Abstract
Municipal biosolids are in widespread use as additives to agricultural soils in the United States. Although it is well known that digested sewage sludge is laden with organic wastewater contaminants, the fate and behavior of micropollutants in biosolids-amended agricultural soils remain unclear. An outdoor mesocosm study was conducted in Baltimore, Maryland, to explore the fate of 72 pharmaceuticals and personal care products (PPCPs) over the course of three years in biosolids/soil mixtures (1:2) that were placed in plastic containers made from polyvinylchloride and kept exposed to ambient outdoor conditions. Of the 72 PPCPs tested for using EPA Method 1694, 15 were initially detected in the soil/biosolids mixtures at concentrations ranging from low parts-per-billion to parts-per-million levels. The antimicrobials triclocarban and triclosan showed the highest initial concentrations at 2715 and 1265 μg kg−1, respectively. Compounds showing no discernable loss over three years of monitoring included diphenhydramine, fluoxetine, thiabendazole and triclocarban. The following half-life estimates were obtained for compounds showing first-order loss rates: azithromycin (408 – 990 d) carbamazepine (462 – 533 d), ciprofloxacin (1155 – 3466 d), doxycycline (533 – 578 d), 4-epitetracycline (630 d), gemfibrozil (224 – 231 d), norfloxacin (990 – 1386 d), tetracycline (578 d), and triclosan (182 – 193 d). Consistent with other outdoor degradation studies, chemical half-lives determined empirically exceeded those reported from laboratory studies or predicted from fate models. Study results suggest that PPCPs shown in the laboratory to be readily biotransformable can persist in soils for extended periods of time when applied in biosolids. This study provides the first experimental data on the persistence in biosolids-amended soils for ciprofloxacin, diphenhydramine, doxycycline, 4-epitetracycline, gemfibrozil, miconazole, norfloxacin, ofloxacin, and thiabendazole.
Keywords: Municipal sludge, land application, PPCPs, half-life, persistence, bioavailability
1. Introduction
Wastewater treatment facilities are responsible for treating large volumes of domestic and industrial sewage containing human waste. The treatment goal is to produce effluents of high enough quality for discharge back into the environment. Sewage sludge is a byproduct of this process and necessitates proper disposal. Biosolids, as defined by the U.S. Environmental Protection Agency (USEPA), are nutrient-rich organic residuals that when treated and processed, may be recycled and applied as fertilizer (USEPA, 2007a). Land application of biosolids combines inexpensive disposal of these abundant materials with the return of valuable nutrients back to the soil which may enhance soil properties and plant yield (USEPA, 2000).
The application of biosolids onto agricultural fields is a farming practice common in many countries such as the US, Canada, and within Europe (Angin and Yaganoglu, 2009; Carballa et al. 2009; Mantovi et al., 2005; Schut, 2008). A national survey on biosolids regulations, quality, end use, and disposal conducted in 2004 reported an annual U.S. production of approximately 6.5 million dry metric tons of sewage sludge of which approximately 49% was applied to soils (NEBRA, 2007). Three quarters of the total mass of land applied biosolids was used on farmlands for agricultural purposes (NEBRA, 2007). In a report on biosolids published in 2002 by the U.S. National Research Council, recommendations were made to the USEPA to investigate the presence of organic wastewater contaminants (OWCs) in biosolids (NRC, 2002).
Pharmaceutical and personal care products (PPCPs) have emerged in recent years as micropollutants in several environmental compartments (Daughton and Ternes, 1999) including, surface water (Hirsch et al., 1999; Kolpin et al., 2002), groundwater (Lindsey et al., 2001, Sacher et al., 2001), drinking water (Stackelberg et al., 2004; Webb et al. 2003), as well as agricultural soils (Hamscher et al., 2004; Kinney et al., 2008). The transformation of PPCPs during the wastewater treatment process varies with the physicochemical properties of the compounds and operating conditions.(Xia et al., 2005). During the different stages of wastewater treatment, the parent PPCPs, conjugates, and metabolites may be (i) completely transformed or mineralized (Richardson and Bowron, 1985), (ii) persistent, implying that a certain amount of the substance, depending on its lipophilicity or other binding possibilities (e.g., ionic bindings), will be retained in the sludge (Jørgensen and Halling-Sørensen, 2000), or (iii) persistent and polar, thus being released with the effluent to aquatic environments (Jørgensen and Halling-Sørensen, 2000).
The USEPA’s recently published Targeted National Sewage Sludge Survey (TNSSS) provides comprehensive data regarding PPCPs in U.S. biosolids collected between 2006 and 2007 (USEPA, 2009b). Uncertainties still surround the fate and potential effects of land applying municipal biosolids that are known to contain micropollutants.
Pharmaceuticals are specifically designed to alter both biochemical and physiological functions of biological systems in humans and animals (Daughton and Ternes 1999; Fent et al., 2006). These features of pharmaceuticals can however unintentionally affect soil and aquatic organisms should their habitats become contaminated with these chemicals (Fent et al., 2006). Acute toxicity tests have been conducted for some PPCPs where the results suggest that at the concentrations found in the environment, organisms are at a low risk for acute toxicity (Fent et al., 2006). What remains relatively unknown are the possible effects of long-term PPCP exposure which may ultimately result in chronic toxicity to soil and aquatic organisms (Chalew and Halden, 2009). Moreover, although side effects resulting from ingestion of multiple pharmaceuticals are well known in humans and animals, little is known about the fate of organisms exposed to similar drug mixtures. For agricultural soils receiving biosolids there is an additional concern that pharmaceutical contaminants may be taken up by food crops (Kumar et al., 2005; Dolliver et al., 2007).
From a human health perspective, potential concerns include physiological effects, elevated rates of cancer, reproductive impairment in humans and other animals, and the development and spread of antimicrobial resistance (Witte, 1998; Heuer et al., 2002; Schwartz et al., 2003; Kümmerer, 2004). With this knowledge or rather lack of knowledge regarding possible detrimental environmental effects, it is imperative to evaluate the presence and environmental fate of PPCPs in biosolids.
In this outdoor mesocosm study, we investigated the occurrence and fate of 72 PPCPs in agricultural soil over the course of three years after a single application of municipal biosolids.
2. Materials and Methods
2.1 Biosolids and Soil Types
Biosolids were obtained from a full-scale activated sludge treatment plant located in the mid-Atlantic region of the U.S. The raw wastewater entering the plant is comprised predominantly of domestic sources with only minor contributions from industry (1.9 %). The plant serves approximately 1.3 million people and has a sewershed of roughly 363 km2. The plant is designed to treat approximately 680 million liters per day. Sludges generated during primary and secondary treatment are blended and thickened with gravity sludge thickeners and a combination of gravity belt thickeners and air flotation thickeners. Subsequently, the sludge is digested anaerobically at 35–37°C with an average solids retention time of 19 days. Finally, the wastewater residuals are dewatered by centrifugation and the resulting digested, dewatered sludges (biosolids) have an average solids content of 20% by weight.
