conditions of retinal image motion while the eyes are moving vs. stationary in observers with IN, it is difficult to determine whether visual sensitivity improves in conjunction with the reduction of perceived motion smear. In normal observers, no direct comparison has been made to assess whether the reduced perception of motion smear during eye movements compared to fixation affects visual functions other than contrast sensitivity.
The evidence presented here indicates that the visual system applies a dual approach to minimize the deleterious effects of visual persistence on the perception of moving retinal images. In section 2, above, we presented evidence that internal, extra-retinal signals are responsible for a reduction of perceived motion smear produced by targets that move against the direction of an eye or head movement. In addition, a reduction of perceived motion smear that is mediated by spatio-temporal interactions between nearby moving visual stimuli occurs regardless of eye or head movements. If these two mechanisms for reducing the extent of perceived motion smear are confirmed to have different functional consequences, it would be appropriate to conclude that their actions occur at different levels of visual processing.
Acknowledgments
We thank the Alexander von Humboldt Transcoop program group for Perception and Action for support. The work described in this article was funded in part by award #003652-0185-2001 from the Texas Advanced Research Program, and grants R01 EY05068 and P30 EY07551 from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Tong et al. (2005) categorized the direction of a target’s retinal image motion as either in the ‘same’ or ‘opposite’ direction of an eye (or head) movement. To reduce the possibility of confusion associated with the inversion of the retinal image, here we use the terminology adopted in subsequent articles, i.e., that the target moved in space ‘with’ or ‘against’ the direction of an eye (or head) movement.
For targets that moved ‘against’ the direction of the head movement, the correlation between the eye velocity and the retinal image velocity was 0.154 and −0.072 during the VOR and VVOR, respectively. For targets that moved ‘with’ the direction of the head movement, the correlation between retinal image velocity and head velocity was 0.054 during the VOR and −0.110 during the VVOR. None of these correlations are significant (smallest p value = 0.135).
The average eye velocity was 0.06 deg/s on trials with 15 deg/s drum rotation and 0.12 deg/s on trials with 30 deg/s drum rotation, with no difference between trials with clockwise and anti-clockwise rotation.
References
- Akao T, Mustari MJ, Fukushima J, Kurkin S, Fukushima K. Discharge characteristics of pursuit neurons in MST during vergence eye movements. Journal of Neurophysiology. 2005;93:2415–2434. doi: 10.1152/jn.01028.2004. [DOI] [PubMed] [Google Scholar]
- Allport DA. Temporal summation and phenomenal simultaneity: Experiments with the radius display. Quarterly Journal of Experimental Psychology. 1970;22:686–701. [Google Scholar]
- Barnes GR, Benson AJ, Prior AR. Visual-vestibular interaction in the control of eye movement. Aviation Space & Environmental Medicine. 1978;49:557–564. [PubMed] [Google Scholar]
- Bedell HE. Perception of a clear and stable visual world with congenital nystagmus. Optometry & Vision Science. 2000;77:573–581. doi: 10.1097/00006324-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Chung ST, Patel SS. Attenuation of perceived motion smear during vergence and pursuit tracking. Vision Research. 2004;44:895–902. doi: 10.1016/j.visres.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell HE, Klopfenstein JF, Yuan NY. Extraretinal information about eye position during involuntary eye movement: optokinetic afternystagmus. Perception & Psychophysics. 1989;46:579–586. doi: 10.3758/bf03208155. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Lott LA. Suppression of motion-produced smear during pursuit eye movements. Current Biology. 1996;6:1032–1034. doi: 10.1016/s0960-9822(02)00650-4. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Ramamurthy M, Patel SS, Subramaniam S, Vu-Yu L-P, Tong J. The temporal impulse response function in infantile nystagmus. Vision Research. 