Abstract
The severe side effects of glucocorticoids prevent long term management of hearing loss. Alternative steroid treatments that minimize or eliminate these effects would significantly benefit therapeutic control of hearing disorders. A steroid treatment study of mouse autoimmune hearing loss was conducted to determine the efficacy of combining aldosterone and prednisolone at low doses. An assessment also was made of low dose fludrocortisone, a synthetic mineralocorticoid that also has a slight glucocorticoid effect. MRL/MpJ-Faslpr mice were tested for baseline ABR thresholds at 3 months of age and then treated with aldosterone (3.0 μg/kg) or prednisolone (1.0 mg/kg) to determine the lowest effective dose of each. Other mice were given the two steroids in combination at doses of Pred 0.5 mg + Aldo 1.5 μg; Pred 1.0 mg + Aldo 3.0 μg; or Pred 1.5 mg + Aldo 5.0 μg. Mice were retested with ABR at one and two months to determine the efficacy of the different steroid treatments in controlling hearing loss. Another series of mice were given the synthetic mineralocorticoid fludrocortisone at low (2.8 μg/kg) or high (10 μg/kg) doses and retested at monthly intervals for three months. Autoimmune hearing loss developed in untreated controls. This threshold elevation was not prevented by prednisolone at 1 mg/kg or by aldosterone at 3 μg/kg when each was given alone. However, the two steroids combined at these doses effectively controlled hearing loss. The fludrocortisone treatments also were effective at low doses in preventing or reversing the autoimmune mouse hearing loss. This efficacy of combined steroids at low doses suggests the potential for reducing the side effects of glucocorticoids in the therapeutic control of hearing disorders.
Keywords: Autoimmune hearing loss, Inner ear, MRL/MpJ-Faslpr autoimmune mice, Prednisolone, Aldosterone, Fludrocortisone, Adrenocorticosteroids
1. Introduction
Because hearing loss is often suspected to result from immune-mediated or inflammatory processes, glucocorticoids (prednisone, prednisolone, dexamethasone) are frequently used to treat such hearing disorders as sudden or rapidly progressive hearing loss, Meniere's disease, and autoimmune inner ear disease. However, in spite of the glucocorticoids' effectiveness, their severe side effects prevent long term management of such inner ear dysfunction. Alternative steroid treatments that minimize or eliminate these side effects would have significant benefit in the therapeutic control of hearing disorders.
This laboratory has been studying the steroid control of hearing loss in the MRL/MpJ-Faslpr autoimmune mouse model to better understand these steroid-responsive mechanisms in the ear. Autoimmune mouse hearing loss progresses with systemic disease development, pathology is limited to the stria vascularis, and treatment with the mineralocorticoid aldosterone is as effective as prednisolone in reversing or preventing hearing loss. The mineralocorticoid receptor-mediated function (ion homeostasis) of glucocorticoids was determined to be as important for hearing control as their glucocorticoid receptor-mediated functions (anti-inflammation and immunosuppression). The autoimmune mouse loses strial endothelial cell tight junction integrity due to immune complexes , which causes a drop in the endocochlear potential and hearing loss. Thus, restoration of strial ion homeostatic processes by mineralocorticoid receptor-mediated processes appears critical for hearing recovery, in spite of the initiating cause being systemic autoimmune disease. This parallels current hypotheses that some human immune-mediated hearing loss is due to circulating inflammatory factors (inflammatory cells, autoantibodies) that disrupt cochlear vasculature.
Given this relevance of the mineralocorticoid function of glucocorticoids for recovery of immune-mediate hearing loss, it is possible that both mineralocorticoid and glucocorticoid steroids could be employed in combination. If their effects are complementary or additive, combining them at lower doses may still provide therapeutic control of hearing loss while reducing glucocorticoid side effects. Therefore, the present study was conducted to determine if the mineralocorticoid aldosterone and the glucocorticoid prednisolone could be combined at low doses to effectively treat hearing loss in the autoimmune mouse model. If reduced levels of each are needed when combined, it would provide important preliminary information regarding optional treatments to better control the detrimental side effects during long term glucocorticoid therapy.