Agricultural soil was obtained from the United States Department of Agriculture Agricultural Research Service (USDA-ARS) Beltsville Agricultural Research Center (BARC). Sandy clay loam soil was taken from plots at a depth of 0 to 20 cm. Larger objects such as plant debris and rocks were removed, and the soil was used without further processing. It consisted of 20% clay, 27% silt, 53% sand, and had an organic carbon content of 1.7% and a pH of 5.6.
2.2 Experimental Setup and Sampling
Biosolids and soil were mixed in a ratio of 1:2 and distributed evenly among six plastic containers made from polyvinylchloride to form a homogenous layer about 25 cm in depth, 30 cm in width and 30 to 80 cm in length. The bottoms of the plastic containers were perforated to allow for drainage of excess water. No attempts were made to capture the leachate. The high content of biosolids was chosen to enable detection of compounds for an extended period of time and thus to facilitate the calculation of environmental half-lives. The application rate was higher (1:2) than what typically is applied in agriculture (e.g., 1:10 after mixing) but lower than the heavy application of pure biosolids customary in forestry (1:1). Control containers filled with 100% soil showed no background levels of PPCPs. Soil/biosolids mixtures and control soils were seeded with tomatoes, bell peppers and green salad in the spring of 2005 at the beginning of the experiment and exposed to the ambient weather conditions prevailing in the Greater Baltimore area, Maryland. The containers were exposed to ambient weather conditions without providing any shelter or artificial irrigation. The 3-year average air temperature was 14°C and the 3-year average monthly precipitation was 91 mm. Results from random sampling over the course of the experiment showed the moisture content of the soils to vary between 14.6 and 35.1%.
At the end of the growing season, grown crops were harvested and stored for future analysis. Crops grown on biosolids-amended soils were much smaller in size and leafy vegetables showed evidence of chemical burn, both plausible effects of the excessive fertilization with biosolids. Initial attempts to analyze for PPCP residues in the crops suffered from analytical limitations and thus are excluded from further discussion. Following cultivation in the growing season of 2005, the soil and remaining plant materials were left exposed to ambient conditions.
Soil sampling was conducted repeatedly over the course of the 994-day experimental period. Each sampling event consisted of sampling three containers with unamended control soils and three containers holding soils that had received a biosolids application at the beginning of the experiment. In order to obtain composite samples representative of the top 20-cm of soil, plant material and leaves were removed from the soil and a soil coring device was pushed into the soil to a depth of 20 cm. Following retrieval of the tool, the soil cores were transferred into plastic whirl-pack bags. Sampling locations were backfilled with soil from the container to avoid pooling of water. Each sampling location was marked and used only once. Three cores were obtained per container and pooled per sampling round. Pooled cores were thoroughly homogenized, and stored at −20°C until the chemical analysis was performed.
2.3 Sample Analysis
Following pooling and homogenization, collected samples (3 for time t = 0; 2 for t = 114 d; 2 for t = 518 d; 2 for t = 858 d, and 3 for t = 994 d) were analyzed for the loss of the 72 PPCPs by AXYS Analytical Services (British Columbia, Canada) according to USEPA Method 1694 (USEPA, 2007b), which involves ultrasonic extraction of solids with acetonitrile followed by solid phase extraction for purification of the diluted extract and reverse phase liquid chromatography with tandem mass spectrometry using two ion transitions per compound for identification and quantitation. Since the majority (>99%) of particulates in the samples investigated had a particle size of 1 mm or less, no grinding of the samples was necessary prior to analysis. Samples subjected to extraction according to Method 1694 had a weight of 1 gram dry weight, as specified for the analysis of soil samples. To facilitate compound detection, the 72 analytes were subdivided into four groups. All analytes were separated by liquid chromatography and detected by tandem mass spectrometry. For compounds where labeled analogs were available, concentrations were determined using the isotope dilution technique and a multipoint calibration of all target analytes. Isotope dilution is an internal calibration method that automatically corrects for non-ideal recovery of the target analytes and for ion suppression during chemical analysis. When a labeled analog was not available for a specific compound, the concentration was determined via the internal standard technique and a multipoint calibration of all the target analytes. Surrogate standards were used to estimate compound recovery. The internal standard technique does not account for analyte losses during sample processing. In contrast to the isotope dilution technique data, concentrations obtained by the internal standard technique were not corrected for recovery. A more detailed description of the analysis protocol and its performance is provided in USEPA method 1694 (USEPA, 2007b).
2.4 Quality Assurance
Prior to sample analysis several tests were carried out to ensure system and laboratory performance. A calibration standard solution with both labeled and native analytes was used to validate calibration accuracy. The retention times of both the native and labeled compounds were required to be within ±15 seconds of the respective retention times determined during the initial calibration. Throughout the analysis precision and recovery were ensured. Lab blanks were analyzed prior to each sample analysis. A duplicate sample analysis was performed by the lab for each batch consisting of 7 to 20 samples.
2.5 Data Analysis
The degradation kinetics were described with a first-order reaction model: C = C0 e−kt, where C0 (mg kg−1) is the initial concentration, C is the concentration at time t (days), and k is the first-order rate constant (days−1). A plot of the natural log of the concentration versus time provided a regression equation with a slope of k. The half-life t1/2 (days) was calculated as t1/2 = ln(2)/k. Fitting of experimental data was performed three times to obtain three separate estimates from datasets representing the average, the minimum and the maximum concentrations detected at each sampling event. Results are reported as mean half-lives and the range obtained from minimum and maximum concentrations fitted.
