Abstract
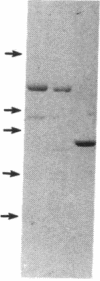
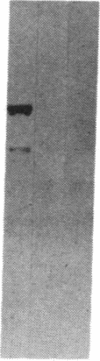
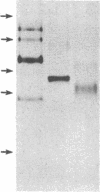
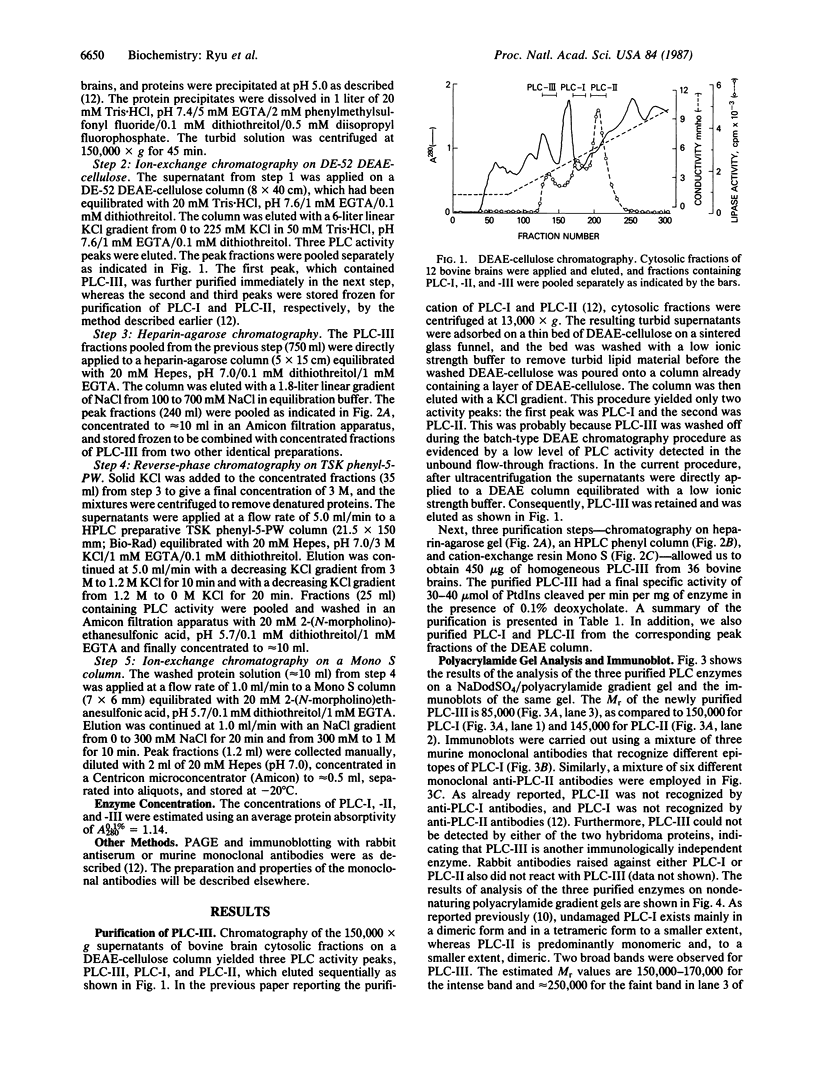
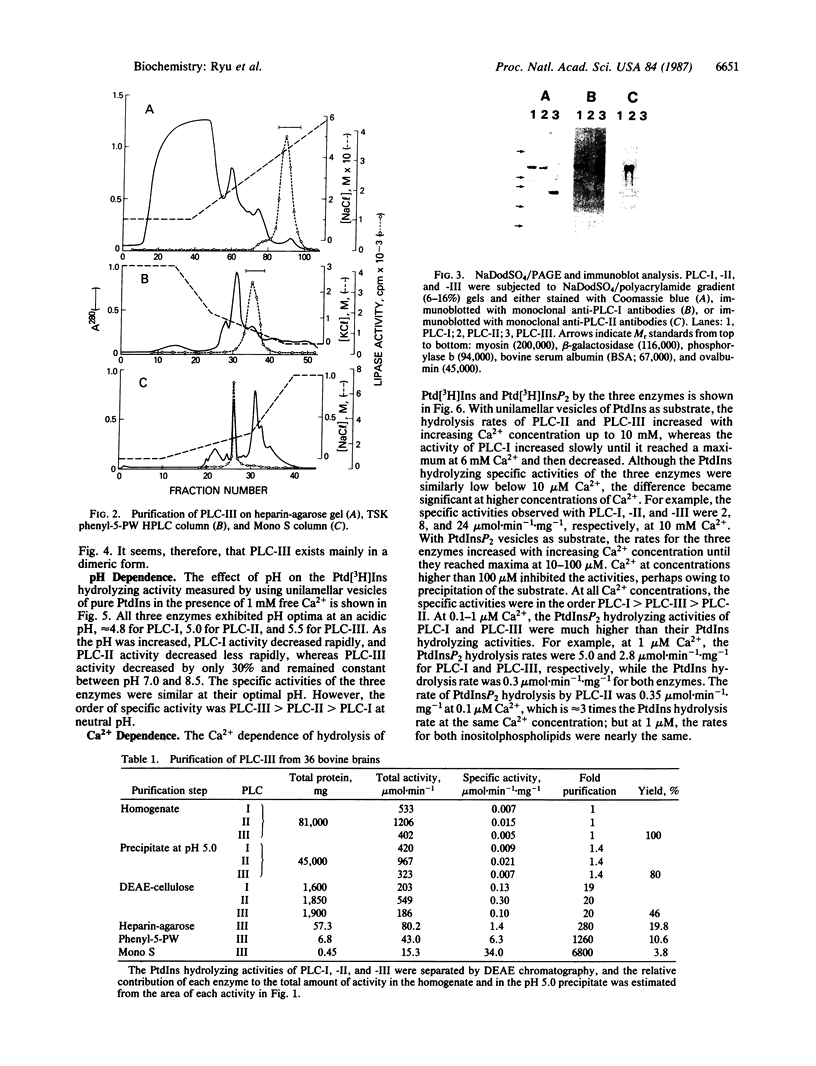
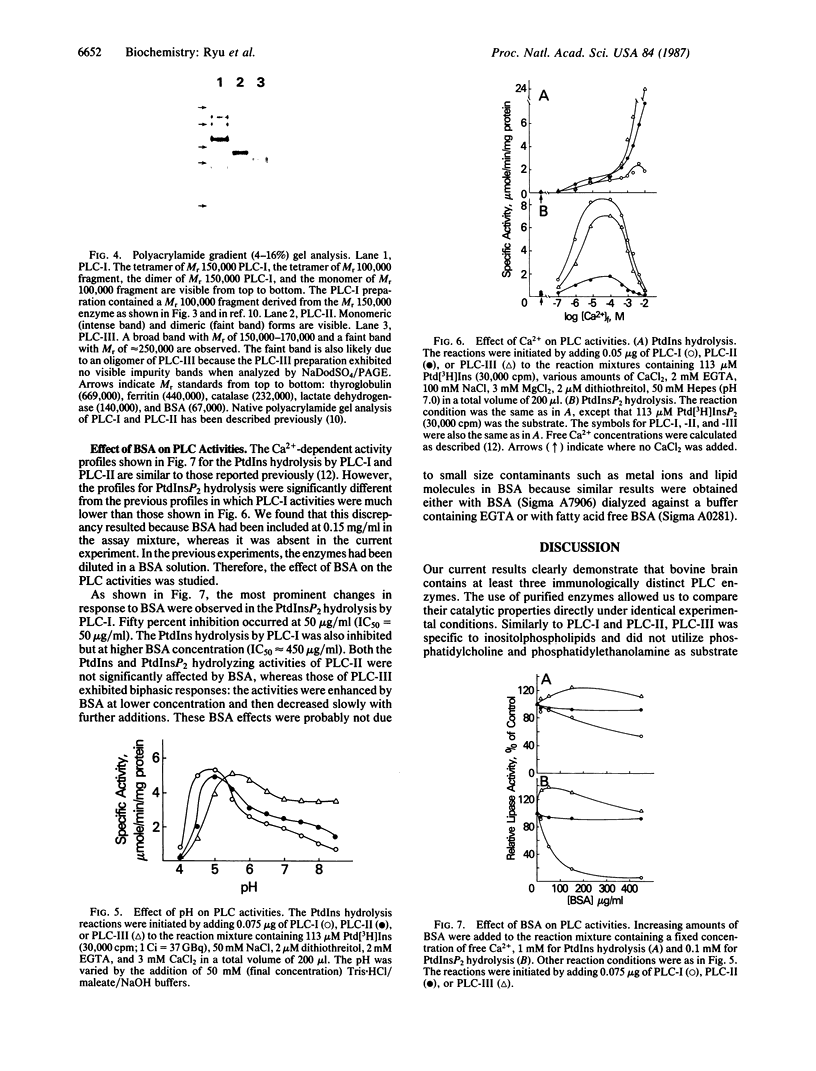
We previously reported that cytosolic fractions of bovine brain contain two immunologically distinct forms of phospholipase C (PLC), PLC-I and PLC-II. We now report the purification of another form of inositolphospholipid-specific phospholipase C from bovine brain cytosol, designated PLC-III, and the comparison of the catalytic properties of the three isozymes. Approximately 450 micrograms of pure PLC-III was obtained from 36 bovine brains, and it had a final specific activity of 30-40 mumol of phosphatidylinositol hydrolyzed per min per mg of enzyme in the presence of 0.1% deoxycholate. PLC-III exhibited an apparent Mr of 85,000 in NaDodSO4/PAGE, which is considerably smaller than the Mr of 150,000 for PLC-I and 145,000 for PLC-II. Neither of the two mixtures of monoclonal antibodies nor the rabbit polyclonal antibodies directed against either PLC-I or PLC-II cross-reacted with PLC-III. The catalytic properties of the three isozymes were studied by using small unilamellar vesicles prepared from either phosphatidylinositol (PtdIns) or phosphatidylinositol 4,5-bisphosphate (PtdInsP2) as substrates. Hydrolysis of both PtdIns and PtdInsP2 by the three enzymes was dependent on Ca2+. However, at low Ca2+ concentration, PtdInsP2 was the preferred substrate for all three enzymes. When PtdIns was the substrate, the three enzymes exhibited similar specific activities at their optimum pH, which was 4.8 for PLC-I, 5.0 for PLC-II, and 5.5 for PLC-III. But at neutral pH, the order of specific activity was