Fludrocortisone acetate (Florinef® acetate) is a synthetic mineralocorticoid given for chronic adrenocortical insufficiency (Addison disease) when low maintenance levels are needed over long periods of time. This drug also was tested to determine if it could suppress hearing loss in this mouse model. If effective, it would offer an alternative therapy in which the mineralocorticoid receptor mediated functions can be obtained with minimal glucocorticoid side effects.
2. Materials and methods
The drugs employed in the present study were the synthetic glucocorticoid prednisolone, the natural mineralocorticoid aldosterone, and the synthetic mineralocorticoid fludrocortisone. Their immune suppressive and sodium transport potencies relative to the natural glucocorticoid cortisol are shown in Table 1. Prednisolone has a predominantly glucocorticoid effect, but has a strong binding affinity for the mineralocorticoid receptor, thus able to mediate both receptor-mediated functions. Aldosterone is a mineralocorticoid with virtually no binding affinity for the glucocorticoid receptor for immune suppression. Fludrocortisone is a synthetic mineralocorticoid with a relatively less glucocorticoid effect, somewhat the opposite of prednisolone. All animal studies were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee.
Table 1.
1Potency Equivalence of Corticosteroids Relative to Natural Glucocorticoid Cortisol
| Corticosteroid | Daily Adrenal Production# |
Anti- inflammation Potency |
Sodium Retention Potency |
Therapeutic Dose (/day) |
Effective Dose Range (70 kg Person) |
|---|---|---|---|---|---|
| Cortisol | 10 mg | 1 | 1 | ||
| Prednisolone | 4 | 0.8 | 5 - 80 mg | 0.1 – 1.2 mg/kg | |
| Aldosterone | 125 μg | 0 | 3000 | 150 - 300 μg | 2.1 – 4.3 μg/kg |
| Fludrocortisone | 10 | 125 | 50 - 200 μg | 0.7 – 3.0 μg/kg |
Adrenals produce 0.15 mg/kg/day of cortisol and 1.8 μg/kg/day of aldosterone.
2.1. Prednisolone – aldosterone combinations
Previous studies of MRL/MpJ-Faslpr autoimmune mice have shown that hearing loss was controlled with a prednisolone dose of 5 mg/kg or an aldosterone dose of 5 μg/kg. These doses are at the approximate therapeutic levels given to patients (Table 1). However, the minimum dose of each that is capable of controlling the mouse hearing loss has not been determined. Furthermore, the efficacy of the two steroids in combination has never been explored. Therefore, a dose response study was employed to assess whether the two steroids in combination would be more effective than their equivalent doses alone.
2.2. Minimum effective dose
MRL/MpJ-Faslpr autoimmune mice normally develop systemic autoimmune disease and hearing loss at 3-4 months of age and approximately 25-30% die of disease by 5 months of age. Therefore, 3-month old mice were tested for baseline ABR thresholds at 4, 8, 16, and 32 kHz. Mice were then treated with steroids in their drinking water for two months according to our previous protocols. They were given either aldosterone (3 μg/kg, N = 8) or prednisolone (1.0 mg/kg; N=7) to determine if these doses were effective individually. Untreated mice (water only, N = 42) served as controls. Mice were retested after one and two months of treatment to determine any change in ABR thresholds.
2.3. Combination Doses
Following the individual steroid treatments, another series of mice was given the two steroids in various combination doses. Following baseline ABR testing, mice were given:
Prednisolone 0.5 mg/kg + Aldosterone 1.5 μg/kg (N=24)
Prednisolone 1.0 mg/kg + Aldosterone 3.0 μg/kg (N=22)
Prednisolone 1.5 mg/kg + Aldosterone 5.0 μg/kg (N=22)
The mice were retested after one and two months to determine the efficacy of each steroid combination treatment in preventing progression of hearing loss. An analysis of variance (ANOVA) was conducted of the threshold shift across all 4 test frequencies for each treatment group. Posthoc tests (Bonferroni) were conducted if treatments effects were significant (P < 0.05) at a particular frequency.
