Abstract
Aims
It is unclear what effect therapeutic hypothermia may have on renal function, because its effect has so far been primarily evaluated in settings in which there may be possible confounding perturbations in cardiovascular and renal physiology, such deep intra-operative hypothermia, general anesthesia, and post-cardiac arrest. We sought to determine if therapeutic hypothermia affects renal function in awake patients with normal renal function who were enrolled into a clinical trial of hypothermia plus intravenous thrombolysis for acute ischemic stroke.
Methods
Eleven patients with normal renal function were cooled to 33°C for 24 hours using an endovascular catheter, and then re-warmed over 12 hours to 36.5°C, while hourly temperature, blood pressure, and fluid status data was recorded. Blood samples for blood urea nitrogen (BUN), creatinine, and hematocrit were drawn prior to treatment (baseline), immediately after hypothermia and re-warming (day 2), and again at day 7 or discharge, and values compared.
Results
On initiation of cooling, temperatures dropped from a median pre-treatment value of 36.1°C (IQR: 35.8 – 36.4°C) to 33.1°C (IQR: 33.1 – 33.4°C). Urine output decreased 5.1 ml/hr for every 1°C decrease in body temperature (p-value = 0.001), with no associated serious adverse events. There were no statistically significant changes in BUN, creatinine, or hematocrit in the hypothermia patients.
Conclusion
Inducing hypothermia in patients with relatively unperturbed renal physiology results in a decrease in urine output that is linearly correlated with the decrease in core temperature. This has important implications for fluid management in patients undergoing therapeutic hypothermia.
Index Medicus medical subject headings: Hypothermia, Induced; Body temperature; Renal Insufficiency, Acute; Kidney Function Tests; Fluid balance; Stroke, Acute
Introduction
After two prospective trials found that therapeutic hypothermia to a target temperature near 33°C significantly improved neurological outcome in comatose survivors of cardiac arrest 1,2, it was adopted into guidelines for treatment of these patients 3,4, and is being implemented with increasing frequency around the world5,6,7,8,9,10,11.
Hypothermia results in a variety of physiological side-effects, such as bradycardia, bronchorrhea, hemoconcentration, and hyperglycemia, and (at temperatures less than 30°C) EKG abnormalities, increased cardiac sensitivity predisposing to ventricular fibrillation, oliguria, and decreased mentation 12,13,14. The Intravascular Cooling for the Treatment of Stroke-Longer window (ICTuS-L) trial is a multi-center, prospective, randomized and controlled trial investigating therapeutic hypothermia to 33°C combined with thrombolysis in patients with acute ischemic stroke. We present here the results of an exploratory sub-study that was triggered by a bedside observation in study patients: hourly urine output appeared to decrease with hypothermia, and return back to normal with re-warming. Unfortunately, a clear understanding of the effect of hypothermia on urine output in humans is obscured by the fact that it has so far been primarily evaluated and described in patients with confounding perturbations in cardiovascular and renal physiology, such as patients exposed to unregulated environmental hypothermia, undergoing deep intraoperative hypothermia with or without circulatory arrest 15,16, under spinal or general anesthesia 17, or who are critically-ill after cardiac arrest 18. We therefore undertook a retrospective study of patients who had been enrolled into the ICTuS-L trial as of the date of the analysis in order to determine how induction of a moderate degree of therapeutic hypothermia affects renal physiology in awake patients with relatively normal renal function and undisturbed physiology.
METHODS AND MATERIALS
Study Design, Setting and Population
The overall ICTuS-L trial design has been published 19. The study was approved by the institutional review boards at each site, and informed consent was obtained from each research subject or an approved surrogate prior to enrollment. This sub-study was a retrospective analysis of recorded data from a subset of consecutively enrolled patients. At the time of data acquisition the ICTuS-L trial had been implemented at five urban university-affiliated institutions with active stroke teams, and the subset of patients in this study were those who had been enrolled from trial initiation (October 2003) to January 2006 (with the exception of one patient who did not have fluid data available). Inclusion criteria have been published previously 19, and include adults age 18 to 80 presenting with acute ischemic stroke within 6 hours of onset. Exclusion criteria, also published previously, include significant comorbidity, heart failure and renal insufficiency or dysfunction.
Hypothermia Protocol
The procedure used to induce and maintain hypothermia in the ICTuS-L study, described previously 19, utilized an endovascular cooling catheter placed in the inferior vena cava (IVC) to rapidly cool patients to a target core temperature of 33°C while shivering was controlled with meperidine and buspirone, with the use of an external warming blanket (kept at a constant setting) to suppress input from cutaneous thermoreceptors. After 24 hours of therapeutic hypothermia induction and maintenance, patients began a precisely controlled re-warm of 0.3°C per hour over about 12 hours, to a target-temperature of 36.5°C.