Additional computer estimates of half-lives in soil were obtained by using the USEPA’s EPI Suite software package (USEPA, 2009a)
3. Results and Discussion
3.1 Quality assurance of sample analysis
Chemical analysis of the samples was performed by the same laboratory the USEPA contracted to develop Method 1694. Stable-isotope labeled analogs were available for 26% of the measured analytes. Recovery rates for all detected analytes varied widely and ranged between 23 and 361% (see Table 1). Analytes exceeding the USEPA defined recovery control limits included azithromycin (23.6% vs. 33 – 120%), carbamezepin (159% vs. 21 – 137%), norfloxacin (144% vs. 50 – 135%), and ofloxacin (361% vs. 50 – 200%). All of these four compounds were determined by internal standard analysis as opposed to isotope dilution. Compared to typical recovery rates reported by the EPA (USEPA, 2007b), the present study was biased toward overestimation of concentrations for azithromycin, norfloxacin and ofloxacin (values greater 100%; see Table 1). Following adjustment for absolute recovery of isotope-labeled internal standards, calculated adjusted recoveries for target compounds quantified with the isotope dilution technique were all within the acceptable range. Recovery rates for isotope dilution analytes ranged from 95.5% (ciprofloxacin) to 109% (triclosan) (Table 1). The results of this study therefore suggest that analytical data from analyses carried out by internal calibration are less reliable with respect to their quantitative value. Quality control samples did not produce any evidence of false positive detections. Use of EPA Method 1694 ensured that results and conclusions drawn from the present work would not be based on the use of a non-standard, customized method of analysis. However, performance of this standard methods potentially could be boosted further by including additional isotope-labeled internal standards and other measures that are known to enhance analytical accuracy and precision.
Table 1.
Detected compounds, their percent recoveries determined from spiked samples and indication if isotope dilution was available.
| Compound | Recovery [%]a | Isotope Dilution |
|---|---|---|
| 4-Epitetracycline | 79.3 | |
| Doxycycline | 67 | |
| Tetracycline | 103 | |
| Gemfibrozil | 105 | yes |
| Triclocarban | 109 | yes |
| Triclosan | 95.5 | yes |
| Azithromycin | 23.6 | |
| Carbamazepine | 159 | |
| Ciprofloxacin | 99.5 | yes |
| Diphenhydramine | 111 | |
| Fluoxetine | 101 | yes |
| Miconazole | 89.7 | |
| Norfloxacin | 144 | |
| Ofloxacin | 361 | |
| Thiabendazole | 83.8 | yes |
Recovery rates shown for compounds determined by the isotope dilution method have been adjusted for recovery of the respective standard.
3.2 PPCPs in Soil-Biosolids Mixtures
Samples taken at the start of the experiment (t = 0) tested positive for 15 of the 72 PPCPs targeted in the study (Table 1). Table 2 lists the 57 compounds that were consistently not detected, and thus either were absent or present below the method detection limit, as defined by the USEPA.
Table 2.
Compounds that were consistently not detected, and thus either were absent or present below the method detection limit.
| Compound | USEPA-defined method detection limit [μg/kg dry weight] |
|---|---|
| 1,7-dimethylxanthine | 138 |
| 4-epianhydrochlortetracycline | 54.5 |
| 4-epianhydrotetracycline | 14.6 |
| 4-epichlortetracycline | 13.6 |
| 4-epioxytetracycline | 5.5 |
| acetaminophen | 54.5 |
| albuterol | 0.3 |
| anhydrochlortetracycline | 13.6 |
| anhydrotetracycline | 13.6 |
| caffeine | 13.6 |
| carbadox | 1.4 |
| cefotaxime | 13.8 |
| chlortetracycline | 5.5 |
| cimetidine | 0.6 |
| clarithromycin | 9.3 |
| clinafloxacin | 16 |
| cloxacillin | 2.7 |
| codeine | 2.7 |
| cotinine | 1.4 |
| dehydronifedipine | 0.5 |
| demeclocycline | 13.6 |
| digoxigenin | 5.5 |
| digoxin | 13.6 |
| diltiazem | 0.3 |
| enrofloxacin | 5.2 |
| erythromycin-H2O | 1.0 |
| flumequine | 1.4 |
| ibuprofen | 13.6 |
| isochlortetracycline | 5.5 |
| lincomycin | 2.7 |
| lomefloxacin | 2.7 |
| metformin | 32.1 |
| minocycline | 54.5 |
| naproxen | 2.7 |
| norgestimate | 2.7 |
| ormetoprim | 0.5 |
| oxacillin | 2.7 |
| oxolinic acid | 0.5 |
| oxytetracyclin | 5.5 |
| penicillin G | 2.7 |
| penicillin V | 2.7 |
| ranitidine | 0.6 |
| roxithromycin | 0.3 |
| sarafloxacin | 13.6 |
| sulfachloropyridazine | 1.4 |
| sulfadiazine | 1.4 |
| sulfadimethoxine | 0.3 |
| sulfamerazine | 0.5 |
| sulfamethazine | 0.5 |
| sulfamethizole | 0.5 |
| sulfamethoxazole | 0.5 |
| sulfanilamide | 1.4 |
| sulfathiazole | 1.4 |
| trimethoprim | 1.4 |
| tylosin | 5.5 |
| virginiamycin | 2.7 |
| warfarin | 1.4 |
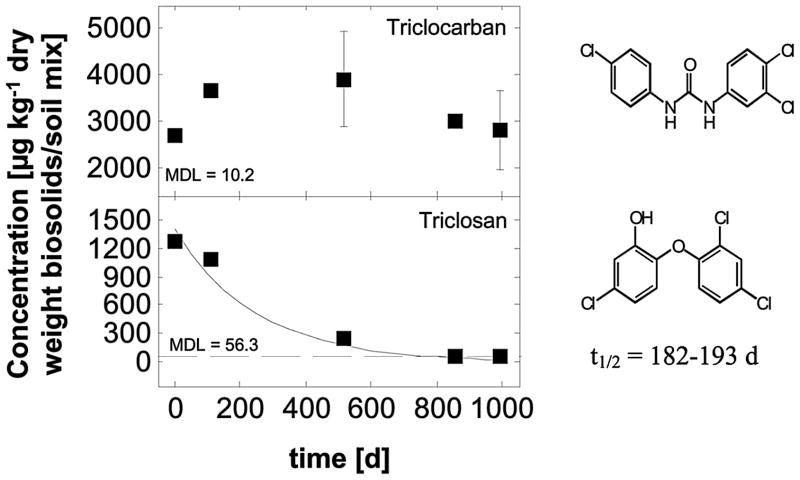
The 15 detected PPCPs are discussed in the following in greater detail. The sanitizing agents triclocarban and triclosan were detected consistently at elevated concentrations (Figure 1). Among the seven antibiotics detected, there were three from the group of tetracyclines (Figure 2), three fluoroquinolones (Figure 3), and the macrolide drug azithromycin (Figure 4). Additional drugs detected included carbamazepine, diphenhydramine, fluoxetine, gemfibrozil, miconazole, and thiabendazole (Figures 4 and 5). Initial concentrations of PPCPs detected in biosolids-amended soils ranged from low parts-per-billion (ppb) for thiabendazole to low parts-per-million (ppm) for triclocarban and triclosan.
Figure 1.
Concentrations of the sanitizing agents triclocarban and triclosan detectable in biosolids-amended soil mesocosms over time and modeled first-order degradation kinetics.