2.4. Fludrocortisone Acetate
Fludrocortisone acetate is a synthetic mineralocorticoid given for chronic adrenocortical insufficiency (Addison disease) when low maintenance levels are needed over long periods of time. It has a significant mineralocorticoid effect, but a smaller glucocorticoid effect (Table 1), making it functionally a combination drug with low dose glucocorticoid impact. The prescribed dose for humans is 0.1 - 0.2 mg per day, which equates to a dosage range of 1.5 – 3.0 μg/kg/day for a 70 kg person. Therefore, mice were treated for three months with either 3.0 μg/kg (N = 17) or 10.0 μg/kg (N = 42) to test the drug effectiveness at controlling progression of the hearing loss. Untreated mice (water, N = 46) served as controls. Hearing thresholds were tested at the end of each month to compare to baseline measures. Thresholds shifts among the groups were compared with ANOVA.
3. Results
3.1. Untreated Controls
Without any steroid treatment (water controls), hearing thresholds continue to rise as systemic disease progresses. One month after treatment began, untreated mice showed slight elevations at 8 and 32 kHz (Fig. 1). Thresholds at these frequencies continued to rise and at two months paired t-test of baseline and two month thresholds showed a significant elevation of thresholds at 4 and 32 kHz (P < 0.05). This pattern of predominantly high frequency threshold shift is typical for the autoimmune mice.
Figure 1.
Impact of various steroid treatments on progression of autoimmune hearing loss. Hearing was not protected in untreated mice (water), or in mice treated with prednisolone at 1 mg/kg or aldosterone at 3 μg/kg. However, when the two steroids at these doses (or higher) were combined, they effectively kept hearing thresholds similar to baseline. Aldo, aldosterone; Pred, prednisolone; statistics were paired t-tests of thresholds at baseline and 2 months: * = p < 0.05.
3.2. Prednisolone and aldosterone minimum effective doses
The objective of the lower doses selected in the present study was to find the dose of each steroid that was ineffective at controlling hearing loss. Neither aldosterone at 3 :g/kg nor prednisolone at 1.0 mg/kg prevented the progression of hearing loss (Fig. 1). Mice treated with each steroid at these doses showed continued elevation of thresholds at one and two months. By two months these thresholds were significantly elevated from baseline at all four frequencies for both steroids (paired t-test; p < 0.05), essentially no different from water controls. This identified the range of steroid concentrations to use in the combination studies below.
3.3. Prednisolone – aldosterone combinations
Hearing loss was not prevented by prednisolone at 1 mg/kg or by aldosterone at 3 μg/kg when each was given alone (Fig. 1). Therefore, combinations of these drugs at the same levels, at slightly higher doses, and at slightly lower doses were employed to determine if their interactions were effective. The two steroids in combination did effectively control hearing loss at the dose of Pred 1 + Aldo 3 (Fig. 1). This was in spite of their not being effective when given alone. Only a slight elevation at 32 kHz was observed (paired t-test, P < 0.05), but all other frequency thresholds were similar to baseline. Thus, the two steroids alone at these doses did not control hearing loss, but did when combined.
The lower combination dose (Pred 0.5 + Aldo 1.5) was not effective in preventing further decline of hearing (Fig. 1) and thresholds were higher at all frequencies, similar to untreated mice (P < 0.05). The higher combination dose of Pred 1.5 + Aldo 5 (Table 1) was effective in preventing hearing loss and showed a threshold pattern that was identical to Pred 1 + Aldo 3 (Fig. 1), slight elevation at 32 kHz only. Thus, if the two steroids are combined, prednisone can be lowered to 1 mg/kg and aldosterone to 3 :g/kg, while each alone is not effective for preserving hearing thresholds.