Blood was drawn from patients immediately prior to treatment with hypothermia (pre-treatment) and then again at 36 hours (post-treatment, day 2, after the patient had completed the re-warm), and at 7 days or discharge, whichever was earlier, and laboratory data entered prospectively into an online database.
Data collection and imputation
Per protocol, all included patients had baseline blood urea nitrogen (BUN), serum creatinine, and estimated glomerular filtration rates (GFRs) in the normal range. Hypothermia patients had foley urinary catheters placed, and the following were recorded on an hourly basis throughout the 36-hour hypothermia and re-warm period: 1) core temperature, 2) urine output and, because exogenous fluid administration can affect urine output, 3) net fluid - the intravenous fluid (IVF) volume minus the urine output. The IVF volume recorded was inclusive of all IV fluids administered, including intravenously administered medications and minimal “to-keep-open” (TKO) drips. In addition, a systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded for each hour by protocol.
Urine output was recorded from the beginning of hypothermia induction (just as cooling was initiated, when patients were still normothermic) through to the end of the re-warming period (when patients had been brought back up to a core temperature of 36.5°C). On occasion, urine output was not recorded, usually because the patient was undergoing a procedure in the ICU. In order to ensure an optimally relevant hour-by-hour comparison of core temperature and urine output, any missing urine output for an hour was interpolated by dividing the volume noted in the urinary collection bag at the next recorded hour by the number of intervening hours since it was last recorded. Time-points for which missing data could not be easily interpolated were excluded, as were any time-points during and immediately after which any of the following specific extraneous factors capable of affecting renal blood flow or function were present: 1) the use of pressors or diuretics, 2) episodes of hypotension or hemodynamic instability.
BUN (mg/dl), serum creatinine (mg/dl), and blood hematocrit (%) were collected for each of the patients before treatment, after treatment (day 2), and - when available - at 7 days post-treatment (or at hospital discharge, whichever was earlier). Hematocrits were evaluated with the assumption that an acute change (hemodilution or hemoconcentration) would be a surrogate for an acute change in fluid status not reflected by a change in BUN or creatinine. In 13 northothermic control patients, the same laboratory data was recorded, but urine output and temperature data was not available with hourly frequency, since the protocol did not mandate ICU admission for these patients.
Statistical analyses
A random effects regression model (with each patient included as a random effect) was used to assess associations between A) temperature and urine output and B) temperature and net fluid during hypothermia and the re-warm. Random intercept and random slope models were fit and the best model was chosen using the likelihood ratio goodness of fit test. As a sensitivity analysis, a nonparametric model was also fit to pooled patient data using re-sampling (bootstrap) techniques with 1000 replicates. The 95% confidence intervals for the coefficient of the linear or quadratic temperature effect were calculated using an accelerated bias-corrected (BCa) method 20.
In all models, temperature was centered by the lowest temperature of all patients such that the intercept terms in models used to assess for associations between temperature and urine output and temperature and net fluid could be interpreted easily. Laboratory values for BUN, serum creatinine (Cr), and blood hematocrit (Hct) are presented as medians with interquartile ranges, and the Wilcoxon signed-rank test was used to make pair-wise comparisons between the three time points studied, with a Hochberg adjustment applied to the p-values for the three multiple comparisons. Lab values at baseline, day 2 and day 7 were compared between hypothermia patients and normothermia patients using a Wilcoxon Rank-sum test.
All statistical analyses were performed using the software R 2.1.1 (www.R-project.org).
Results
Baseline demographics
Baseline demographics are outlined in Table 1. There were no differences in median age, cardiovascular risk factors or stroke severity between hypothermia and normothermia patients.
Table 1.
Baseline demographics of hypothermia and normothermia patients.