2008;48:1575–1583. doi: 10.1016/j.visres.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell HE, Patel SS. Attenuation of perceived motion smear during the vestibulo-ocular reflex. Vision Research. 2005;45:2191–2200. doi: 10.1016/j.visres.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Tong J, Patel SS, White JM. Perceptual influences of extraretinal signals for normal eye movements and infantile nystagmus. In: Leigh RJ, Devereaux MW, editors. Advances in Understanding Mechanisms and Treatment of Infantile Forms of Nystagmus. London: Oxford University Press; 2008. pp. 11–22. [Google Scholar]
- Bedell HE, Tong J. Asymmetrical perception of motion smear in infantile nystagmus. Vision Research. 2009;49:262–267. doi: 10.1016/j.visres.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Bedell HE, Yang J. The attenuation of perceived image smear during saccades. Vision Res. 2001;41:521–528. doi: 10.1016/s0042-6989(00)00266-2. [DOI] [PubMed] [Google Scholar]
- Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Visual Neuroscience. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- Bidwell S. Curiosities of light and sight. London: Swan Sonnenschein; 1899. [Google Scholar]
- Blommaert FJ, Roufs JA. Prediction of thresholds and latency on the basis of experimentally determined impulse functions. Biological Cybernetics. 1987;56:329–344. doi: 10.1007/BF00319513. [DOI] [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Dieterich M. Reciprocal inhibitory visual-vestibular interaction: visual motion stimulation deactivates the parieto-vestibular cortex. Brain. 1998;121:1749–1758. doi: 10.1093/brain/121.9.1749. [DOI] [PubMed] [Google Scholar]
- Brandt T, Dichgans J, Koenig E. Differential effects of central versus peripheral vision on egocentric and exocentric motion perception. Experimental Brain Research. 1973;16:476–491. doi: 10.1007/BF00234474. 1973. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Pekel M, Lappe M, Hoffmann KP. Linear vestibular self-motion signals in monkey medial superior temporal area. Annals of the New York Academy of Sciences. 1999;871:272–281. doi: 10.1111/j.1749-6632.1999.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Brenner E, van den Berg AV. Judging object velocity during smooth pursuit eye movements. Experimental Brain Research. 1994;99:316–324. doi: 10.1007/BF00239598. [DOI] [PubMed] [Google Scholar]
- Bridgeman B. A review of the role of efference copy in sensory and oculomotor control systems. Annals of Biomedical Engineering. 1995;23:409–422. doi: 10.1007/BF02584441. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Stark L. Ocular proprioception and efference copy in registering visual direction. Vision Research. 1991;31:1903–1913. doi: 10.1016/0042-6989(91)90185-8. [DOI] [PubMed] [Google Scholar]
- Bryan AS, Angelaki DE. Optokinetic and vestibular responsiveness in the macaque rostral vestibular and fastigial nuclei. Journal of Neurophysiology. 2009;101:714–720. doi: 10.1152/jn.90612.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. Motion smear. Nature. 1980;284:164–165. doi: 10.1038/284164a0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morgan MJ. Motion deblurring in human vision. Proceedings of the Royal Society of London B. 1997;264:431–436. doi: 10.1098/rspb.1997.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Morgan MJ, Morrone MC. Saccadic suppression precedes visual motion analysis. Current Biology. 1999;9:1207–1209. doi: 10.1016/S0960-9822(00)80028-7. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC. Impulse-response function for chromatic and achromatic stimuli. Journal of the Optical Society of America A. 1993;10:1706–1713. [Google Scholar]