3.4. ANOVA
The results above imply that some treatments were effective in preventing hearing loss progression with systemic autoimmune disease. To confirm this, it was necessary to compare treatment effects across all groups with ANOVA. However, because the onset, degree, and progression of systemic autoimmune disease is variable, mice may have slightly different thresholds at the initiation of treatment. Therefore, an ANOVA was conducted of the change in threshold over the two month treatment period. This permitted incorporation of all treatments and mice together for total variance in a more conservative statistical evaluation of treatment effects. Subsequent posthoc analyses would then provide a statistical basis for determining what treatments groups were different from untreated water controls at one month of treatment and at two months of treatment.
An ANOVA comparison was first made of baseline thresholds at the beginning of treatments to determine if there were significant differences at the frequencies tested (Table 2). These results show the baseline thresholds were not statistically different at any of the 4 frequencies (P < 0.05). However, the starting thresholds at 4 kHz were close to significance (P = 0.055), suggesting considerable variability at this frequency. Therefore, to eliminate the potential for threshold variability to be a factor, the group comparisons were made of change in threshold over the treatment periods rather than absolute thresholds.
Table 2.
Oneway ANOVA of Group Treatment × Time Effects
| Frequency | Baseline Threshold | Baseline to 1 Month | Baseline to 2 Months | |||
|---|---|---|---|---|---|---|
| F ratio | Probability | F ratio | Probability | F ratio | Probability | |
| 4 | 2.21 | 0.055 | 2.51 | 0.031 | 5.83 | <0.001 |
| 8 | 1.45 | 0.206 | 1.69 | 0.138 | 6.06 | <0.001 |
| 16 | 1.42 | 0.218 | 2.12 | 0.064 | 4.50 | 0.001 |
| 32 | 1.46 | 0.205 | 4.42 | 0.001 | 7.07 | <0.001 |
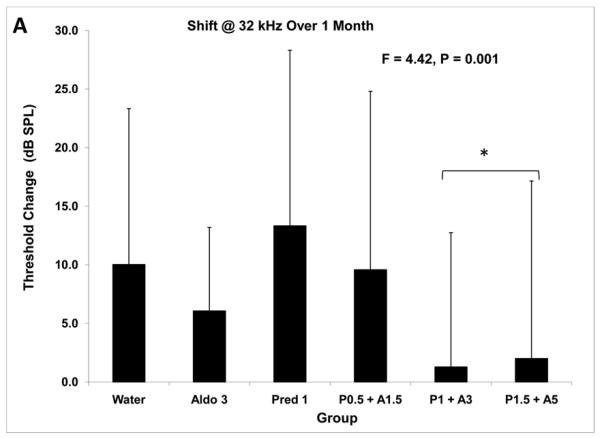
The ANOVA results of group comparisons showed that there were significant differences at both one month and two months of treatment (Table 2). Groups differed in the change in thresholds at one month at both 4 kHz (F = 2.5; P = 0.031) and 32 kHz (F = 4.4; P = 0.001), suggesting significant treatment effects. At two months, there were significant treatment effects at all four frequencies (Table 2; P < 0.001). Posthoc tests to determine differences among the treatment groups showed consistently that the Pred 1 + Aldo 3 and the Pred 1.5 + Aldo 5 groups were similar to each other and different from the others. Because 32 kHz is the frequency showing the greatest impact of systemic autoimmune disease, it also would be the frequency most likely to show an impact of treatments if there were any. Posthoc results at this frequency showed these two combination treatments were different from the other treatment groups at both one month and two months (Fig. 2A, B). At one month the change in threshold in these treatments was 2 dB or less, while at two months they were 13.7 dB or less. In both cases these shifts were significantly less than the other groups. These results further demonstrate that the Pred 1 + Aldo 3 combination was effective while individually the same doses had no effect.