| Hypothermia | Normothermia | p-value | |
|---|---|---|---|
| Age | 74 (IQR:63.00–77.50) | 67 (IQR:49.00– 75.00) |
0.164 |
| Gender | > 0.9999 | ||
| Male | 6 (56%) | 6 (46%) | |
| Female | 5 (46%) | 7 (54%) | |
| Race/ethnicity | 0.149 | ||
| Caucasian | 11 (100%) | 9 (69%) | |
| Black | 0 (0%) | 3 (23%) | |
| Asian | 0 (0%) | 1 (8%) | |
|
Cardiovascular risk factors |
|||
| History of hypertension |
10 (77%) | 9 (82%) | > 0.9999 |
| History of diabetes |
1 (9%) | 4 (31%) | 0.327 |
| History of cerebrovascular disease |
2 (18%) | 2 (15%) | > 0.9999 |
| Smoking | 6 (55%) | 8 (62%) | > 0.9999 |
|
Baseline neurological deficit |
|||
| Baseline NIHSS | 14.0 (IQR: 10.0–14.0 16.0) |
16.0 (IQR: 12.5– 19.5) |
0.45 |
|
Pre-stroke debility |
0.458 | ||
| mRS 0– 2 (mild) | 10 (91%) | 13 (100%) | |
| mRS 3–6 (more severe) |
1 (9%) | 0 (0%) |
Data is presented as medians with interquartile ranges (IQR) or as absolute numbers with percentages (n(%)). NIHSS = National Institutes of Health Stroke Scale score, a measure of stroke severity on a scale from 0 (no deficit) to 42 (severe deficit). mRS = modified Rankin Score, a measure of baseline pre-stroke debility on a scale from 0 (no symptoms) to 6 (severe debility).
Temperatures
Eleven patients had undergone the therapeutic hypothermia protocol as of the implementation of this retrospective sub-study. On initiation of cooling, their temperatures dropped from a median pre-treatment temperature of 36.1°C (IQ range: 35.8 – 36.4°C), to a median plateau value (defined previously 19) of 33.5°C (IQ range: 33.2 – 33.9°C) in a median time of 2 hours (IQ range: 1–2 hours), with a median average post-plateau temperature during the cooling phase of 33.9°C (IQ range: 33.3 – 34.5°C). The median lowest temperature reached was 33.1°C (IQ range: 33.1 – 33.4°C). In the 13 normothermic patients, the median temperature during the first 24 hours post-enrollment was 36.6°C (IQ range: 36.2 – 37.6°C).
Urine output and fluid status
A total of 398 hours of data were available in the hypothermia group. Urine output was interpolated in the manner described in Methods for 33 of them. Sixteen of the 398 hourly time-points of data were excluded for the following reasons: six due to diuretic use, eight consecutive time-points in one patient due to use of “renal-dose” dopamine, and two consecutive time-points in another patient due to hemodynamic instability which developed during the last two hours of the re-warm period (hours 35 and 36). An observational correlation between lower temperature and lower urine output was frequently seen in individual patients (Figure 1A). The urine output fell below 20 ml/hr in 93 of the 382 remaining hourly time-points, with complete cessation of urine output (0 ml/hr) in 21 of them (seen in four of the eleven hypothermia patients). Over the 24 hrs of hypothermia and 12 hours of controlled re-warming, hypothermia patients accrued a median net positive fluid balance (more IV fluid in than urine out) of 3.7 liters (IQR: 3.1 – 4.8 L). All had resolution of oliguria by the end of the re-warm period (hour 36), except for one, who recovered normal urine output after that time point. The statistical analysis revealed a significant linear association between urine output and core temperature (coefficient=5.095, p-value = 0.0012) in which the urine output decreased 5.10 ml/hr for every 1°C decrease in body temperature (Figure 2). There were no serious adverse events directly attributable to this. While in some individual patients there also appeared to be a gross correlation between temperature and net fluid status, with lower temperatures being associated with a tendency to be in a positive fluid balance (Figure 1B), a statistically significant relationship was not detected for the group as a whole.
Figure 1.
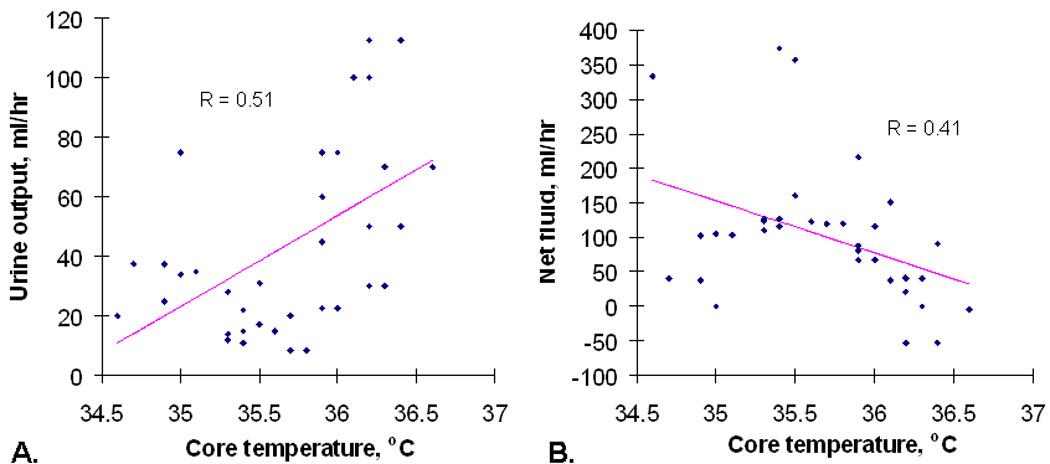
A, Hourly urine output as correlated with core temperature (with a regression line) in one of the hypothermia patients. B, Hourly net fluid status (the intravenous fluid volume minus the urine output) as correlated with core temperature for the same patient.