- Burr DC, Morrone MC. Temporal impulse response functions for luminance and color during saccades. Vision Research. 1996;36:2069–2078. doi: 10.1016/0042-6989(95)00282-0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994;371:511–513. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Burr DC, Ross J. Direct evidence that “speedlines” influence motion mechanisms. Journal of Neuroscience. 2002;22:8661–8664. doi: 10.1523/JNEUROSCI.22-19-08661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr DC, Ross J, Morrone MC. Seeing objects in motion. Proceedings of the Royal Society of London B. 1986;227:249–265. doi: 10.1098/rspb.1986.0022. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Movshon JA. Linearity and normalization in simple cells of the macaque primary visual cortex. Journal of Neuroscience. 1997;17:8621–8644. doi: 10.1523/JNEUROSCI.17-21-08621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castet E. Effect of the ISI on the visible persistence of a stimulus in apparent motion. Vision Research. 1994;34:2103–2114. doi: 10.1016/0042-6989(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Castet E, Lorenceau J, Bonnet C. The inverse intensity effect is not lost with stimuli in apparent motion. Vision Research. 1993;33:1697–1708. doi: 10.1016/0042-6989(93)90035-u. [DOI] [PubMed] [Google Scholar]
- Chen S, Bedell HE, Ögmen H. A target in real motion appears blurred in the absence of other proximal moving targets. Vision Research. 1995;35:2315–2328. doi: 10.1016/0042-6989(94)00308-9. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Lisberger SG. Relationship between extraretinal component of firing and eye speed in area MST of macaque monkeys. Journal of Neurophysiology. 2005;94:2416–2426. doi: 10.1152/jn.00195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans J, Brandt T. Visual-vestibular interaction: Effects on self-motion perception and postural control. In: Held R, Leibowitz HW, Teuber HL, editors. Handbook of Sensory Physiology, Vol. VIII, Perception. New York: Springer; 1978. pp. 755–804. [Google Scholar]
- Di Lollo V, Hogben JH. Suppression of visible persistence. Journal of Experimental Psychology. Human Perception & Performance. 1985;11:304–316. doi: 10.1037/0096-1523.11.3.304. [DOI] [PubMed] [Google Scholar]
- Dixon NE, Hammond EJ. The attenuation of visual persistence. British Journal of Psychology. 1972;63:243–254. doi: 10.1111/j.2044-8295.1972.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Duffy CJ. MST neurons respond to optic flow and translational movement. Journal of Neurophysiology. 1998;80:1816–1827. doi: 10.1152/jn.1998.80.4.1816. [DOI] [PubMed] [Google Scholar]
- Edwards M, Crane MF. Motion streaks improve motion detection. Vision Research. 2007;47:828–833. doi: 10.1016/j.visres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Farrell JE, Pavel M, Sperling G. The visible persistence of stimuli in stroboscopic motion. Vision Research. 1990;30:921–936. doi: 10.1016/0042-6989(90)90058-s. [DOI] [PubMed] [Google Scholar]
- Flipse JP, Maas AJ. Visual processing during high frequency head oscillation. Aviation Space and Environmental Medicine. 1996;67:625–632. [PubMed] [Google Scholar]
- Flipse JP, van der Wildt GJ, Rodenburg M, Keemink CJ, Knol PGM. Contrast sensitivity for oscillating sine wave gratings during ocular fixation and pursuit. Vision Research. 1988;28:819–826. doi: 10.1016/0042-6989(88)90029-6. [DOI] [PubMed] [Google Scholar]
- Freeman TCA. Transducer models of head-centered motion perception. Vision Research. 2001;41:2741–2755. doi: 10.1016/s0042-6989(01)00159-6. [DOI] [PubMed] [Google Scholar]
- Gauthier GM, Nommay D, Vercher J-L. The role of ocular muscle proprioception in visual localization of targets. Science. 1990;249:58–61. doi: 10.1126/science.2367852. [DOI] [PubMed] [Google Scholar]
- Geisler WS. Motion streaks provide a spatial code for motion direction. Nature. 1999;400:65–69. doi: 10.1038/21886. [DOI] [PubMed] [Google Scholar]
- Georgeson MA. Temporal properties of spatial contrast vision. Vision Research. 1987;27:765–780. doi: 10.1016/0042-6989(87)90074-5. [DOI] [PubMed] [Google Scholar]
- Geremek A, Stürzel F, da Pos O, Spillmann L. Masking, persistence, and transfer in rotating arcs. Vision Research. 2002;42:2509–2519. doi: 10.1016/s0042-6989(02)00201-8. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. In: 1962, Treatise on Physiological Optics. Southall JPC, editor. Volume 3. New York: Dover; 1866. [Google Scholar]
- Hogben JH, Di Lollo V. Suppression of visible persistence in apparent motion. Perception & Psychophysics. 1985;38:450–460. doi: 10.3758/bf03207176. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstädt H. The principal of reafference: interactions between the central nervous system and the peripheral organs. In: Dodwell PC, editor. 1971, Perceptual Processing: Stimulus Equivalence and Pattern Recognition. New York: Appleton Century Crofts; 1950. pp. 41–71. [Google Scholar]