Figure 2.
Threshold shift at 32 kHz with steroid treatments. 2A: After one month of treatment, ANOVA showed an overall group difference with regard to change from baseline. Posthoc tests demonstrated the two higher steroid combinations changed little and this change was different from that of the other treatment groups. 2B: Threshold shifts after 2 months of treatment showed a similar pattern. The two higher combination doses showed shifts that were significantly less than the other treatment groups. * = p < 0.05.
3.5. Fludrocortisone acetate
Results of the fludrocortisone treatments suggested the drug had a positive effect on the progression of hearing loss in the autoimmune mice. ANOVA of the change in thresholds by frequency showed there was a treatment effect at both 1 and 2 months (Table 3). Post-hoc analysis showed the higher dose was more effective than the lower dose in most cases. The change in thresholds at 3 months did not show a statistical group effect at any frequency. These data reflect a considerable amount of variability since all animals alive at each time point are incorporated in the analysis, but some alive at 2 months may not be alive for the 3 month assessment. Also, there was considerable variation in age of treatment onset, some starting at approximately 3 months of age (prior to disease), while others were started at 6 months of age (disease well established). Thus, the younger mice would be assessing fludrocortisone's impact on hearing loss prevention, while the older mice would be a determination of reversing hearing loss once it was established.
Table 3.
Oneway ANOVA of Fludrocortisone Treatment × Time Effects
| Frequency | Baseline to 1 Month | Baseline to 2 Months | Baseline to 3 Months | |||
|---|---|---|---|---|---|---|
| F ratio | Probability | F ratio | Probability | F ratio | Probability | |
| 4 | 2.62 | 0.075 | 2.82 | 0.063 | 2.23 | 0.115 |
| 8 | 3.4 | 0.035 | 6.16 | 0.003 | 0.79 | 0.459 |
| 16 | 1.44 | 0.239 | 0.98 | 0.377 | 0.6 | 0.554 |
| 32 | 3.77 | 0.025 | 1.58 | 0.209 | 2.02 | 0.14 |
Therefore, to better differentiate the impact of treatment timing, dose, and group differences, the mean treatment group change in threshold across all frequencies was compared in the three groups. Also, the groups were split further into age of treatment onset, those started on fludrocortisones at <100 days of age (prevention) versus >100 days (reversal or slowing further progression). ANOVA of these changes showed that the impact of fludrocortisone differed among groups depending on the age at which they began treatment (Fig. 3). Those mice younger than 100 days of age at treatment onset showed little effect of the drugs until 3 months of treatment (P < 0.05). At that time, the higher dose fludrocortisone was more efficacious in preventing hearing loss. On the other hand, those mice started at ages greater than 100 days showed an immediate slowing of hearing loss at all time assessments (Fig. 3). Group differences were significant at one month (P < 0.05), two months (P < 0.05), and three months (P < 0.001). While both fludrocortisone doses were similarly effective at one month, the higher dose appeared less effective as time went on. The lower dose fludrocortisone maintained hearing quite well, leading to a shift of less than 5 dB per frequency after three months of treatment. Thus, the fludrocortisone treatments were effective, with the lower dose having the greatest effect on preventing further hearing loss.
Figure 3.
ANOVA of threshold change from baseline for mice treated with fludrocortisone at 3 μg/kg and 10 μg/kg. The impact of fludrocortisone differed depending on the age at which the groups began treatment. Mice started on the drug when younger than 100 days of age showed little effect of the drug until 3 months of treatment. At that time, the higher dose fludrocortisone was more effective in preventing hearing loss. On the other hand, those mice started at ages greater than 100 days showed an immediate slowing of hearing loss at all time assessments.