Figure 2.
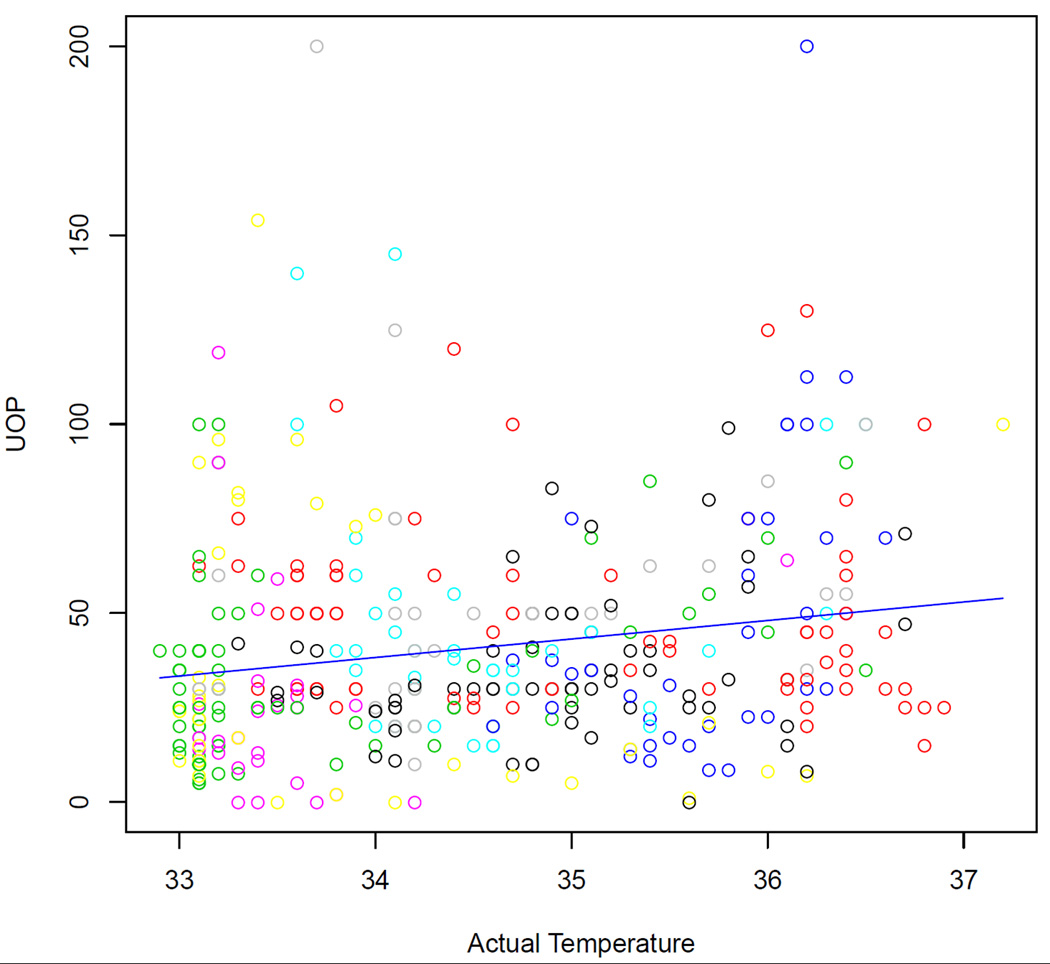
Hourly urine output (UOP) as correlated with centralized temperature (defined as the patient's temperature minus the lowest temperature of all patients in the study) for the entire group of hypothermia patients.
Blood pressures
Two of the 398 hourly time-points of data were excluded due to hemodynamic instability (in a single patient). The median of all the remaining hourly systolic blood pressures (SBPs) in the hypothermia patients was 139 mmHg (interquartile range (IQR): 125 – 155). The median hourly diastolic blood pressure (DBP) was 73 mmHg (IQR: 64 −83). The median mean arterial pressure (MAP), calculated with the formula MAP= DBP + 1/3(SBP – DBP) at each time-point, was 94 mmHg (IQR: 86 – 107). The lowest SBP seen at any time-point in any patient during the active hypothermia treatment phase (hours 1 through 24) was 97 mmHg, and the lowest MAP was 65 mmHg.