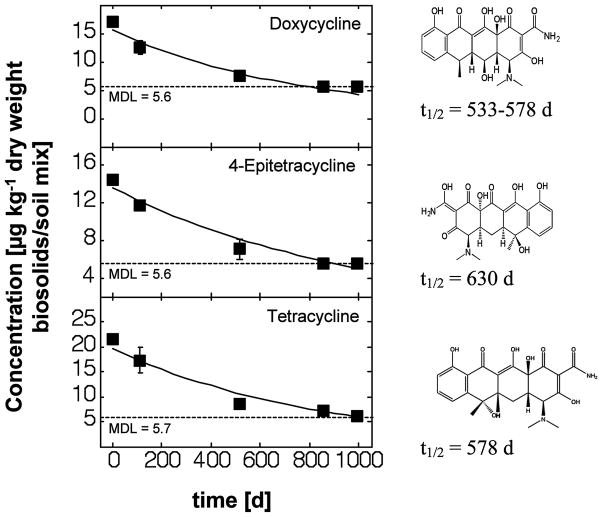
Figure 2.
Concentrations of various tetracycline antibiotics detectable in biosolids-amended soil mesocosms over time and modeled first-order degradation kinetics.
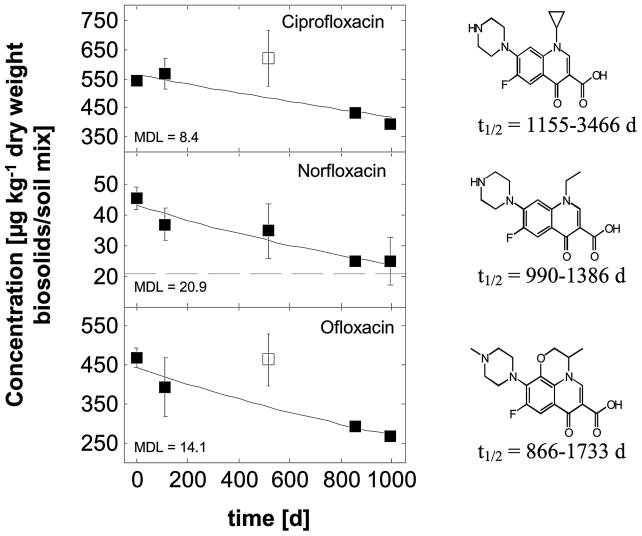
Figure 3.
Concentrations of various quinolone antibiotics detectable in biosolids-amended soil mesocosms over time and modeled first-order degradation kinetics. Both solid and empty data points represent average values of two samples per campaign. Error bars indicate the minimum and maximum concentrations. Data points with empty symbols were treated as outliers during data fitting.
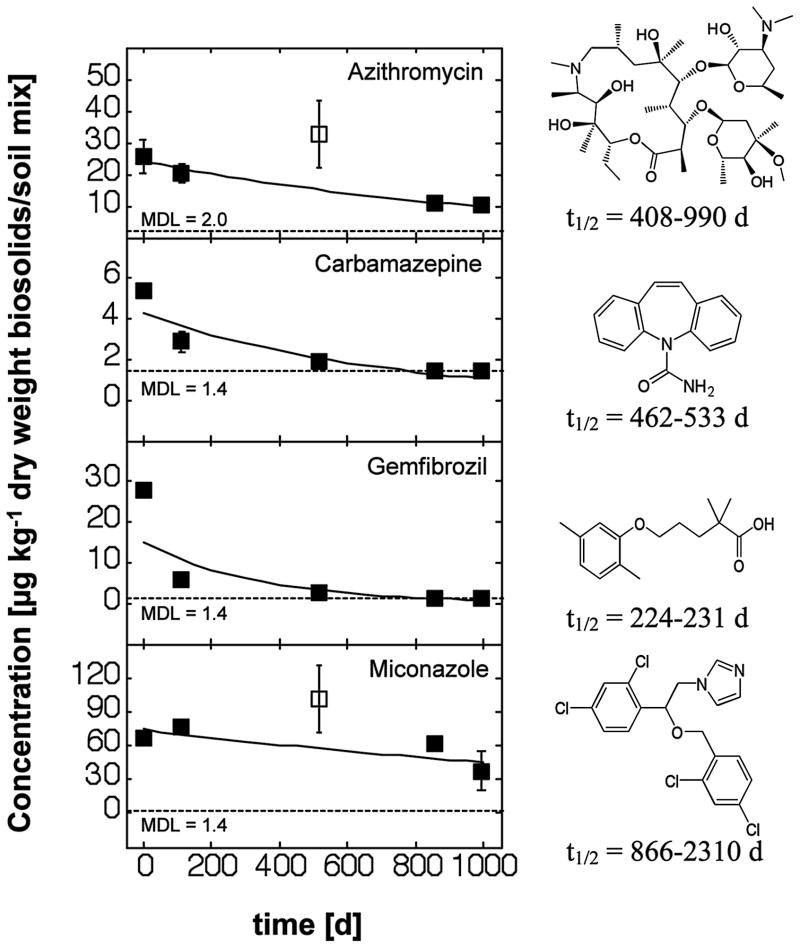
Figure 4.
Concentrations in biosolids-amended soil microcosms of various pharmaceuticals and modeled first-order degradation kinetics. Both solid and empty data points represent average values of two samples per campaign. Error bars indicate the minimum and maximum concentrations. Data points with empty symbols were treated as outliers during data fitting.
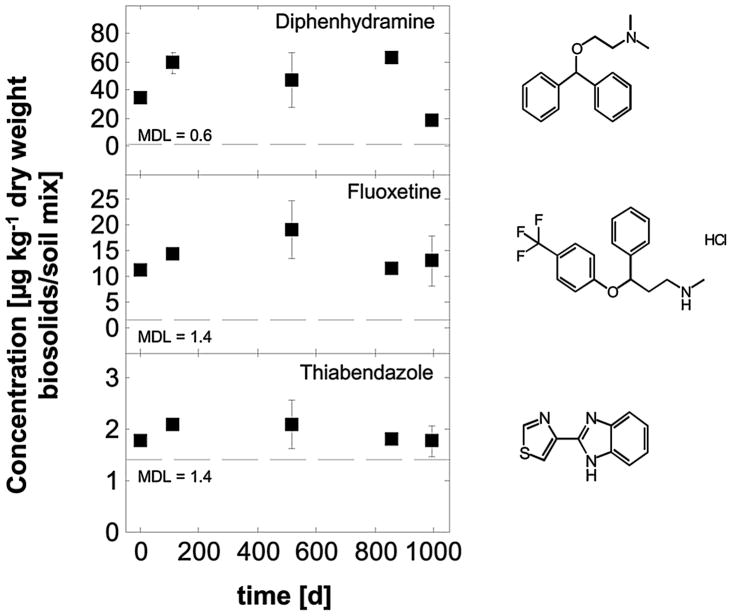
Figure 5.