4. DISCUSSION
These studies indicate that mouse autoimmune hearing loss in mice can be effectively controlled by mineralocorticoids and glucocorticoids in combination at lower doses than what is required for either steroid alone. Although the basis for this is undetermined, it is presumably the additive effects of the mineralocorticoid aldosterone to the mineralocorticoid impact of prednisolone. Previous studies have shown aldosterone alone will protect hearing in these mice and the glucocorticoids are effective because of their binding to the mineralocorticoid receptor (Trune and Kempton, 2009). Thus, the additional aldosterone, combined with a level of prednisolone that by itself was not enough (1.0 mg), may have sufficiently increased mineralocorticoid receptor functions sufficiently to preserve normal cochlear ion transport functions. The significance of this finding is that it allowed the glucocorticoid prednisolone to be given at 1 mg/kg instead of 5 mg/kg. This 80% reduction in the glucocorticoid dose required represents a major pharmacokinetic shift and raises the possibility for lowered doses and fewer side effects in the management of hearing loss.
The major inner ear pathology in the autoimmune mouse is breakdown of the stria vascularis , due presumably to the immunoglobulin and/or immune complexes sequestered there during systemic disease. This places at risk the stria function of Na+ and K+ transport and maintenance of their respective endolymph balances that are critical for hearing. The stria vascularis and its endocochlear potential are capable of regeneration following insult. Thus, spontaneous or steroid-induced recovery of sensorineural hearing loss may involve such restoration of strial function. Glucocorticoids, in addition to their role in immune suppression, have a significant mineralocorticoid receptor-mediated function that includes Na+ and K+ transport. Others also have shown these glucocorticoid-mediated ion transport functions are important in the ear. Recent studies also suggest that the glucocorticoid receptor itself may mediate epithelial Na+ channel (ENaC) production. This distinction between glucocorticoid receptor- and mineralocorticoid receptor-driven processes is blurring further with the recent identification of serum- and glucocorticoid-regulated kinase-1, which controls the abundance of ENaC in the cell membrane. Both glucocorticoids and mineralocorticoids upregulate this kinase, which in turn controls production of numerous ion channels that occur in the inner ear. Thus, either steroid group may have the same ultimate effect of restoring cochlear ion homeostasis processes to improve hearing. Therefore, traditional glucocorticoid (dexamethasone, prednisone) treatment for numerous inner ear diseases may restore hearing through both immune suppression and enhanced ion transport.
Efficacy also was demonstrated for fludrocortisone, which is a very low dose mineralocorticoid with minimal glucocorticoid effects. Fludrocortisone (9α–fluorohydrocortisone, 9α–fluorocortisol) has not previously been demonstrated as a therapy for hearing loss. Its predominant binding for the mineralocorticoid receptor makes it a synthetic form of aldosterone. However, it does have a minor binding affinity for the glucocorticoid receptor, albeit at a very low dose. Several decades ago it was reported that adrenocortical insufficiency patients suffering from hearing loss were improved by several different adrenocortical steroids. Although no actual audiometric data were provided for fludrocortisone, it was claimed by the authors that fludrocortisone combined with glucocorticoids effectively restored thresholds in this patient group. The present study is the first to experimentally test the effectiveness of fludrocortisone for these presumed ion transport deficiencies in the inner ear as an alternative to aldosterone. Currently mineralocorticoids are not routinely employed in the treatment of hearing loss, although it has been reported that low levels of aldosterone are correlated with presbycusis.
Currently many forms of hearing loss are treated with glucocorticoids, even though often there is no suspected immunologic basis. As our understanding of the cellular bases for these clinical disorders improves, we may determine they have disrupted ion transport processes. If this is the case, their response to glucocorticoid treatment may be due to its ion transport functions and mineralocorticoid treatment may be equally effective. On the other hand, combination steroid therapy may benefit patients requiring immune suppression by glucocorticoids and enhanced cochlear ion transport by both glucocorticoids and mineralocorticoids. This may be most applicable to known hearing disorders of ion transport dysfunction (e.g., Meniere's disease) and potentially involved in others (sudden hearing loss). Furthermore, the potential use of glucocorticoids at lower doses raises an exciting potential for long term management of some forms of steroid-responsive hearing loss without the morbid side effects.