Blood urea nitrogen, serum creatinine and hematocrit
The median BUNs, creatinines, and hematocrits in hypothermic and normothermic patients at each time-point collected are presented in Table 2, with trends and group comparisons presented there and in Figures 3 – 5. There were no statistically significant differences between time-points in the hypothermia group. In addition, there were no statistically significant differences in BUN/Cr ratios between the time-points in the hypothermia group, with median (and interquartile ranges, or IQR) values of 15.83 (IQR: 14.49 – 20.42), 17.65 (IQR: 13.45 – 25.86), and 16.67 (IQR: 10.00 – 18.38) at pre-treatment, post-treatment (day 2), and day 7 respectively. In normothermic group, on the other hand, the median post-treatment (day 2) BUN and creatinine were lower than those at pre-treatment (p = 0.025 and p = 0.0049 for BUN and creatinine, respectively) and those in the hypothermia group at day 2 (p= 0.032 and p = 0.006, respectively, Figure 3).
Table 2.
The median values (with interquartile ranges) of blood urea nitrogen (BUN), creatinine (Cr) and hematocrit (Hct) in hypothermic and normothermic patients at each time point.
| Hypothermia | Normothermia | |
|---|---|---|
| BUN (mg/dl) | ||
| Baseline | 19 (IQR: 15 – 25) | 19 (IQR: 16.0 – 20.0) |
| Day 2 | 23 (IQR: 16.5 – 28) | 14 (IQR: 8.0 – 17.0) ** †† |
| Day 7 | 15 (IQR: 9.0 – 16.5) | 14 (IQR: 12.0 – 17.0) |
| Cr (mg/dl) | ||
| Baseline | 1.1 (IQR: 0.95 – 1.2) | 1.0 (IQR: 0.9 – 1.3) |
| Day 2 | 1.1 (IQR: 1.05 –1.3) | 1.0 (IQR: 0.8 – 1.1) * † |
| Day 7 | 0.9 (IQR: 0.8 – 0.95) | 1.0 (IQR: 0.7 – 1.2) |
| Hct (%) | ||
| Baseline | 39.30 (IQR: 36.35 – 44.40) | 40.2 (IQR: 37.7 −41.8) |
| Day 2 | 38.80 (IQR: 33.40 – 41.30) | 38.4 (IQR: 32.5 – 40.0) †† |
| Day 7 | 38.45 (IQR: 37.23 – 40.65) | 35.4 (IQR: 31.3 – 40.5) |
statistically significant between groups with a p-value <0.05
(p-value <0.01);
statistically significant between time-points within the group, with a p-value <0.05
(p-value <0.01).
Figure 3.
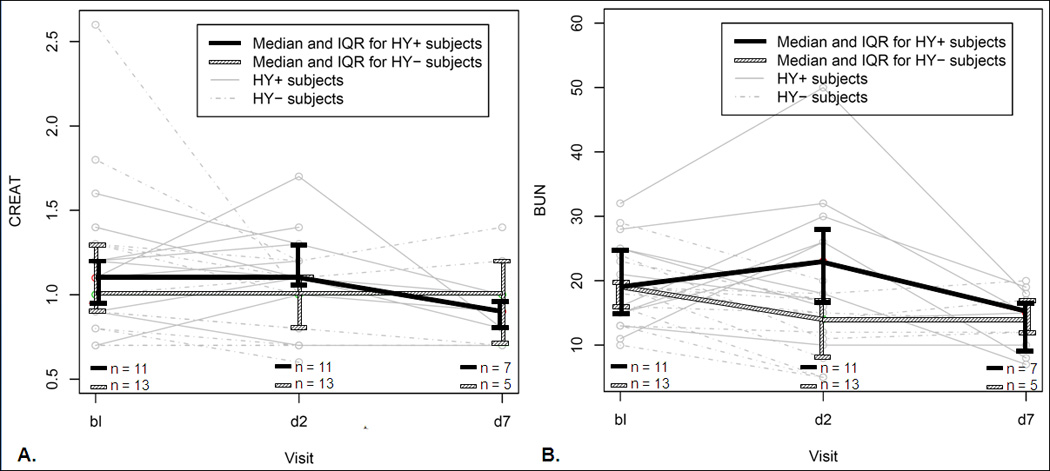
A) Comparison between normothermic control and hypothermia patients’ serum creatinine (CREAT), and B) serum blood urea nitrogen (BUN). Individual patients are shown in grey in the background (solid lines, hypothermia; dashed lines, normothermia controls). Each thick line represents a group (black, hypothermia; hatched, normothermia), with median and interquartile range values shown at each time-point. Bl, baseline; d2, day 2 (post-treatment, at completion of hypothermia and re-warm); d7, day 7.