- Ikeda M. Temporal summation of positive and negative flashes in the visual system. Journal of the Optical Society of America. 1965;55:1527–1534. doi: 10.1364/josa.55.000560. [DOI] [PubMed] [Google Scholar]
- Kelly DH. Theory of flicker and transient responses. I. Uniform fields. Journal of the Optical Society of America. 1971;61:537–546. doi: 10.1364/josa.61.000537. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Thilo KV, Büchel C, Gresty MA, Bronstein AM, Frackowiak RS. Neural correlates of visual-motion perception as object- or self-motion. Neuroimage. 2002;16:873–882. doi: 10.1006/nimg.2002.1181. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. 3rd Edition. New York: Oxford University Press; 1999. pp. 19–150. [Google Scholar]
- McDougall W. The sensations excited by a single momentary stimulation of the eye. British Journal of Psychology. 1904;1:78–113. [Google Scholar]
- Meng H, Angelaki DE. Responses of ventral posterior thalamus neurons to three-dimensional vestibular and optic flow stimulation. Journal of Neurophysiology. 2010;103:817–826. doi: 10.1152/jn.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslisch H, Tweed D, Fetter M, Dichgans J, Vilis T. Interaction of smooth pursuit and the vestibuloocular reflex in three dimensions. Journal of Neurophysiology. 1996;75:2520–2532. doi: 10.1152/jn.1996.75.6.2520. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Benton S. Motion-deblurring in human vision. Nature. 1989;340:385–386. doi: 10.1038/340385a0. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Tripathy SP, Barrett BT. Loss of positional information when tracking multiple moving objects: the role of visual memory. Vision Research. 2009;49:10–27. doi: 10.1016/j.visres.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatdu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Nishida S, Watanabe J, Kuriki I, Tomimoto T. Human visual system integrates color signals along a motion trajectory. Current Biology. 2007;17:366–372. doi: 10.1016/j.cub.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Pääkkönen AK, Morgan MJ. Effects of motion on blur discrimination. Journal of the Optical Society of America A. 1994;11:992–1002. [Google Scholar]
- Pola J. Models of the mechanism underlying perceived location of a perisaccadic flash. Vision Research. 2004;44:2799–2813. doi: 10.1016/j.visres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Pola J. A model of the mechanism for the perceived location of a single flash and two successive flashes presented around the time of a saccade. Vision Research. 2007;47:2798–2813. doi: 10.1016/j.visres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Ögmen H, Chen S, Bedell HE. Motion deblurring in a neural network model of retino-cortical dynamics. Vision Research. 1998;38:1827–1842. doi: 10.1016/s0042-6989(97)00350-7. [DOI] [PubMed] [Google Scholar]
- Ramamurthy M, Bedell HE, Patel SS. Stereothresholds for moving line stimuli for a range of velocities. Vision Research. 2005;45:789–799. doi: 10.1016/j.visres.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The visibility of transient changes of luminance. Journal of Physiology. 1970;210:165–186. doi: 10.1113/jphysiol.1970.sp009202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppas JB, Usrey WM, Reid RC. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron. 2002;35:961–974. doi: 10.1016/s0896-6273(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Vestibular and optokinetic symbiosis: an example of explaining by modelling. In: Baker R, Berthoz A, editors. Control of Gaze by Brainstem Neurons. New York: Elsevier/North Holland Biomedical Press; 1977. pp. 49–58. [Google Scholar]
- Roufs JAJ. Dynamic properties of vision. II. Theoretical relationships between flicker and flash thresholds. Vision Research. 1972;12:279–292. doi: 10.1016/0042-6989(72)90118-6. [DOI] [PubMed] [Google Scholar]
- Sachs D, Nasiri S, Goehl D. Image stabilization technology overview. [retrieved 30 April, 2010];2007 http://invensense.com/mems/gyro/documents/whitepapers/ImageStabilizationWhitepaper_051606.pdf.