Acknowledgments
Research supported by NIH-NIDCD R01 DC05593 and NIDCD P30 DC005983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexiou C, Arnold W, Fauser C, Schratzenstaller B, Gloddek B, Fuhrmann S, Lamm K. Sudden sensorineural hearing loss: does application of glucocorticoids make sense? Arch Otolaryngol Head Neck Surg. 2001;127:253–258. doi: 10.1001/archotol.127.3.253. [DOI] [PubMed] [Google Scholar]
- Aoki D, Takegoshi H, Kikuchi S. Evaluation of super-high-dose steroid therapy for sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2006;134:783–787. doi: 10.1016/j.otohns.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Barna BP, Hughes GB. Autoimmune inner ear disease--a real entity? Clin Lab Med. 1997;17:581–594. [PubMed] [Google Scholar]
- Barrs DM. Intratympanic corticosteroids for Meniere's disease and vertigo. Otolaryngol Clin North Am. 2004;37:955–972, v. doi: 10.1016/j.otc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Couloigner V, Sterkers O, Ferrary E. What's new in ion transports in the cochlea? Pflugers Arch. 2006;453:11–22. doi: 10.1007/s00424-006-0103-4. [DOI] [PubMed] [Google Scholar]
- Dornhoffer JL, Arenberg JG, Arenberg IK, Shambaugh GE., Jr. Pathophysiological mechanisms in immune inner ear disease. Acta Otolaryngol Suppl. 1997;526:30–36. doi: 10.3109/00016489709124018. [DOI] [PubMed] [Google Scholar]
- Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu Rev Med. 1997;48:231–240. doi: 10.1146/annurev.med.48.1.231. [DOI] [PubMed] [Google Scholar]
- Garcia-Berrocal JR, Ramirez-Camacho R. Sudden sensorineural hearing loss: supporting the immunologic theory. Ann Otol Rhinol Laryngol. 2002;111:989–997. doi: 10.1177/000348940211101107. [DOI] [PubMed] [Google Scholar]
- Harris JP, Ryan AF. Fundamental immune mechanisms of the brain and inner ear. Otolaryngol Head Neck Surg. 1995;112:639–653. doi: 10.1016/s0194-5998(95)70170-2. [DOI] [PubMed] [Google Scholar]
- Hellier WP, Wagstaff SA, O'Leary SJ, Shepherd RK. Functional and morphological response of the stria vascularis following a sensorineural hearing loss. Hear Res. 2002;172:127–136. doi: 10.1016/s0378-5955(02)00553-1. [DOI] [PubMed] [Google Scholar]
- Henkin RI, Daly RL. Auditory detection and perception in normal man and in patients with adrenal cortical insufficiency: effect of adrenal cortical steroids. J Clin Invest. 1968;47:1269–1280. doi: 10.1172/JCI105819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin RI, McGlone RE, Daly R, Bartter FC. Studies on auditory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids. J Clin Invest. 1967;46:429–435. doi: 10.1172/JCI105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am J Physiol Cell Physiol. 2009;296:C544–557. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Marcus DC. Endolymphatic sodium homeostasis by extramacular epithelium of the saccule. J Neurosci. 2009;29:15851–15858. doi: 10.1523/JNEUROSCI.3044-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Lang F, Vallon V, Knipper M, Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol. 2007;293:C1187–1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- Lee JH, Marcus DC. Nongenomic effects of corticosteroids on ion transport by stria vascularis. Audiol Neurootol. 2002;7:100–106. doi: 10.1159/000057657. [DOI] [PubMed] [Google Scholar]
- Lin DW, Trune DR. Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol Head Neck Surg. 1997;117:530–534. doi: 10.1016/S0194-59989770026-3. [DOI] [PubMed] [Google Scholar]