Inspection of trends in individual patients revealed a decrease in hematocrit between baseline and day 2, a trend that almost reached statistical significance in hypothermia patients as a group (p=0.058) and was statistically significant in normothermic control patients as a group (p = 0.0051). While one of the hypothermia patients was eventually diagnosed with chronic anemia resulting from a gastric carcinoma, none of the patients had a documented episode of acute blood loss significant enough to explain the acute decrease in hematocrits.
Discussion
We found that moderate hypothermia in stroke patients with no other cardiovascular or physiological derangements was associated with a reduction in hourly urine output that correlated linearly with core temperature. There were no documented blood pressure perturbations that could have accounted for this. Dehydration is very unlikely to have been present or contributory because patients were given intravenous fluids, and ended up with net fluid balances that were several liters positive. While no patient appeared to suffer an adverse effect from the temporary oliguria, and there were no changes in BUN, creatinine and hematocrit that were specific to hypothermia, the oliguria seen was at times close to or at 0 ml/hr for consecutive hours.
The results of our study are unique because they highlight the effect of a moderate degree of therapeutic hypothermia on urine output in awake patients with no other physiological or hemodynamic disturbances that might affect the kidneys, the presence of which has previously confounded a clear assessment of its effect. In a prior study, hypothermia to around 30°C in endotracheally intubated, anesthestized patients significantly decreased glomerular filtration rate (GFR) and renal plasma flow, and slightly decreased urinary flow, but the anesthesia and spinal blocks used, in and of themselves, were shown to have similar though less marked effects 17. In another study, profound hypothermia to 20°C in patients undergoing cardiopulmonary bypass increased GFR and increased flow of hypo-osmolar urine 15, but general anesthesia used has itself been found to confounding effects on renal function and urine output 21,22. Cardiac arrest survivors treated with hypothermia to 32–34°C had a relative delay in the post-arrest return of normal creatinine clearance 18, but renal dysfunction is a common occurrence in survivors of cardiac arrest, and manifests as acute renal failure in up to a third of patients 23,24. Cold water immersion results in a diuresis of dilute urine 25,26, but immersion in water itself, regardless of temperature, induces humoral and hemodynamic changes that may increase urine output more significantly than the hypothermia itself 26,27,28.
The decrease in urine output we found with therapeutic hypothermia could have been specific to hypothermia-related interventions used in our protocol, such as the use of a warming blanket, the use of an endovascalur catheter, or administration of meperidine and buspirone. Diuresis from hypothermia may be due to a vasoconstriction of the peripheral vasculature that shifts blood into to the central circulation 17,28, and the warming blanket we used may have lessened the degree of re-distributive peripheral vasoconstriction that might have otherwise occurred with hypothermia. However, this would account for an abolition of a cold-induced diuresis, but not for the oliguria noted. The catheter sitting in the inferior vena cava may have directly imparted a local temperature effect on the adjacent retroperitoneal space and kidneys, independently affecting urine output. This is unlikely, however, because 1) the liters of vena caval blood flowing past the catheter every minute are likely to have quickly “washed out” any local temperature gradients and 2) temperature-sensing urinary catheters, used for confirmatory core temperature assessments as part of the protocol, revealed temperatures that were closely matched with those from the temperature probe built into the endovascular catheter (data not shown), and did not reveal a profound hypothermic effect in the retroperitoneal compartment. Finally, an oliguria side-effect from meperidine and busipirone is possible and cannot be definitively excluded; but this is unlikely, as oliguria is not a clearly documented side-effect of these medications.
While hypothermia was associated with oliguria in our study, it was not associated with a statistically significant increase in BUN or creatinine. The drop in BUN and creatinine seen in normothermic controls on day 2 may represent the possibility that a majority of our stroke patients were dehydrated (i.e., had relatively elevated BUNs and creatinines) when they presented, and that, with customary administration of maintenance intravenous fluids, this dehydration was reversed by day 2. Dehydration, which affects blood viscosity and coagulability, has been identified as a possible precipitant risk factor for acute ischemic stroke 29,30,31,32,33. The decrease in hematocrit noted between pre-treatment and day 2 may also be accounted for by dehydration; i.e., patients may have been hemoconcentrated when they presented, and were rehydrated and relatively hemodiluted by day 2.