PLC-III greater than PLC-II greater than PLC-I. In contrast, the order of specific activity was PLC-I greater than PLC-III greater than PLC-II for PtdInsP2 hydrolysis, which means that PLC-I is the most specific for PtdInsP2. The three enzymes were affected differently by bovine serum albumin: inhibition of PLC-I and activation of PLC-III were observed, whereas PLC-II was unaffected. This observation suggests that any putative protein effectors for PLC should be critically scrutinized.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banno Y., Nakashima S., Nozawa Y. Partial purification of phosphoinositide phospholipase C from human platelet cytosol; characterization of its three forms. Biochem Biophys Res Commun. 1986 Apr 29;136(2):713–721. doi: 10.1016/0006-291x(86)90498-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi V., Porciatti F., Pasquali F., Bruni P. Transformation of BALB/3T3 cells with EJ/T24/H-ras oncogene inhibits adenylate cyclase response to beta-adrenergic agonist while increases muscarinic receptor dependent hydrolysis of inositol lipids. Biochem Biophys Res Commun. 1985 Nov 15;132(3):900–907. doi: 10.1016/0006-291x(85)91892-3. [DOI] [PubMed] [Google Scholar]
- Chueh S. H., Gill D. L. Inositol 1,4,5-trisphosphate and guanine nucleotides activate calcium release from endoplasmic reticulum via distinct mechanisms. J Biol Chem. 1986 Oct 25;261(30):13883–13886. [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Heterogeneity of the calcium-dependent phosphatidylinositol-phosphodiesterase of rat liver kidney, as revealed by column chromatofocusing. Biochem Biophys Res Commun. 1982 Jul 30;107(2):533–537. doi: 10.1016/0006-291x(82)91524-8. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem. 1982 Dec 10;257(23):14359–14364. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Characteristics of inositol trisphosphate-mediated Ca2+ release from permeabilized hepatocytes. J Biol Chem. 1986 Nov 5;261(31):14658–14664. [PubMed] [Google Scholar]