- Schlack A, Hoffmann KP, Bremmer F. Interaction of linear vestibular and visual stimulation in the macaque ventral intraparietal area (VIP) European Journal of Neuroscience. 2002;16:1877–1886. doi: 10.1046/j.1460-9568.2002.02251.x. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Braun DI, Gegenfurtner KR. Improved visual sensitivity during smooth pursuit eye movements. Nature Neuroscience. 2008;11:1211–1216. doi: 10.1038/nn.2194. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Braun DI, Gegenfurtner KR. Improved visual sensitivity during smooth pursuit eye movements: temporal and spatial characteristics. Visual Neuroscience. 2009a;26:329–340. doi: 10.1017/S0952523809990083. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Braun DI, Gegenfurtner KR. Chromatic sensitivity during optokinetic nystagmus, visually enhanced vestibulo-ocular reflex, and smooth pursuit eye movements. Journal of Neurophysiology. 2009b;101:2317–2327. doi: 10.1152/jn.91248.2008. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Braun DI, Gegenfurtner KR. Contrast sensitivity during the initiation of smooth pursuit eye movements. Vision Research. 2007;47:2767–2777. doi: 10.1016/j.visres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Schütz AC, Delipetkos E, Braun DI, Kerzel D, Gegenfurtner KR. Temporal contrast sensitivity during smooth pursuit eye movements. Journal of Vision. 2007;7(13):1–15. doi: 10.1167/7.13.3. [DOI] [PubMed] [Google Scholar]
- Shinomori K, Werner JS. The temporal impulse response of S-cone pathways in detection of increments and decrements. Visual Neuroscience. 2008;25:341–347. doi: 10.1017/S0952523808080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souman JL, Hooge IT, Wertheim AH. Frame of reference transformations in motion perception during smooth pursuit eye movements. Journal of Computational Neuroscience. 2006;20:61–76. doi: 10.1007/s10827-006-5216-4. [DOI] [PubMed] [Google Scholar]
- Squatrito S, Maioli MG. Encoding smooth pursuit direction and eye position by neurons of area MSTd of macaque monkey. Journal of Neuroscience. 1997;17:3847–3860. doi: 10.1523/JNEUROSCI.17-10-03847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Angel R, Yeates H. Visual suppression during smooth following and saccadic eye movements. Vision Research. 1969;9:195–197. doi: 10.1016/0042-6989(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Swanson WH, Ueno T, Smith VC, Pokorny J. Temporal modulation sensitivity and pulse-detection thresholds for chromatic and luminance perturbations. Journal of the Optical Society of America A. 1987;4:1992–2005. doi: 10.1364/josaa.4.001992. [DOI] [PubMed] [Google Scholar]
- Stromeyer CF, 3rd, Martini P. Human temporal impulse response speeds up with increased stimulus contrast. Vision Research. 2003;43:285–298. doi: 10.1016/s0042-6989(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Terao M, Watanabe J, Yagi A, Nishida S. Smooth pursuit eye movements improve temporal resolution for color perception. PLoS ONE. 2010;5(6):e11214. doi: 10.1371/journal.pone.0011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier P, Erickson RG. Vestibular input to visual-tracking neurons in area MST of awake rhesus monkeys. Annals of the New York Academy of Sciences. 1992;656:960–963. doi: 10.1111/j.1749-6632.1992.tb25307.x. [DOI] [PubMed] [Google Scholar]
- Tong J, Aydin M, Bedell HE. Direction-of-motion discrimination is facilitated by visible motion smear. Perception & Psychophysics. 2007a;69:48–55. doi: 10.3758/bf03194452. [DOI] [PubMed] [Google Scholar]