Concentrations of various persistent pharmaceuticals that persisted in biosolids-amended soil microcosms.
Sanitizing Agents
The antimicrobials triclocarban and triclosan were detected at the highest concentrations of all compounds investigated with averages of 2715 and 1265 μg kg−1 dry weight or 52% and 24%, respectively, of the entire PPCP mass present (5270±380 μg kg−1). These elevated concentrations can be attributed to their intense usage and the strong tendency of the two antimicrobials to partition into and persist in sewage sludge. Halden and Paull (2005) provided conservative usage estimates for triclocarban and triclosan discharged into U.S. sewage of >330,000 and >300,000 kg yr−1, respectively. For the city of Baltimore, Maryland, the annual per-capita usage of both compounds was reported at 1130 mg yr−1for triclocarban and 1030 mg yr−1for triclosan (Halden and Paull, 2005). Logarithmic octanol-water partitioning coefficients (log KOW) were estimated by USEPA EPI Suite software at 4.9 and 4.8 for triclocarban and triclosan, respectively, at neutral pH.
Throughout the experiment detection of triclocarban showed some scatter, but overall levels appeared to remain constant (Figure 1). After 994 days, the compound was still present at ppm levels. These data indicate that triclocarban can persist for extended periods of time in biosolids-amended soils without any apparent degradation. Triclocarban previously had been observed to persist in agricultural soils (Cha and Cupples, 2009) and application of the compound in biosolids was demonstrated to extend its environmental persistence (Al-Rajab et al. 2009). Similarly, triclocarban was observed to persist in anaerobic estuarine environments with a half-life on the order of decades (Miller et al. 2008). Two separate laboratory experiments indicated an environmental half-life of triclocarban in soil of 87 – 231 days (Wu et al., 2009) and 108 days (Ying et al., 2007).
Triclosan, remained detectable only until day 858 (Figure 1) at a detection limit of 56.3 μg kg−1. Whether the compound was merely transformed or completely mineralized is uncertain. It has been shown that triclosan can be transformed to another, more environmentally persistent degradate, methyl triclosan (Balmer et al., 2004; Boehmer et al., 2004; Coogan et al., 2007). A log KOW of 5.2 has been reported for methyl triclosan, which makes it more lipophilic than its parent compound triclosan (Boehmer et al., 2004). Evidence of methyl triclosan production from triclosan has been found in wastewater treatment plants, as well as in lakes and a river in Switzerland (Lindström et al., 2002; McAvoy et al., 2002). The method used in this study did not include this degradate.
The first-order degradation curve showed a half-life for triclosan of 188 days in biosolids-amended soil. (Figure 1; Table 3). A laboratory degradation study performed under aerobic conditions on different soil types with and without biosolids amendments yielded half-lives for triclosan of 20 – 58 days (Wu et al., 2009). An additional laboratory degradation study conducted on agricultural soils never having been amended with biosolids reported a half-life for triclosan of 18 days (Ying et al., 2007). The half-life of both triclocarban and triclosan in soil as estimated by USEPA’s EPI Suite Software (USEPA, 2009a) is 120 days.
Table 3.
Half-lives calculated for tested samples, as well as values found in the literature and estimated with the USEPA’s EPI Suite software.
| Compound | Half-life [days] | Source |
|---|---|---|
| Azithromycin | 770 ± 181 | this study, experimental |
| 360a | this study, predicted | |
| Carbamazepine | 495 ± 36 | this study, experimental |
| >60c | (Monteiro and Boxall, 2009) | |
| 75a | this study, predicted | |
| Ciprofloxacin | 2310 ± 1155 | this study, experimental |
| 120a | this study, predicted | |
| Diphenhydramine | >1000c | this study, experimental |
| 75a | this study, predicted | |
| Doxycycline | 533 ± 23 | this study, experimental |
| 120a | this study, predicted | |
| 4-Epitetracycline | 630 | this study, experimental |
| 120a | this study, predicted | |
| Fluoxetine | >1000c | this study, experimental |
| 120a | this study, predicted | |
| >60c | (Monteiro and Boxall, 2009) | |
| >60c | (Rowland et al. 2008) | |
| Gemfibrozil | 231 ± 4 | this study, experimental |
| 75a | this study, predicted | |
| Miconazole | 1386 ± 722 | this study, experimental |
| 360a | this study, predicted | |
| Norfloxacin | 1155 ± 198 | this study, experimental |
| 120a | this study, predicted | |
| Ofloxacin | 1386 ± 434 | this study, experimental |
| 360a | this study, predicted | |
| Tetracycline | 578 | this study, experimental |
| 55–105b | (Winckler and Grafe, 2001) | |
| 120a | this study, predicted | |
| Thiabendazole | 30a | this study, predicted |
| Triclocarban | >1000c | this study, experimental |
| 87 – 231 | (Wu et al., 2009) | |
| 108 | (Ying et al., 2007 | |
| 120a | this study, predicted | |
| Triclosan | 187 ± 6 | this study, experimental |
| 20 – 58a | (Wu et al., 2009) | |
| 18a | (Ying et al., 2007) | |
| 120a | this study, predicted |
soil;
pig manure;
no detectable degradation over 3 years
Tetracycline Antibiotics
Among 12 tetracycline antibiotics monitored, three were detected consistently in biosolids-amended soils (Figure 2). Concentration levels were in the low ppb-range and approached the limit of detection after 518 days of weathering in the mesocosms. Despite their polar structure and potential biodegradability, these compounds persisted for extended periods of time in the biosolids amended soil. The estimated mean half-lives in biosolids-amended soils ranged from 533 days for doxycycline to 578 days for tetracycline to 630 days for 4-epitetracycline (Table 3). Despite the very low concentration levels there was no observable scatter in the data. The estimated half-lives were about 5 times greater than estimates obtained using USEPA’s EPI Suite software (USEPA, 2009a) and as experimental measurements obtained for tetracycline in pig manure (Winckler and Grafe, 2001). The present study produced the first experimental data for the fate of doxycycline and 4-epitetracycline in biosolids-amended soils and thus, at present no comparison is feasible.