- Lee K. Y., Ryu S. H., Suh P. G., Choi W. C., Rhee S. G. Phospholipase C associated with particulate fractions of bovine brain. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5540–5544. doi: 10.1073/pnas.84.16.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. Regulation of phosphoinositide breakdown by guanine nucleotides. Life Sci. 1986 Jul 21;39(3):187–194. doi: 10.1016/0024-3205(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Cox A. C. Characterization of multiple forms of phosphoinositide-specific phospholipase C purified from human platelets. Biochem J. 1986 Jul 1;237(1):139–145. doi: 10.1042/bj2370139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Carroll R. C., Weglicki W. B. Multiple forms of phosphoinositide-specific phospholipase C of different relative molecular masses in animal tissues. Evidence for modification of the platelet enzyme by Ca2+-dependent proteinase. Biochem J. 1984 Aug 1;221(3):813–820. doi: 10.1042/bj2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Nomura H., Kikkawa U., Kishimoto A., Nishizuka Y. Rat brain and liver soluble phospholipase C: resolution of two forms with different requirements for calcium. Biochem Biophys Res Commun. 1985 Oct 30;132(2):582–590. doi: 10.1016/0006-291x(85)91173-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Two forms of phosphatidylinositol-specific phospholipase C from bovine brain. Biochem Biophys Res Commun. 1986 Nov 26;141(1):137–144. doi: 10.1016/s0006-291x(86)80345-x. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]