The data reported above has to be taken in context of certain limitations of our study. This was a partially retrospective study with a small number of patients. We did not have serial urine output data from the normothermia control group to compare to, although there were no reports of clinically significant oliguria occurring in these patients that were brought to the attention of the investigators by nursing staff. The changes in hematocrit, BUN and creatinine we used are only surrogate measures of volume status, and we did not have confirmatory data from invasive cardiovascular monitoring, since there was typically no indication for this in our relatively stable patients. In addition, we did not collect laboratory data in the period between day 2 and day 7, and so cannot definitively say that there wasn’t a transient alteration in laboratory parameters of renal function that might have developed and resolved within that period. We did not find a correlation between core temperature and net fluid balance. This may be because fluid management was not specifically proscribed by protocol, and was implemented as per clinician discretion, with a typical response to decreased urine output observed at the bedside being the use of intermittent fluid boluses. Since these fluid boluses tended to produce large, intermittent, high-amplitude spikes in hourly net fluid, resulting in a significantly more prominent hourly variation in net fluid when compared to urine output (which tended to have a noticeably smoother, lower-amplitude hourly variation; data not shown), the detection of an effect may have been obscured.
Conclusion
Inducing hypothermia in patients with relatively unperturbed renal physiology and hemodynamic status results in a decrease in urine output that is linearly correlated with the decrease in core temperature. This has important implications for fluid management in patients undergoing therapeutic hypothermia. Further study is required to confirm this effect, but our data suggest that urine output and fluid status should be carefully monitored in these patients.
Acknowledgements
This work was funded with monetary support from a National Institutes of Health (NIH) grant, number NIH P50N5044148.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentation:
Early portions of this work were presented at the 32th International Stroke Conference, San Francisco, CA at a poster session, February 7, 2007, and at the 4th Mediterranean Emergency Medicine Congress (MEMC IV), Sorrento, Italy, in an oral session, September 19, 2007
Conflict of interest statement
No conflict of interest declared.
Contributor Information
Kama Z. Guluma, Department of Emergency Medicine, University of California, San Diego Medical Center, San Diego, California.
Lin Liu, Department of Biostatistics and Bioinformatics, University of California San Diego, San Diego, California.
Thomas M. Hemmen, Department of Neurosciences, University of California San Diego, San Diego, California.
Aninda B. Acharya, Department of Neurology, Souers Stroke Institute, Saint Louis University School of Medicine.
Karen S. Rapp, Department of Neurosciences, University of California San Diego, San Diego, California.
Rema Raman, Department of Biostatistics and Bioinformatics, University of California San Diego, San Diego, California.
Patrick D. Lyden, Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California.
REFERENCES
- 1.Hozler M for The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, Jones BM, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Morley PT, Hoek TL, Hickey RW. Advancement Life support Task Force of the International Liaison committee on Resuscitation. Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation. 2003;57(3):231–235. doi: 10.1016/s0300-9572(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 4.ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(24 Suppl) doi: 10.1161/CIRCULATIONAHA.105.166550. IV1-203. [DOI] [PubMed] [Google Scholar]
- 5.Laver SR, Padkin A, Atalla A, Nolan JP. Therapeutic hypothermia after cardiac arrest: a survey of practice in intensive care units in the United Kingdom. Anaesthesia. 2006;61(9):873–877. doi: 10.1111/j.1365-2044.2006.04552.x. [DOI] [PubMed] [Google Scholar]
- 6.Merchant RM, Soar J, Skrifvars MB, Silfvast T, Edelson DP, Ahmad F, Huang KN, Khan M, Vanden Hoek TL, Becker LB, Abella BS. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006 Jul;34(7):1935–1940. doi: 10.1097/01.CCM.0000220494.90290.92. [DOI] [PubMed] [Google Scholar]
- 7.Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34(7):1865–1873. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PubMed] [Google Scholar]
- 8.Arrich J. European Resuscitation Council Hypothermia After Cardiac Arrest Registry [Google Scholar]
- 9.Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand. 2007;51(2):137–142. doi: 10.1111/j.1399-6576.2006.01209.x. [DOI] [PubMed] [Google Scholar]