- Tong J, Aydin M, Bedell HE. Direction and extent of perceived motion smear during pursuit eye movement. Vision Research. 2007b;47:1011–1019. doi: 10.1016/j.visres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Tong J, Patel SS, Bedell HE. Asymmetry of perceived motion smear during head and eye movements: evidence for a dichotomous neural categorization of retinal image motion. Vision Research. 2005;45:1519–1524. doi: 10.1016/j.visres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Tong J, Patel SS, Bedell HE. The attenuation of perceived motion smear during combined eye and head movements. Vision Research. 2006;46:4387–4397. doi: 10.1016/j.visres.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Ramamurthy M, Patel SS, Vu-Yu LP, Bedell HE. The temporal impulse response function during smooth pursuit. Vision Research. 2009;49:2835–2842. doi: 10.1016/j.visres.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Stevenson SB, Bedell HE. Signals of eye-muscle proprioception modulate perceived motion smear. Journal of Vision. 2008;8(14):7, 1–6. doi: 10.1167/8.14.7. [DOI] [PubMed] [Google Scholar]
- Turano KA, Heidenreich SM. Eye movements affect the perceived speed of visual motion. Vision Research. 1999;39:1177–1187. doi: 10.1016/s0042-6989(98)00174-6. [DOI] [PubMed] [Google Scholar]
- Turano KA, Masoff RW. Nonlinear contribution of eye velocity in motion perception. Vision Research. 2001;41:385–395. doi: 10.1016/s0042-6989(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Uchikawa K, Sato M. Saccadic suppression of achromatic and chromatic responses measured by increment-threshold spectral sensitivity. Journal of the Optical Society of America. 1995;12:661–666. doi: 10.1364/josaa.12.000661. [DOI] [PubMed] [Google Scholar]
- Volkmann FC, Riggs LA, White KD, Moore RK. Contrast sensitivity during saccadic eye movements. Vision Research. 1978;18:1193–1199. doi: 10.1016/0042-6989(78)90104-9. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Experimental Brain Research. 1977;27:523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. The velocity response of vestibular nucleus neurons during vestibular, visual, and combined angular acceleration. Experimental Brain Research. 1979;37:337–347. doi: 10.1007/BF00237718. [DOI] [PubMed] [Google Scholar]
- Wall MB, Smith AT. The representation of egomotion in the human brain. Current Biology. 2008;18:191–194. doi: 10.1016/j.cub.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SN. Visible persistence is reduced by fixed trajectory motion but not by random motion. Perception. 1992;21:791–802. doi: 10.1068/p210791. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Nishida S. Veridical perception of moving colors by trajectory integration of input signals. Journal of Vision. 2007;7(11):3, 1–16. doi: 10.1167/7.11.3. [DOI] [PubMed] [Google Scholar]
- Watson AB, Nachmias J. Patterns of temporal integration in the detection of gratings. Vision Research. 1977;17:893–902. doi: 10.1016/0042-6989(77)90063-3. [DOI] [PubMed] [Google Scholar]
- Watt RJ, Morgan MJ. The recognition and representation of edge blur: evidence for spatial primitives in human vision. Vision Research. 1983;23:1465–1477. doi: 10.1016/0042-6989(83)90158-x. [DOI] [PubMed] [Google Scholar]
- Wertheim AH, van Gelder P. An acceleration illusion caused by underestimation of stimulus velocity during pursuit eye movements: Aubert-Fleischl revisited. Perception. 1990;19:471–482. doi: 10.1068/p190471. [DOI] [PubMed] [Google Scholar]
- Wikipedia contributors, Image stabilization. [retrieved 28 April 2010];Wikipedia, The free encyclopedia. http://en.wikipedia.org/w/index.php?title=Image_stabilization&oldid=357692933.
- Zhang Z-L, Cantor CRL, Schor CM. Effects of luminance and saccadic suppression on perisaccadic spatial distortions. Journal of Vision. 2008;8(14):22, 1, 8. doi: 10.1167/8.14.22. [DOI] [PubMed] [Google Scholar]