Quinolone Antibiotics
Among nine quinolone antibiotics monitored, three were consistently detected over the course of the experiment (Figure 3). Ciprofloxacin and ofloxacin were initially found at the highest concentrations (542 and 470 μg kg−1 dry weight, respectively); however, ofloxacin had an extremely high recovery value of 361% which indicates low data quality for this quantitative information. After 994 days of weathering, both antibiotics were still present at over 390 and 267 μg kg−1 dry weight, respectively, thus indicating their environmental persistence. Potential reasons for their prolonged detection may include strong electrostatic sorption to the biosolids and thus low bioavailability as well as chemical aging, again resulting in reduced bioavailability. The aging mechanism is understood to entail the migration of molecules into very small sites within the soil matrix (e.g., nanopores) (Alexander, 2000). As chemicals become lodged in these small pores, microorganisms are unable to access the chemicals due to size exclusion. The main sorbent for hydrophobic molecules is the organic matter that comprises soils. Thus, hydrophobic contaminants become tightly bound within small pores rich in organic matter after an extended residence time in soil (Alexander, 2000).
Environmental half-lives for the three quinolones were in the range of 1000 days, which is at least three times longer than predictions obtained using USEPA’s EPI Suite software (USEPA, 2009a) (Table 3). No prior data were available in the peer-reviewed literature for the environmental persistence in biosolids-amended soils of these three quinolones. However, the estimate for ofloxacin carries great uncertainty since the recovery rate for this compound was extremely poor (Table 1).
Other Classes of PPCPs
In addition to the sanitizing agents and antibiotics, the following compounds were found in order of decreasing concentration: miconazole (fungicide), diphenhydramine (antihistamine), gemfibrozil (lipid regulator), fluoxetine (antidepressant), carbamazepine (anticonvulsant), and thiabendazole (fungicide), ranging between 70 and 2 μg kg−1 dry weight. Mean environmental half-lives could be calculated for azithromycin (770 d), carbamezepin (495 d), gemfibrozil (231 d), and miconazole (1386 d) (Table 3). These experimental data exceeded predictions obtained with USEPA’s EPI Suite software (USEPA, 2009a) by a factor of 2.8 (azithromycin) to 6.6 (carbamezepin). However, the study by Löffler et al. (2005) presented a half-life of carbamazepine in a water/sediment system of 328 days, similar to the value determined in this study. A more recent study showed that carbamazepine persisted in soils, biosolids, and soil-biosolids mixtures without observable degradation for a period of 60 days (Monteiro and Boxall, 2009). The present study furnishes the first experimental data for the environmental half-lives in biosolids-amended soils for azithromycin, gemfibrozil, and miconazole.
Three additional drugs showed no detectable loss from the mesocosms over the three-year monitoring period (Figure 5). These compounds included the antihistamine diphenhydramine, the antidepressant fluoxetine, and the parasiticide/agricultural fungicide thiabendazole. The observed persistence of fluoxetine is consistent with two prior reports (Monteiro and Boxall, 2009; Rowland et al. 2008). The present study furnishes the first experimental information on the fate of diphenhydramine and thiabendazole in biosolids-amended soils.
Overall, the environmental half-lives determined in this study tended to be higher than comparable values obtained in controlled laboratory experiments or half-lives in soil calculated with chemical fate computer models (Table 3). This is expected. Recent studies indicate that the biodegradability of compounds decreases when they are introduced into soils in the form of biosolids (Al-Rajab et al. 2009; Monteiro and Boxall, 2009). Potential reasons for this observation include a reduced bioavailability of compounds that have become sequestered in biosolids and the presence of complex mixtures that may inhibit microbial activity and limit degradation (Al-Ahmad et al. 1999). An additional reason for the prolonged half-lives of the studied PPCPs might be that other sources of e.g. carbon and sulfur were more bioavailable to the soil biota in comparison to the traces of PPCPs. Other factors influencing a chemical’s half-life include the water content of the matrix, the amount of readily available nutrients, temperature, adaptation processes, and the initial micropollutant concentration, as well as the presence of co-contaminants, including substances that inhibit microbial activity.
On average, the environmental half-lives determined in this study exceeded model predictions for fate in soil by a factor of 8.5 with a range of 1.6 (triclosan) to 33 (thiabendazole). If inhibitory effects of co-contaminants such as antimicrobials and antibiotics indeed play a role, then one would expect the half-lives of chemicals to increase with increasing biosolids content of the soils. If this untested assumption holds true, the half-lives reported in this study may underestimate environmental persistence of PPCPs applied in forestry, where biosolids applications are heavy and undiluted. Conversely, the reported half-lives would overestimate environmental persistence for PPCPs in agricultural soils receiving biosolids, since agricultural mixing ratios of biosolids to soils are lower than the ratio of 1:2 chosen in this study (e.g., 1:10 or greater). Data presented in Figures 2 – 5 illustrate that for the purpose of this study use of more dilute biosolids mixtures would not have been desirable, as many of the analyte concentrations would have dropped below the method detection limits.
3.3 Risks, Concerns, and Data Gaps
Although the presence of micropollutants in biosolids destined for land application is known, the effects of these compounds on terrestrial ecosystems remain relatively uninvestigated (Liu et al., 2009). The majority of available ecotoxicity data deal solely with aquatic organisms. To perform a proper risk assessment for soil-dwelling organism, half-life data for PPCPs are indispensable and should come from field studies rather than from laboratory experiments. Although concentrations of eleven of the 15 PPCPs detected in this study decreased over time with first-order kinetics, all 15 compounds were still present after 518 days of weathering. This suggests that environmental risk assessments should consider not only acute, but also chronic toxic effect threshold values of PPCPs on non-target organisms due to chemical longevity in soil and opportunities for extended or even life-time exposures of terrestrial biota (Chalew and Halden, 2009).
Another area requiring further research is the potential for plants and crops to take up microcontaminants from biosolids-amended soils. For example, it has been documented that certain antibiotics, specifically tetracyclines and fluoroquinolones, can be taken up by crop plants (Migliore et al., 2003; Kumar et al., 2005).
The environmental persistence in biosolids of a number of antimicrobials and antibiotics observed here suggests a need for determining potential risks posed by these substances through the promotion of antimicrobial drug resistance in soil microorganisms residing on agricultural soils and the crops produced. The presence of antibiotics at sub-lethal concentrations in the environment may negatively impact soil microbial communities. Moreover, their presence can promote the formation of resistance, and even cross-resistance and multiple drug resistance in microorganisms (Wegener et al., 1998; Al-Ahmad et al., 1999).
Another area of concern is the potential for bioaccumulation and biomagnifications of microcontaminants throughout the food chain. Lipophilic contaminants selectively partition into sludge during wastewater treatment, which can result in concentrations of the antimicrobials triclocarban and triclosan in biosolids, for example, as high as 441 ppm and 133 ppm, respectively (USEPA 2009b). Bioaccumulation in worms was recently demonstrated for these two biocides in aquatic and terrestrial environments (Higgins et al. 2009; Kinney et al., 2008). Transfer of persistent hydrophobic PPCPs from biosolids-amended soils and agricultural runoff into soil and aquatic organisms opens a pathway for the potential biomagnifications of these compounds in birds, fish and mammals.