- Loveman DM, de Comarmond C, Cepero R, Baldwin DM. Autoimmune sensorineural hearing loss: clinical course and treatment outcome. Semin Arthritis Rheum. 2004;34:538–543. doi: 10.1016/j.semarthrit.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Mathews J, Kumar BN. Autoimmune sensorineural hearing loss. Clin Otolaryngol Allied Sci. 2003;28:479–488. doi: 10.1046/j.0307-7772.2003.00738.x. [DOI] [PubMed] [Google Scholar]
- Mitchell CR, Kempton JB, Creedon TA, Trune DR. The use of a 56-stimulus train for the rapid acquisition of auditory brainstem responses. Audiol Neurootol. 1999;4:80–87. doi: 10.1159/000013824. [DOI] [PubMed] [Google Scholar]
- Nadel DM. The use of systemic steroids in otolaryngology. Ear Nose Throat J. 1996;75:502–505. 509-510, 511-502 passim. [PubMed] [Google Scholar]
- Niparko JK, Wang NY, Rauch SD, Russell GB, Espeland MA, Pierce JJ, Bowditch S, Masuda A, Gulya AJ, Gantz BJ, Hughes GB, Brookhouser PE, Hannley MT, Telian SA, Harris JP. Serial audiometry in a clinical trial of AIED treatment. Otol Neurotol. 2005;26:908–917. doi: 10.1097/01.mao.0000185081.28598.5c. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol. 2004;286:F1127–1135. doi: 10.1152/ajprenal.00387.2003. [DOI] [PubMed] [Google Scholar]
- Rauch SD. Intratympanic steroids for sensorineural hearing loss. Otolaryngol Clin North Am. 2004;37:1061–1074. doi: 10.1016/j.otc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Fas mouse. Otolaryngol Head Neck Surg. 1999;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, van Steensel B, Spengler D, Soder M, Berning B, Holsboer F, Damm K. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol. 1993;247:145–154. doi: 10.1016/0922-4106(93)90072-h. [DOI] [PubMed] [Google Scholar]
- Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. New York. McGraw-Hill; 2006. pp. 1587–1612. [Google Scholar]
- Schuetz F, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K(+) channels KCNQ2/3 and KCNQ3/5 by serum- and glucocorticoid-regulated kinase-1. Am J Physiol Cell Physiol. 2008;295:C73–80. doi: 10.1152/ajpcell.00146.2008. [DOI] [PubMed] [Google Scholar]
- Sismanis A, Wise CM, Johnson GD. Methotrexate management of immune-mediated cochleovestibular disorders. Otolaryngol Head Neck Surg. 1997;116:146–152. doi: 10.1016/S0194-59989770316-4. [DOI] [PubMed] [Google Scholar]
- Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: a possible protective hormone against presbycusis. Hear Res. 2005;209:10–18. doi: 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Trune DR. Cochlear immunoglobulin in the C3H/lpr mouse model for autoimmune hearing loss. Otolaryngol Head Neck Surg. 1997;117:504–508. doi: 10.1016/s0194-5998(97)70022-6. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Fas(lpr) autoimmune mice. Hear Res. 2001;155:9–20. doi: 10.1016/s0378-5955(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB. Blocking the glucocorticoid receptor with RU-486 does not prevent glucocorticoid control of autoimmune mouse hearing loss. Audiol Neurootol. 2009;14:423–431. doi: 10.1159/000241899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Harrison AR, Wobig JL. Glucocorticoid impact on cochlear function and systemic side effects in autoimmune C3.MRL-Faslpr and normal C3H/HeJ mice. Hear Res. 2007;226:209–217. doi: 10.1016/j.heares.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Yehudai D, Shoenfeld Y, Toubi E. The autoimmune characteristics of progressive or sudden sensorineural hearing loss. Autoimmunity. 2006;39:153–158. doi: 10.1080/08916930500499599. [DOI] [PubMed] [Google Scholar]