- 10.Oksanen T, Pettila V, Hynynen M, Varpula T Intensium Consortium study. Therapeutic hypothermia after cardiac arrest: implementation and outcome in Finnish intensive care units. Acta Anaesthesiol Scand. 2007;51(7):866–871. doi: 10.1111/j.1399-6576.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 11.Bouwes A, Kuiper MA, Hijdra A, Horn J. Induced hypothermia and determination of neurological outcome after CPR in ICUs in the Netherlands: Results of a survey. Resuscitation. 2010 Jan 30; doi: 10.1016/j.resuscitation.2009.12.032. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Granberg PO. Human physiology under cold exposure. Arct Med Res. 1991;50(S6):23–27. [PubMed] [Google Scholar]
- 13.Mallet ML. Pathophysiology of accidental hypothermia. QJM. 2002;95(12):775–785. doi: 10.1093/qjmed/95.12.775. [DOI] [PubMed] [Google Scholar]
- 14.Kempainen RR, Brunette DD. The evaluation and management of accidental hypothermia. Respir Care. 2004;49(2):192–205. [PubMed] [Google Scholar]
- 15.Gil-Rodriguez JA, O'Gorman P. Renal function during profound hypothermia. Br J Anaesth. 1970;42(6):557. [PubMed] [Google Scholar]
- 16.Boodhwani M, Rubens FD, Wozny D, Nathan HJ. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg. 2009;87(2):489–495. doi: 10.1016/j.athoracsur.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 17.Morales P, Carbery Y, Morello A, Morales G. Alterations in renal function during hypothermia in man. Ann Surg. 1957;145(4):488–499. doi: 10.1097/00000658-195704000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeiner A, Sunder-Plassmann G, Sterz F, Holzer M, Losert H, Laggner AN, Müllner M. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation. 2004;60(3):253–261. doi: 10.1016/j.resuscitation.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Guluma KZ, Hemmen TM, Olsen SE, MD, Rapp KS, Lyden PD. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Academic Emergency Medicine. 2006;13(8):820–827. doi: 10.1197/j.aem.2006.03.559. [DOI] [PubMed] [Google Scholar]
- 20.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 21.Halperin BD, Feeley TW. The effect of anesthesia and surgery on renal function. Int Anesthesiol Clin. 1984;22(1):157–168. doi: 10.1097/00004311-198405000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Sladen RN. Effect of anesthesia and surgery on renal function. Crit Care Clin. 1987;3(2):373–393. [PubMed] [Google Scholar]
- 23.Mattana J, Singhal PC. Prevalence and determinants of acute renal failure following cardiopulmonary resuscitation. Arch Intern Med. 1993;153:235–239. [PubMed] [Google Scholar]
- 24.Domanovits H, Schillinger M, Müllner M, Thoennissen J, Sterz F, Zeiner A, Druml W. Acute renal failure after successful cardiopulmonary resuscitation. Intensive Care Med. 2001;27(7):1194–1199. doi: 10.1007/s001340101002. [DOI] [PubMed] [Google Scholar]
- 25.Hoar PF, Raymond LW, Langworthy HC, Johnsonbuagh RE, Sode J. Physiological responses of men working in 25.5”C water, breathing air or helium tri-mix. J. Appl. Physiol. 1976;40:605–610. doi: 10.1152/jappl.1976.40.4.605. [DOI] [PubMed] [Google Scholar]
- 26.Knight DR, Horvath SM. Urinary responses to cold temperature during water immersion. Am J Physiol. 1985;248(5 Pt 2):R560–R566. doi: 10.1152/ajpregu.1985.248.5.R560. [DOI] [PubMed] [Google Scholar]
- 27.Epstein M. Renal effects of head-out water immersion in man: implications for an understanding of volume homeostasis. Physiol. Rev. 1978;58:529–581. doi: 10.1152/physrev.1978.58.3.529. [DOI] [PubMed] [Google Scholar]
- 28.Srámek P, Simecková M, Janský L, Savlíková J, Vybíral S. Human physiological responses to immersion into water of different temperatures. Eur J Appl Physiol. 2000;81(5):436–442. doi: 10.1007/s004210050065. [DOI] [PubMed] [Google Scholar]
- 29.Larsen SF, Zaric D, Boysen G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol Scand. 1988;32(8):698–701. doi: 10.1111/j.1399-6576.1988.tb02811.x. [DOI] [PubMed] [Google Scholar]
- 30.Yasaka M, Yamaguchi T, Miyashita T, Park YD, Sawada T, Omae T. Predisposing factors of recurrent embolization in cardiogenic cerebral embolism. Stroke. 1990;21(7):1000–1007. doi: 10.1161/01.str.21.7.1000. [DOI] [PubMed] [Google Scholar]
- 31.Niazi GA, Awada A, al Rajeh S, Larbi E. Hematological values and their assessment as risk factor in Saudi patients with stroke. Acta Neurol Scand. 1994;89(6):439–445. doi: 10.1111/j.1600-0404.1994.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto T, Kawaguchi M, Kurehara K, Kitaguchi K, Furuya H, Karasawa J. Postoperative neurological deterioration following the revascularization surgery in children with moyamoya disease. J Neurosurg Anesthesiol. 1998;10(1):37–41. doi: 10.1097/00008506-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Nadav L, Gur AY, Korczyn AD, Bornstein NM. Stroke in hospitalized patients: are there special risk factors? Cerebrovasc Dis. 2002;13(2):127–131. doi: 10.1159/000047762. [DOI] [PubMed] [Google Scholar]