4. Conclusions
In this mesocosm study, EPA Method 1694 (USEPA, 2007b) was used to monitor 72 PPCPs in soil-biosolids mixtures. Weathering of biosolids-amended soils under outdoor conditions in Maryland indicated that although many PPCPs degrade over time, some compounds persist in agricultural soils years after their application in the form of biosolids. The work underscores the necessity of conducting experimental studies in addition to simply predicting half-lives using computer models that utilize soil as a surrogate matrix for biosolids-soil mixtures. Available models presently do not take into account degradation limiting parameters such as reduced bioavailability and the presence of co-contaminants, including antimicrobials, antibiotics and similar substances that are known to inhibit microbial communities and delay biodegradation (Al-Ahmad et al. 1999; Al-Rajab et al. 2009). In addition, the analytical results presented in this study re-emphasize the formidable analytical challenges associated with the determination of trace contaminants in biosolids. Four of the 15 PPCPs detected in this study showed recovery rates outside of the quality control boundaries specified by the USEPA method. Average performance data reported by the USEPA (2007b) for biosolids showed typical recoveries of as low as 22.6% for digoxin and as high as 359.7% for triclosan. Taken together with the results of the present work, it is evident that there is a need for continuing method development efforts to furnish reliable data for risk assessment studies.
Supplementary Material
Acknowledgments
We thank Yakov Pachepsky from the United States Department of Agriculture Agricultural Research Service (USDA-ARS) Beltsville Agricultural Research Center for kindly providing the soil samples. We also thank Thayer Young for his help with sample handling and shipping. This work was supported in part by the National Institute of Environmental Health Sciences by NIEHS grant 1R01ES015445 and by the Johns Hopkins University Center for a Livable Future.
Abbreviations
- DL
Detection Limit
- EPA
Environmental Protection Agency
- PPCP
Pharmaceuticals and Personal Care Products
- TNSSS
Targeted National Sewage Sludge Survey
Footnotes
Additional details regarding the temperature and precipitation for the three-year test period can be found in the Supplemental Information accompanying this article. This information is available free of charge via the Internet at http://pubs.acs.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ahmad A, Daschner FD, Kümmerer K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch Environ Contam Toxicol. 1999;37:158–163. doi: 10.1007/s002449900501. [DOI] [PubMed] [Google Scholar]
- Al-Rajab AJ, Sabourin L, Scott A, Lapen DR, Topp E. Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarban in an agricultural soil. Sci Total Environ. 2009;407(23):5978–5985. doi: 10.1016/j.scitotenv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Alexander M. Aging, Bioavailability, and Overestimation of Risk from Environmental Pollutants. Environmental Science and Technology. 2000;34(20):4259–4265. [Google Scholar]
- Angin I, Yaganoglu AV. Application of Sewage Sludge as a Soil Physical and Chemical Amendment. ECOLOJI. 2009;19(73):39–47. [Google Scholar]
- Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, Müller MD, Buser HR. Occurrence of Methyl-Triclosan, a transformation product of the Batericide Triclosan, in Fish from Various Lakes in Switzerland. Environ Sci and Technol. 2004;38:390–395. doi: 10.1021/es030068p. [DOI] [PubMed] [Google Scholar]
- Boehmer W, Ruedel H, Wenzel A, Schroeter-Kermani C. Retrospective monitoring of triclosan and methyl-triclosan in fish: results from the German environmental specimen bank. Organohalogen Compd. 2004;66:1516–1521. [Google Scholar]
- Carballa M, Omil F, Lema JM. Influence of Different Pretreatments on Anaerobically Digested Sludge Characteristics: Suitability for Final Disposal. Water Air Soil Pollut. 2009;199(1–4):311–321. [Google Scholar]
- Cha J, Cupples AM. Detection of antimicrobials triclocarban and triclosan in agricultural soils following land application of municipal biosolids. Water Research. 2009;43:2522–2530. doi: 10.1016/j.watres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Chalew T, Halden RU. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Triclocarban. J Am Water Res Assoc. 2009;45(1):3–13. doi: 10.1111/j.1752-1688.2008.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan MA, Edziyie RE, La Point TW, Venables BJ. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere. 2007;67:1911–1918. doi: 10.1016/j.chemosphere.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Daughton CG, Ternes TA. Pharmaceutical and Personal Care Products in the Environment: Agents of Subtle Change? Environ Health Perspectives. 1999;107(6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolliver H, Kumar K, Gupta S. Sulfamethazine uptake by plants from manure-amended soil. J Environ Qual. 2007;36:1224–1230. doi: 10.2134/jeq2006.0266. [DOI] [PubMed] [Google Scholar]
- Fent K, Weston AA, Caminada D. Ecotoxicology of human pharmaceuticals. Aquatic Toxicology. 2006;(76):122–159. doi: 10.1016/j.aquatox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Halden RU, Paull DH. Co-Occurrence of Triclocarban and Triclosan in U.S. Water Resources. Environ Sci Technol. 2005;39:1420–1426. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- Hamscher G, Pawelzick HT, Hoper H, Nau H. Antibiotics in soil: Routes of entry, environmental concentrations, fate and possible effects. In: Kümmerer K, editor. Pharmaceuticals in the environment - Sources, fate, effects, and risks. 2. Chapter 11. Springer; Berlin, Germany: 2004. pp. 139–148. [Google Scholar]
- Heuer H, Krogerrecklenfort E, Wellingon EMH, Egan S, van Elsas JD, van Overbeek L, Collard JM, Guillaume G, Karagouni AD, Nikolakupoulou TL, Smalla K. Gentamicin resistance genes in environmental bacteria: Presence and transfer. FEMS Microbiol Ecol. 2002;42:289–302. doi: 10.1111/j.1574-6941.2002.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Higgins CP, Paesani ZJ, Chalew TEA, Halden RU. Bioaccumulation of Triclocarban in Lumbriculus variegates. Environ Toxicol Chem. 2009;65:141–148. doi: 10.1897/09-013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R, Ternes T, Haberer K, Kratz KL. Occurrence of antibiotics in the aquatic environment. Science of the Total Environment. 1999;225:109–118. doi: 10.1016/s0048-9697(98)00337-4. [DOI] [PubMed] [Google Scholar]
- Jørgensen SE, Halling-Sørensen B. Drugs in the environment. Chemosphere. 2000;40:691–699. doi: 10.1016/s0045-6535(99)00438-5. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, Bossio JP, Benotti MJ. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol. 2008;42(6):1863–1870. doi: 10.1021/es702304c. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ Sci Technol. 2002;36(6):1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kumar K, Gupta SC, Baidoo SK, Chander Y, Rosen CJ. Antibiotic uptake by plants from soil fertilized with animal manure. J Environ Qual. 2005;(34):2082–2085. doi: 10.2134/jeq2005.0026. [DOI] [PubMed] [Google Scholar]
- Kümmerer K. Resistance in the Environment. J Antimicrob Chemother. 2004;54:311–320. doi: 10.1093/jac/dkh325. [DOI] [PubMed] [Google Scholar]
- Lindsey ME, Meyer MT, Thurman EM. Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal Chem. 2001;73(19):4640–4646. doi: 10.1021/ac010514w. [DOI] [PubMed] [Google Scholar]
- Lindström A, Buerge IJ, Poiger T, Bergqvist PA, Müller MD, Buser HR. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol. 2002;36(11):2322–2329. doi: 10.1021/es0114254. [DOI] [PubMed] [Google Scholar]
- Liu F, Ying GG, Zhou QX. Terrestrial ecotoxicological effects of the antimicrobial agent triclosan. Ecotox Environ Safety. 2009;72:86–92. doi: 10.1016/j.ecoenv.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Löffler D, Römbke J, Meller M, Ternes TA. Environmental Fate of Pharmaceuticals in Water/Sediment Systems. Environ Sci Technol. 2005;39(14):5209–5218. doi: 10.1021/es0484146. [DOI] [PubMed] [Google Scholar]
- Mantovi P, Baldoni G, Toderi G. Reuse of liquid, dewatered, and composted sewage sludge on agricultural land: Effects of long-term application on soil and crop. Water Research. 2005;39:289–296. doi: 10.1016/j.watres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- McAvoy DC, Schatowitz B, Jacob M, Hauk A, Eckhoff WS. Measurement of triclosan in wastewater treatment systems. Environmental Toxicological Chemistry. 2002;21:1323–1329. [PubMed] [Google Scholar]
- Migliore L, Cozzolino S, Fiori M. Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere. 2003;52:1233–1244. doi: 10.1016/S0045-6535(03)00272-8. [DOI] [PubMed] [Google Scholar]
- Miller TR, Heidler J, Chillrud SN, DeLaquil A, Ritchie JC, Mihalic JN, Halden RU. Fate of Triclosan and Triclocarban in Estuarine Sediment. Environ Sci Technol. 2008;42:4570–4576. doi: 10.1021/es702882g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro SC, Boxall ABA. Factors affecting the degradation of pharmaceuticals in agricultural soils. Environ Tox Chem. 2009;28(12):2546–2554. doi: 10.1897/08-657.1. [DOI] [PubMed] [Google Scholar]
- NEBRA, North East Biosolids and Residuals Association. A National Biosolids Regulation, Quality, End Use, and Disposal Survey - Final Report. 2007 http://www.nebiosolids.org/uploads/pdf/NtlBiosolidsReport-20July07.pdf.
- NRC, National Research Council. Biosolids applied to land: Advancing standards and practices. 2002 Retrieved June 2009, from http://www.epa.gov/waterscience/biosolids/nas/complete.pdf.
- Richardson ML, Bowron JM. The fate of pharmaceutical chemicals in the aquatic environment - A review. J Pharm Parmacol. 1985;37:1–12. doi: 10.1111/j.2042-7158.1985.tb04922.x. [DOI] [PubMed] [Google Scholar]
- Rowland S, Ciije M, Reshaw C, Talbot H. Low biodegradability of fluoxetine HCl, diazepam, and their human metabolites in sewage sludge-amended soil. J Soils Sed. 2008;8:217–230. [Google Scholar]
- Sacher F, Lange FT, Brauch HJ, Blankenhorn I. Pharmaceuticals in groundwaters: Analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatography A. 2001;938:199–210. doi: 10.1016/s0021-9673(01)01266-3. [DOI] [PubMed] [Google Scholar]
- Schut L. Sewage biosolids - Managing urban nutrients responsibly for crop production. 2008 Retrieved June 2009, from http://www.omafra.gov.on.ca/english/nm/nasm/info/brochure.htm.
- Schwartz T, Kohnen W, Jansen B, Obst U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol. 2003;43:325–335. doi: 10.1111/j.1574-6941.2003.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB. Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water treatment plant. Sci Total Environ. 2004;329(1–3):99–113. doi: 10.1016/j.scitotenv.2004.03.015. [DOI] [PubMed] [Google Scholar]
- USEPA. Biosolids Technology Fact Sheet: Land Application of Biosolids. 2000. EPA832-F-00-064. [Google Scholar]
- USEPA. Biosolids: Frequently Asked Questions. 2007a Retrieved June 2009, from http://www.epa.gov/owm/mtb/biosolids/genqa.htm.
- USEPA. Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. 2007b. EPA821-R-08-002. [Google Scholar]
- USEPA. Estimation Programs Interface Suite™ for Microsoft® Windows v. 4.00. 2009a. [Google Scholar]
- USEPA. Targeted National Sewage Sludge Survey Report. 2009b. EPA822-F-08-006. [Google Scholar]
- Webb S, Ternes T, Gibert M, Olejniczak K. Indirect human exposure to pharmaceuticals via drinking water. Toxicol Lett. 2003;142(3):157–167. doi: 10.1016/s0378-4274(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Wegener HC, Aarestrup FM, Jensen LB, Hammerum AM, Bager F. The association between the use of antimicrobial growth promoters and development of resistance in pathogenic bacteria towards growth promoting and therapeutic antimicrobials. J Animal Feed Sci. 1998;7:7–14. [Google Scholar]
- Winckler C, Grafe A. Use of veterinary drugs in intensive animal production: evidence for persistence of tetracycline in pig slurry. J Soils Sed. 2001;1:66–70. [Google Scholar]
- Witte W. Medical Consequences of Antibiotic Use in Agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- Wu C, Sponberg AL, Witter JD. Adsorption and Degradation of Triclosan and Triclocarban in Soils and Biosolids-Amended Soils. J Agri Food Chem. 2009;57(11):4900–4905. doi: 10.1021/jf900376c. [DOI] [PubMed] [Google Scholar]
- Xia K, Bhandari KD, Pillar G. Occurrence and Fate of Pharmaceuticals and Personal Care Products (PPCPs) in Biosolids. J Environ Qual. 2005;34:91–104. doi: 10.2134/jeq2005.0091. [DOI] [PubMed] [Google Scholar]
- Ying GG, Yu XY, Kookana RS. Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut. 2007;150:300–305. doi: 10.1016/j.envpol.2007.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.